In this issue, Li and colleagues describe the growth and characterization of a specific subpopulation of dendritic cells with the ability to support the expansion of hematopoietic progenitor cells in vitro and induce immune tolerance in vivo.
Dendritic cells (DCs), named for their multiple projections, have multiple roles in hematopoiesis and the immune system.1 Previous work by this group and others has shown that dendritic cells play a critical role in hematopoietic stem cell (HSC) engraftment and can induce either tolerance to or rejection of transplanted HSCs.2,3 In the thymic microenvironment dendritic cells are potent antigen-presenting cells,4 and dendritic cells are also a component of the hematopoietic microenvironment, where their specific role has not been defined.2 One of many questions in this field is whether DCs are a relatively homogenous population of cells with multiple functions or whether specific subclasses of DCs are responsible for their various activities.1
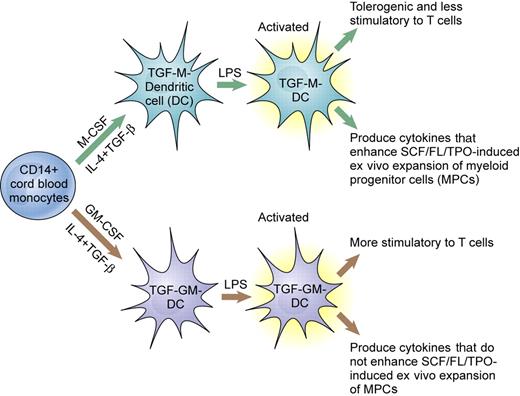
The paper by Li and colleagues, in the context of ongoing work in other labs, addresses this question. Li et al shows that human cord blood mononuclear cells treated with TGF-β, IL-4, and either M-CSF or GM-CSF give rise to phenotypically identical DCs that can be activated by exposure to lipopolysaccharide. But the 2 populations of DCs had markedly different and nonoverlapping activities. As shown previously, the GM-DCs were highly stimulatory to allogeneic T cells. Furthermore, conditioned medium from these cells had no ability to promote the expansion of human CD34+ progenitor cells in liquid culture. In contrast, the M-DCs were poor stimulators of allogeneic T cells (tolerogenic) and produced cytokines that promoted a robust expansion of progenitor cells in culture (see the figure).
Differentiation of human monocytes into dendritic cells. Depending on whether the cells are cultured with M-CSF or GM-CSF, dendritic cells with different activities are produced. Illustration by Kenneth X. Probst.
Differentiation of human monocytes into dendritic cells. Depending on whether the cells are cultured with M-CSF or GM-CSF, dendritic cells with different activities are produced. Illustration by Kenneth X. Probst.
The long-term impact of these findings is the opportunity to further dendritic cell re-search in many different directions. Clinically it needs to be demonstrated whether M-DCs can enhance HSC engraftment. It will be important to identify the cytokines produced by the M-DCs so that their role(s) in hematopoiesis can be better defined. Immunologists will want to compare the 2 populations of DCs for their ability to present antigens and their roles in tolerance induction. For this investigator, the critical question is whether the 2 populations of dendritic cells have a common ancestor that responds differently to M-CSF and GM-CSF depending on their microenvironment or whether they represent the progeny 2 already distinct progenitor cells. Although none of the answers to these questions is provided in the paper by Li et al, the demonstration that DCs with different activities can be cultured provides an excellent place to begin work in each of these different directions.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal