To the editor:
Study of inherited polycythemia/erythrocytosis has revealed defects in the oxygen-sensing pathway and provided insights into the regulation of erythropoietin (Epo) synthesis by hypoxia inducible factor (HIF).1 HIF is a dimer consisting of α and β subunits. Two different α isoforms exist: HIF-1α, which is ubiquitously expressed, and HIF-2α or EPAS1, which is limited to specific tissues. The constitutively expressed HIF-β subunit is not hypoxia regulated.2 In normoxia, the HIF-1α and HIF-2α proteins are rapidly degraded through a complex interaction with one of several iron-containing prolyl hydroxylases (PHDs), leading to the binding of the von Hippel Lindau protein (pVHL), ubiquitination, and degradation by the proteasome. This interaction constitutes the “hypoxia sensing” since hypoxia and iron deprivation leads to a posttranslational increase in both HIF-1α and HIF-2α proteins and increased transcription of an array of their target genes.2
The recent discovery of an iron-responsive element (IRE) in the 5′ untranslated region (UTR) of HIF2A reveals a novel regulatory link between iron availability and HIF2A expression.3 In iron deficiency, the iron regulatory proteins (IRPs) repress the translation of HIF-2α protein by binding to its 5′ IRE.3 Recent data implicates HIF-2α in the control of renal, hepatic, and brain Epo production in vivo.4,,–7 Altogether, these data indicate new molecular connections between HIF2A expression, erythropoiesis, and iron availability.
Polycythemia/erythrocytosis can be associated with raised Epo levels, which are indicative of a deregulated oxygen-sensing pathway. Indeed, defects in VHL and PHD2 have been described in a minority of patients.8,–10 We hypothesized that germ-line mutations in the 5′ IRE of the HIF2A gene would uncouple HIF-2α synthesis from negative translational control, predicting polycythemia/erythrocytosis with increased Epo levels (Figure 1). Consequently, a group of 147 such individuals referred to Belfast or Salt Lake City was screened for defects in the 5′ IRE by PCR-direct sequencing (Figure 1). The sequence in all 294 alleles was in full concordance with the published genomic sequence of HIF2A (EPAS1, RefSeq; AC016696, nucleotide 81298–81330).11 However, 4 single-nucleotide polymorphisms (SNPs) were detected (Figure 1B). According to the Entrez SNP database,12 the T allele for the rs17039192 SNP (nucleotide 81261; Figure 1) is present in the Japanese population at a frequency of 0.14. Within the group of 120 patients referred to Belfast, 3 T alleles were detected in individuals from China and Vietnam. Of the 27 patients with polycythemia studied in Salt Lake City, this T allele was not detected. However, we found 3 previously unreported SNPs at 3′ of the IRE at nucleotides 81405, 81411, and 81424; their allele frequencies in 27 patients with polycythemia were 0.85, 0.69, and 0.37, respectively; these did not meaningfully differ from 25 control participants, whose frequencies were 0.58, 0.20, and 0.28. We conclude that the loss of iron-regulated control of HIF-2α translation by the IRP/IRE regulatory system is not the cause of polycythemia/erythrocytosis in a large collection of patients collected from the United Kingdom, Europe, and North and Central America.
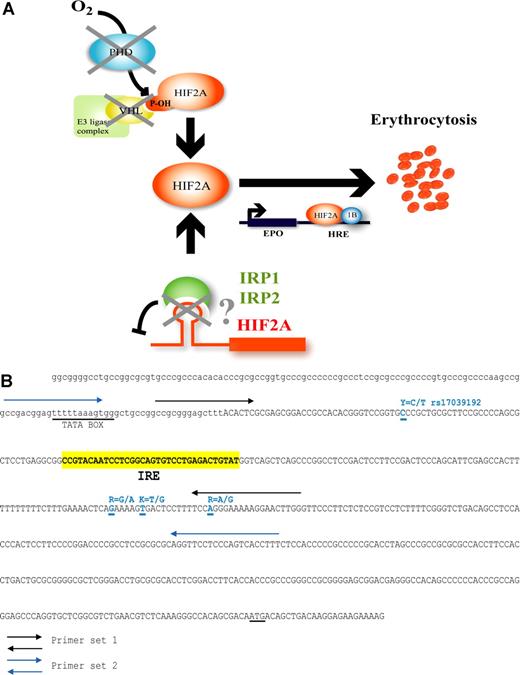
Hypothesis for HIF-2α deregulation through putative mutations present in its 5′ IRE and mutational sequence screening of the 5′ UTR of HIF2A. (A)Iron and oxygen regulation of HIF-2α (EPAS1) in oxygen homeostasis. Availability of iron influences the level of HIF-induced Epo gene transcription via binding to the 5′ IRE of HIF2A. In iron deficiency, the IRE is bound by iron regulatory proteins (IRPs), which prevent HIF-2α translation, but when iron is replete, the IRPs cannot bind the IRE and do not impede HIF-2α protein expression. In hypoxia, HIF-2α is free to escape proteasomal degradation and induce Epo gene expression. The loss of translational inhibition due to mutations in the HIF2A IRE may result in inappropriately high HIF-2α levels with deregulated Epo production, thus allowing for the development of erythrocytosis. (B) Sequence of the 5′ UTR region of HIF2A. The IRE region is indicated in yellow (nucleotides 81298 to 81330; RefSeq AC016696). Primer sets 1 and 2 are as indicated by black and blue arrows, respectively. All SNPs are indicated in blue. Described SNP rs17039192 is labeled Y and located at nucleotide 81261. The 3 uncharacterized SNPs are indicated by R, K, and R, and are located at nucleotides 81405, 81411, and 81424. TATA box and ATG sequences are underlined.
Hypothesis for HIF-2α deregulation through putative mutations present in its 5′ IRE and mutational sequence screening of the 5′ UTR of HIF2A. (A)Iron and oxygen regulation of HIF-2α (EPAS1) in oxygen homeostasis. Availability of iron influences the level of HIF-induced Epo gene transcription via binding to the 5′ IRE of HIF2A. In iron deficiency, the IRE is bound by iron regulatory proteins (IRPs), which prevent HIF-2α translation, but when iron is replete, the IRPs cannot bind the IRE and do not impede HIF-2α protein expression. In hypoxia, HIF-2α is free to escape proteasomal degradation and induce Epo gene expression. The loss of translational inhibition due to mutations in the HIF2A IRE may result in inappropriately high HIF-2α levels with deregulated Epo production, thus allowing for the development of erythrocytosis. (B) Sequence of the 5′ UTR region of HIF2A. The IRE region is indicated in yellow (nucleotides 81298 to 81330; RefSeq AC016696). Primer sets 1 and 2 are as indicated by black and blue arrows, respectively. All SNPs are indicated in blue. Described SNP rs17039192 is labeled Y and located at nucleotide 81261. The 3 uncharacterized SNPs are indicated by R, K, and R, and are located at nucleotides 81405, 81411, and 81424. TATA box and ATG sequences are underlined.
Authorship
The first three authors contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Melanie J. Percy, Department of Haematology, Floor C, Tower Block, Belfast City Hospital, Lisburn Road, Belfast, BT9 7AB, Northern Ireland; e-mail: melanie.percy@belfasttrust.hscni.net.