Sickle red cell (SS RBC) adhesion is believed to contribute to the process of vaso-occlusion in sickle cell disease (SCD). We previously found that the LW RBC adhesion receptor can be activated by epinephrine to mediate SS RBC adhesion to endothelial αvβ3 integrin. To determine the contribution of LW activation to vaso-occlusive events in vivo, we investigated whether in vitro treatment of SS RBCs by epinephrine resulted in vaso-occlusion in intact microvasculature after RBC infusion into nude mice. Epinephrine enhanced human SS but not normal RBC adhesion to murine endothelial cells in vitro and to endothelium in vivo, promoting vaso-occlusion and RBC organ sequestration. Murine sickle RBCs also responded to epinephrine with increased adhesion to postcapillary endothelium in nude mice. Epinephrine-induced SS RBC adhesion, vaso-occlusion, and RBC organ trapping could be prevented by the β-adrenergic receptor (β-AR) antagonist, propranolol. Infusion of soluble recombinant LW also significantly reduced adhesion and vaso-occlusion. In addition, epinephrine-treated SS RBCs induced activation of murine leukocyte adhesion to endothelium as well. We conclude that LW activation by epinephrine via β-AR stimulation can promote both SS RBC and leukocyte adhesion as well as vaso-occlusion, suggesting that both epinephrine and LW play potentially pathophysiological roles in SCD.
Introduction
Abnormal sickle red blood cell (SS RBC) adhesion to the vascular endothelium has been postulated to be important in the initiation and/or progression of vaso-occlusion in sickle cell disease (SCD).1,–3 Vaso-occlusive episodes are often associated with a variety of infectious and noninfectious stressors. Infection leads to increased levels of proinflammatory cytokines, which may induce activation of endothelial cells (ECs) and leukocytes, resulting ultimately in SS RBC adhesion, vaso-occlusion, and hypoxia/reperfusion-associated tissue injury. Patients with SCD also frequently report the development of vaso-occlusive symptoms after emotional and psychological stresses, changes in temperature, and physical exertion.4,–6 The molecular mechanism(s) by which these forms of stress may predispose to painful vaso-occlusive episodes has remained largely unexplored.
Catecholamines released during stress stimulate adrenergic receptors (ARs), including the β-AR. These receptors, archetypal members of the G protein–coupled receptor superfamily, are expressed by RBCs7 as well as by a variety of tissues throughout the body. β-ARs signal via stimulation of the heterotrimeric Gs protein, mediating activation of adenylate cyclase (AC)8 and subsequent generation of cAMP.9 AR stimulation with supraphysiological concentrations of epinephrine has been previously shown to alter normal RBC filterability.10 Recently, we showed that epinephrine induces activation of the LW glycoprotein on human SS but not normal RBCs to mediate adhesion to cultured ECs in vitro via activation of protein kinase A (PKA).11 We hypothesized that catecholamines associated with stress in vivo could induce activation of LW on SS RBCs, promoting or even initiating vaso-occlusion. Therefore, we sought to determine whether activation of LW on SS RBCs by epinephrine could induce pathophysiologically significant adhesion and initiate vaso-occlusion in vivo.
Materials and methods
Endothelial cells
The murine endothelial cell line EOMA (American Type Culture Collection [ATCC], Manassas, VA), which exhibits properties characteristic of microvascular endothelial cells, was grown as monolayers in Dulbecco modified Eagle media (DMEM) (Celprogen, San Pedro, CA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA). Human umbilical vein endothelial cells ([HUVECs] ATCC) were grown as previously described.11
Mice
All animal experiments were carried out in accordance with protocols approved by the Duke University Animal Care and Use Committee. Female athymic homozygous nude mice (nu-/nu-) were between 8 and 12 weeks of age (Charles River Laboratories, Wilmington, MA). Sickle12 and wild-type C57 black mice were obtained from Jackson Laboratories (Bar Harbor, ME).
Collection and preparation of RBCs
The Institutional Review Board of Duke University Medical School approved of obtaining patient and normal donor red cells for this study. Informed consent was obtained in accordance with the Declaration of Helsinki. SCD patient donors had not received transfusions for at least 3 months and were not on hydroxyurea. Murine and human blood samples were collected into citrate tubes. RBCs were separated from the buffy coat by gravity at 4°C for at least 2 hours. Plasma and buffy coat were aspirated, and RBCs were washed 4 times in sterile PBS with 1.26 mM Ca2+ and 0.9 mM Mg2+ (pH 7.4). Packed RBCs were analyzed for leukocyte and platelet contamination using an Automated Hematology Analyzer K-1000 (Sysmex America, Mundelein, IL).
Treatment of RBCs
Packed RBCs were fluorescently labeled for in vitro and in vivo adhesion studies as previously described.11,13 Dil or DiO (Molecular Probes, Eugene, OR) dyes used for in vivo studies have no effect on RBC suspension viscosity and RBC survival in the circulation.13 Cell morphology was checked by microscopy. Using conditions previously optimized for in vitro adhesion assays,11 human RBCs were sham-treated with buffer and vehicle alone, or treated at 37°C with 0.2 mM phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, St. Louis, MO) and 80 μM forskolin (Sigma) for 1 hour, or treated with 20 nM epinephrine (Sigma) for 1 minute. Cells were then washed 3 times with 5 mL PBS with Ca2+ and Mg2+. Murine normal or sickle RBCs were similarly sham or epinephrine treated.
In vitro adhesion assays
Assays of adhesion to ECs were performed in graduated-height flow chambers as described previously.11 To identify whether LW was involved in adhesion to EOMA cells, human SS RBCs were preincubated for 30 minutes with 10 μg/mL anti-LW (BS46),14 anti-CD47 (RBC thrombospondin receptor),15 or murine myeloma protein P3x63/Ag8 (nonreactive control murine immunoglobulin),16 washed, and then treated with epinephrine prior to adhesion assays.
To identify the AR subtype involved in SS RBC stimulation by epinephrine, SS RBCs were treated for 15 minutes with 10 μM phenoxybenzamine, propranolol, atenolol, or butoxamine (Sigma), which are α-, β-, β1-, and β2-AR antagonists, respectively, followed by treatment with epinephrine prior to adhesion assays.
Flow cytometric analysis
To detect possible interaction of murine immunoglobulin with human RBCs, blood samples were collected from mice 40 minutes after infusion of red fluorescent-labeled sham- or epinephrine-treated human normal RBCs, and RBCs were incubated with an FITC-conjugated antibody against murine immunoglobulins (Jackson ImmunoResearch Laboratories, West Grove, PA). To determine the percent of circulating human RBCs, Dil- and DiO-labeled RBCs from a single donor were sham or epinephrine treated, respectively, resuspended as a 1:1 mixture, and then infused into mice. Blood samples were collected 1, 5, 10, and 20 minutes after injection and analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Preparation and purification of soluble LW
The cDNA construct encoding recombinant soluble LW (sLW) was prepared by deleting the transmembrane and cytoplasmic domains of the full-length cDNA encoding LW17 by polymerase chain reaction (PCR). Primers used were the following: forward, 5′-CTTTTTGCCATGGGGTCTCTGT-3′; reverse, 5′-GCTCCAAGCGAGCATCAGTGT-3′. The amplified fragment was cloned into pcDNA 3.1/V5-His TOPO expression vector (Invitrogen) and transfected with TransFast reagent (Promega, Madison, WI) into 293 cells, which were then grown in MEME medium (Invitrogen) supplemented with 10% FBS and 500 μg/mL G418 (Invitrogen). sLW-transfected 293 cells were transferred to serum-free 293 SFM II medium (Invitrogen) with G418. sLW protein was purified with ProBond (Invitrogen).
Window chamber surgery
General anesthesia was achieved by intraperitoneal injection of 100 mg/kg ketamine (Abbott Laboratory, Chicago, IL) and 10 mg/kg xylazine (Bayer, Shawnee Mission, KS). A double-sided titanium frame window chamber was surgically implanted into the dorsal skin fold under sterile conditions using a laminar flow hood.18,–20 Surgery involved carefully removing the epidermal and dermal layers of one side of a dorsal skin fold, exposing the blood vessels of the subcutaneous tissue adjacent to the striated muscles of the opposing skin fold, and then securing the 2 sides of the chamber to the skin using stainless steel screws and sutures. A glass window was placed in the chamber to cover the exposed tissue and secured with a snap ring. Subsequently, animals were kept at 32°C to 34°C until in vivo studies were performed 3 days after surgery.
RBC infusions and intravital microscopy
Anesthetized animals with window chambers were placed on the stage of an Axoplan microscope (Carl Zeiss, Thornwood, NY); temperature was maintained at 37°C using a thermostatically controlled heating pad. All infusions were through the dorsal tail vein. Labeled treated human or murine RBCs (300 or 150 μL, respectively; hematocrit [Hct] 0.50 [50%] in PBS with Ca2+ and Mg2+) were infused, and RBC adhesion and blood flow dynamics were observed in subdermal vessels for at least 30 minutes using LD Achroplan 20×/0.40 Korr and Fluar 5×/0.25 objectives. Microcirculatory events and cell adhesion were simultaneously recorded using a Trinitron Color video monitor (PVM-1353 MD; Sony, Tokyo, Japan) and JVC video cassette recorder (BR-S3784; VCR King, Durham, NC) connected to a digital video camera C2400 (Hamamatsu Photonics KK, Hamamatsu City, Japan). Thirty segments of venules were examined for each set of conditions. Arterioles were distinguished from venules based on (1) observation of divergent flow as opposed to convergent flow; (2) birefringent appearance of vessel walls using transillumination, which is characteristic of arteriolar vascular smooth muscle; and (3) relatively straight vessel trajectory without evidence of tortuosity.21
Measurement of red cell flux and adhesion was performed by examining videotapes produced using × 20 magnification. Cell adherence was quantitated by considering cells attached to the vessel walls and immobile for 1 minute. The percentage of the length of vessels with diameters up to 25 μm or more than 25 μm, occupied by SS RBCs, was quantified as follows: % venular length occupied by SS RBCs = (length of vessel wall with adherent cells/total length of the vessel segments analyzed) × 100. Changes in RBC flux were calculated as follows: flux = number of circulating fluorescent human RBCs crossing a single point marked on vessels less than 50 μm in diameter per minute.
For β-AR blockade, SS RBCs were incubated at 37°C for 15 minutes with 10 μM propranolol alone, epinephrine alone, or propranolol followed by epinephrine. SS RBCs were then washed 3 times before infusion.
To identify whether RBC LW was involved in adhesion, 500 μg sLW or sCD44 (generously provided by Dr Barton F. Haynes, Duke University) was infused into anesthetized mice 30 minutes before infusion of epinephrine-treated SS RBCs.
Acridine orange (2 mg/kg; Sigma) was infused to detect whether murine leukocytes were adherent to endothelium as a result of inflammation due to window chamber implantation, in the absence of infused RBCs. To detect murine leukocyte adhesion associated with RBC infusion, circulating murine leukocytes were labeled by infusion of 100 μg rat FITC-conjugated antimouse CD45 (ATCC) or LFA-1 (ATCC) 30 minutes prior to infusion of red fluorescently labeled human SS RBCs.
Histology
Animals were killed 30 minutes after injection of labeled RBCs, and organs were collected and snap-frozen in OCT media. Sections measuring 40 μm were cut from 4 standardized locations in each organ, mounted, and examined via inverted fluorescence microscopy (Axioskop 2 Plus, Zeiss) using Fluar 20×/0.75 objective. Three random fields were imaged for each section of each organ (Axiocam, Zeiss), and fluorescence intensity for each field was quantified using Adobe Photoshop CS2 software (Adobe Systems, San Jose, CA). The values were averaged for the 3 fields to obtain mean fluorescence intensity. The mean fluorescence values were averaged among groups of animals (n = 3) for statistical analysis using a paired t test. Figures were prepared using Photoshop CS 2 software.
Radioligand binding
RBCs were lysed as previously described,11 and total membrane protein concentrations were determined by Bradford assay corrected for residual hemoglobin content. To determine the level of β-AR expression on RBCs, saturation-binding experiments were performed using 125I-cyanopindolol (125I-cyp) (PerkinElmer Life Sciences, Boston, MA) as described previously.22
Statistical analysis
Results using sham and treated RBCs were compared only with results using the same donor sample, because RBC adhesion varies greatly among SCD patients.11 Data were compared using parametric analyses (GraphPad Prism 4 Software, San Diego, CA), including repeated and nonrepeated measures of analysis of variance (ANOVA). One-way and 2-way ANOVA analyses were followed by Bonferroni corrections for multiple comparisons (multiplying the P value by the number of comparisons). A P value below .05 was considered significant.
Results
Epinephrine increases human and murine SS RBC adhesion to murine ECs in vitro.
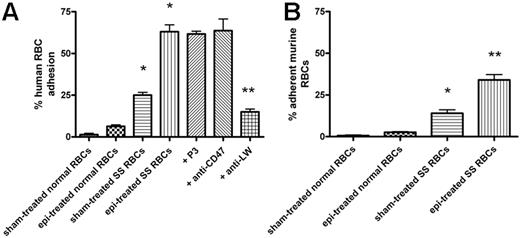
We first determined whether epinephrine, at a physiologic “stress” dose (20 nM),10 up-regulates SS RBC interaction with mouse ECs. Sham-treated human and murine SS RBCs adhered to some degree to EOMA cells at a shear stress of 2 dyne/cm2 (Figure 1). Epinephrine enhanced human and murine SS RBC adhesion by 2.6 (± 0.2)-fold and 2.5 (± 0.2)-fold, respectively, over baseline sham-treated cells. In contrast, epinephrine did not significantly increase adhesion of normal human or murine RBCs when compared with adhesion of sham-treated normal cells.
Epinephrine induces increased human and murine SS RBC adhesion to murine EOMA cells in vitro. Results are presented as percent adherent RBCs at a shear stress of 2 dynes/cm2; error bars show SEM of 3 different experiments. RBCs were sham treated or stimulated with 20 nM epinephrine (epi) for 1 minute. (A) Human RBCs: Inhibition of adhesion with antibody was performed as described in “Materials and methods.” *P < .001 compared with sham-treated normal RBCs; **P < .001 compared with epi-treated SS RBCs. (B) Murine normal and SS RBC adhesion: *P < .01 compared with sham-treated normal RBCs; **P < .001 compared with sham-treated SS RBCs.
Epinephrine induces increased human and murine SS RBC adhesion to murine EOMA cells in vitro. Results are presented as percent adherent RBCs at a shear stress of 2 dynes/cm2; error bars show SEM of 3 different experiments. RBCs were sham treated or stimulated with 20 nM epinephrine (epi) for 1 minute. (A) Human RBCs: Inhibition of adhesion with antibody was performed as described in “Materials and methods.” *P < .001 compared with sham-treated normal RBCs; **P < .001 compared with epi-treated SS RBCs. (B) Murine normal and SS RBC adhesion: *P < .01 compared with sham-treated normal RBCs; **P < .001 compared with sham-treated SS RBCs.
Human RBCs in nude mice
To validate the use of nude mice, we showed that there was insignificant murine immunoglobulin bound to circulating sham- or epinephrine-treated human normal RBCs 40 minutes after infusion, as shown by the paucity of double-labeled RBCs (Figure 2A).
Immune interactions and survival of human RBCs in nude mice. (A) Anesthetized nude mice were infused with Dil (rhodamine)–labeled sham-treated or epi-treated normal RBCs. Blood samples collected 40 minutes after infusion were analyzed for murine immunoglobulin, which was detected using FITC-labeled antimurine immunoglobulin, bound to human RBCs. Insignificant murine immunoglobulin was bound to circulating sham- or epi-treated human normal RBCs compared with noninfused cells. One representative experiment is shown (n = 3). (B,C) Anesthetized mice were injected with a 1:1 mixture of Dil-labeled sham- (■) and DiO-labeled epi-treated (□) RBCs ([B] normal, [C] SS RBCs; n = 3 for each). Error bars show SEM of 3 different experiments. The percentages of sham- and epi-treated normal RBCs circulating in the bloodstream of animals at all times were not significantly different. A significantly greater percentage of sham-treated than epi-treated SS RBCs was retained in the circulation at all times (P < .001). (D,E) Normal RBCs were sham treated or epi treated. Sham-treated normal RBCs were detected to some degree in the spleen. Epi treatment did not lead to a significant increase in splenic trapping of normal RBCs. (F,G) Epi treatment had a significant effect on trapping of SS RBCs in the spleen compared with sham-treated cells. *P < .05 compared with sham-treated. Scale bar for panels D and F = 100 μm.
Immune interactions and survival of human RBCs in nude mice. (A) Anesthetized nude mice were infused with Dil (rhodamine)–labeled sham-treated or epi-treated normal RBCs. Blood samples collected 40 minutes after infusion were analyzed for murine immunoglobulin, which was detected using FITC-labeled antimurine immunoglobulin, bound to human RBCs. Insignificant murine immunoglobulin was bound to circulating sham- or epi-treated human normal RBCs compared with noninfused cells. One representative experiment is shown (n = 3). (B,C) Anesthetized mice were injected with a 1:1 mixture of Dil-labeled sham- (■) and DiO-labeled epi-treated (□) RBCs ([B] normal, [C] SS RBCs; n = 3 for each). Error bars show SEM of 3 different experiments. The percentages of sham- and epi-treated normal RBCs circulating in the bloodstream of animals at all times were not significantly different. A significantly greater percentage of sham-treated than epi-treated SS RBCs was retained in the circulation at all times (P < .001). (D,E) Normal RBCs were sham treated or epi treated. Sham-treated normal RBCs were detected to some degree in the spleen. Epi treatment did not lead to a significant increase in splenic trapping of normal RBCs. (F,G) Epi treatment had a significant effect on trapping of SS RBCs in the spleen compared with sham-treated cells. *P < .05 compared with sham-treated. Scale bar for panels D and F = 100 μm.
Clearance of human RBCs from the circulation 20 minutes after injection of sham- and epinephrine-treated RBC mixtures was also measured. The percentage of sham-treated and epinephrine-treated normal RBCs remaining in the bloodstream were similar (84% ± 10.7% and 90% ± 4.1%, respectively) (Figure 2B). The percentage of remaining sham-treated SS RBCs was 55% ± 3.6% (Figure 2C), not significantly lower than the percentage of sham-treated normal RBCs (P > 0.05). The modestly enhanced clearance of sham-treated sickle cells is likely due to factors such as adhesion, membrane rigidity, sensitivity of cells to hemolysis, and/or SS RBC trapping by the reticuloendothelial system. However, epinephrine markedly lowered the percentage of SS RBCs remaining in the circulation to 20% (± 2.9%); Figure 2C; P < .001 for epinephrine-treated SS versus normal [sham- or epinephrine-treated], and P < .05 for epinephrine-treated SS versus sham-treated SS) despite the fact that sham- and epinephrine-treated SS RBCs were mixed and coinjected, which could reflect both increased adhesion to vessel walls as well as organ sequestration.
Clearance of xenogeneic RBCs has been attributed to RBC uptake by splenic macrophages,23 which may involve innate cellular recognition of RBC carbohydrates by lectins.24 We therefore determined the degree to which human RBCs were sequestered in the spleen of animals at 40 minutes. Trapping of both sham-treated normal (Figure 2D,E) and SS RBCs (Figure 2F,G) was detected to some degree in the spleen. Epinephrine significantly increased SS but not normal RBC trapping by 3-fold compared with sham-treated cells (P < .05), reflecting in part the decreased survival of these cells in the bloodstream. In view of these data, we felt that observations made during the first 30 minutes after infusion would be informative, because the percentages of circulating xenogeneic normal RBCs 20 minutes after injection remained high and epinephrine did not alter splenic sequestration of normal human RBCs during that period.
Other investigators have shown that infusion of a small fraction (7.8%) of labeled human RBCs into rats remained rheologically normal, had the same velocity as the total cell population, and caused no change in vascular diameters.25 In our studies, the quantity of human and murine RBCs did not exceed 10% and 5% of the total circulating RBCs, respectively, assuming that the mouse blood volume is 1.5 mL, thereby minimizing any possible rheologic effects attributable to increased Hct.26 RBCs in these small concentrations should also not influence vascular regulatory mechanisms or O2 delivery.
Epinephrine induces both human and murine SS RBC adhesion to vascular endothelium in vivo
These experiments were designed specifically to study the effect of epinephrine on activation of SS RBC adhesion to the endothelium and vaso-occlusion in the absence of potentially confounding non-RBC signals, such as those derived from stimulation of murine endothelium or leukocytes, and to determine whether LW has pathophysiological significance in mediating RBC adhesion. We first determined whether dorsal skin-fold window chamber implantation induced persistent inflammation and, therefore, leukocyte adhesion to endothelium prior to RBC infusion. Acridine orange staining failed to show murine leukocyte-endothelial interactions in dermal vasculature as a result of window chamber implantation (data not shown).
Normal and SS human RBC preparations contained 0.24 × 1012/L (± 0.9 × 1012/L) RBCs and 0.1 × 1012/L (± 0.01 × 1012/L) (0.24 × 106/μL ± 0.9 × 106/μL RBCs and 0.1 × 106/μL ± 0.01 × 106/μL) RBCs, respectively, and both showed unmeasurable (0 cells per microliter) leukocytes or platelets, making it unlikely that human leukocytes and platelets could participate in SS RBC adhesion and vaso-occlusion in our model.27
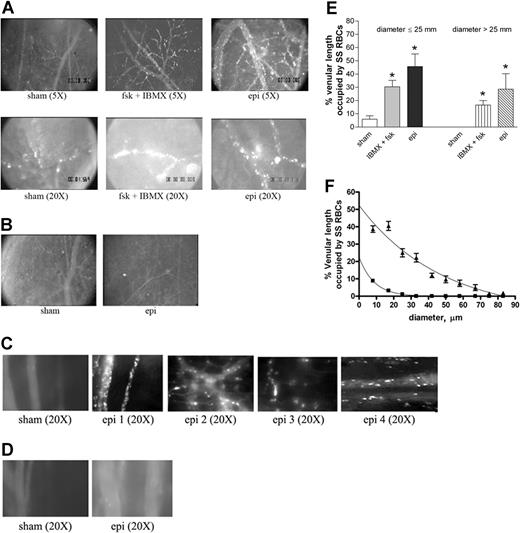
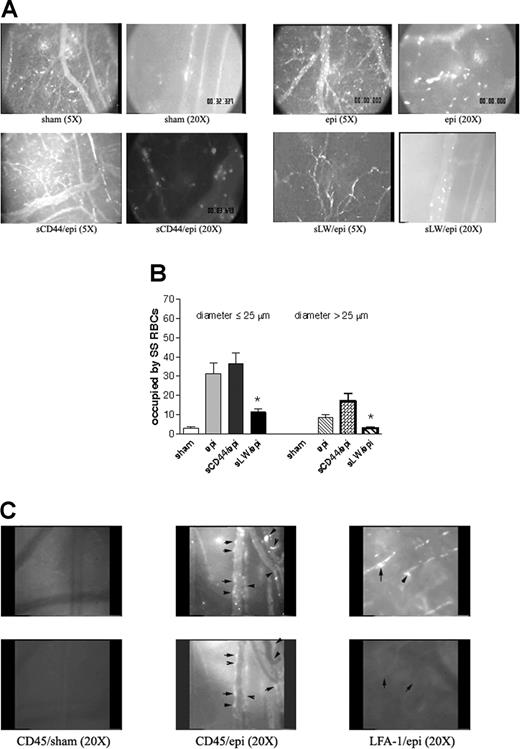
Epinephrine increases intracellular RBC cAMP.28 To test the effect of up-regulation of intracellular cAMP on sickle cell adhesion in vivo, all human and murine RBCs were fluorescently labeled, exposed to stimuli known to increase endogenous cAMP or sham treated in vitro, and then washed again prior to infusion into animals for observation within native intact vessels. The conditions and procedures used to manipulate RBCs were previously shown to be optimal in in vitro adhesion assays and have been shown not to mediate their effects through stimulation of ECs.11 Sham-treated human SS RBCs showed very little adhesion to vascular endothelium and adhered only occasionally to small postcapillary venules not larger than 25 μm in diameter (Figure 3A; Videos S1,S2, available on the Blood website; see the Supplemental Videos link at the top of the online article). In sharp contrast, infusion of SS RBCs treated with IBMX, a phosphodiesterase inhibitor known to prevent degradation of intracellular cAMP, and forskolin, a direct activator of AC, resulted in marked RBC adhesion in vessels 9 to 25 μm in diameter, predominantly in postcapillary venules, with intermittent occlusion of vessels as large as 17 μm in diameter and permanent blockage of some vessel segments, especially at junctions (Figure 3A; Videos S3,S4). Adhesion of IBMX and forskolin-treated SS RBCs was also noted in larger vessels with diameters larger than 17 μm and up to 83 μm.
Epinephrine stimulates activation of SS, but not normal, RBC adhesion to vessel walls in nude mice. (A-D) Microscopic observations of postcapillary venules were conducted through implanted window chambers after infusion of RBCs into the tail vein of nude mice using × 5 and × 20 magnification to observe human RBCs and × 20 magnification to observe murine RBCs. Vessels without adherent cells appear gray due to rapidly moving fluorescent RBCs. (A) Infusion of sham-treated (n = 8), forskolin (fsk) + IBMX–treated (n = 3) or epi-treated (n = 8) human SS RBCs. Sham-treated human SS RBCs showed little adhesion to vessel walls, whereas fsk + IBMX–treated and, to a greater extent, epi-treated human SS RBCs showed marked adhesion to postcapillary venules, with intermittent vaso-occlusion, as indicated by arrows. (B) Infusion of sham-treated (n = 3) or epi-treated (n = 3) normal human RBCs. Sham-treated human normal RBCs showed no adhesion to venule walls. Epi had little to no effect on human normal RBC adhesion to vessel walls. Scale bar = 200 μm. (C) Infusion of sham-treated (n = 4) or epi-treated (n = 4) murine sickle cells. Sham-treated murine SS RBCs showed very weak adhesion to vessel walls. Epi increased adhesion of murine SS RBCs to postcapillary venules. (D) Infusion of sham-treated (n = 3) or epi-treated (n = 3) normal murine RBCs. Sham- or epi-treated normal murine RBCs showed no adhesion to venule walls. (E) Fsk + IBMX or epi enhances human SS RBC occupation of venular length. SS RBCs were sham treated, IBMX + fsk treated, or epi treated (n = 3 for each treatment) prior to infusion into the tail vein of nude mice. The values of 30 segments of vessels analyzed were averaged among groups of animals to represent the mean percent venular length occupied by SS RBCs. Error bars show SEM of 3 different experiments for each treatment. *P < .05 compared with sham-treated regardless of the vessel diameter within the ranges specified. (F) Percentage venular length occupied by human SS RBCs related to venular diameter. Animals were injected with sham- (■) or epi-treated (▴) SS RBCs. Error bars show SEM for 6 different experiments for each treatment. *P < .001 compared with sham-treated. Data were compared using 1-way ANOVA analysis followed by Bonferroni corrections for multiple comparisons.
Epinephrine stimulates activation of SS, but not normal, RBC adhesion to vessel walls in nude mice. (A-D) Microscopic observations of postcapillary venules were conducted through implanted window chambers after infusion of RBCs into the tail vein of nude mice using × 5 and × 20 magnification to observe human RBCs and × 20 magnification to observe murine RBCs. Vessels without adherent cells appear gray due to rapidly moving fluorescent RBCs. (A) Infusion of sham-treated (n = 8), forskolin (fsk) + IBMX–treated (n = 3) or epi-treated (n = 8) human SS RBCs. Sham-treated human SS RBCs showed little adhesion to vessel walls, whereas fsk + IBMX–treated and, to a greater extent, epi-treated human SS RBCs showed marked adhesion to postcapillary venules, with intermittent vaso-occlusion, as indicated by arrows. (B) Infusion of sham-treated (n = 3) or epi-treated (n = 3) normal human RBCs. Sham-treated human normal RBCs showed no adhesion to venule walls. Epi had little to no effect on human normal RBC adhesion to vessel walls. Scale bar = 200 μm. (C) Infusion of sham-treated (n = 4) or epi-treated (n = 4) murine sickle cells. Sham-treated murine SS RBCs showed very weak adhesion to vessel walls. Epi increased adhesion of murine SS RBCs to postcapillary venules. (D) Infusion of sham-treated (n = 3) or epi-treated (n = 3) normal murine RBCs. Sham- or epi-treated normal murine RBCs showed no adhesion to venule walls. (E) Fsk + IBMX or epi enhances human SS RBC occupation of venular length. SS RBCs were sham treated, IBMX + fsk treated, or epi treated (n = 3 for each treatment) prior to infusion into the tail vein of nude mice. The values of 30 segments of vessels analyzed were averaged among groups of animals to represent the mean percent venular length occupied by SS RBCs. Error bars show SEM of 3 different experiments for each treatment. *P < .05 compared with sham-treated regardless of the vessel diameter within the ranges specified. (F) Percentage venular length occupied by human SS RBCs related to venular diameter. Animals were injected with sham- (■) or epi-treated (▴) SS RBCs. Error bars show SEM for 6 different experiments for each treatment. *P < .001 compared with sham-treated. Data were compared using 1-way ANOVA analysis followed by Bonferroni corrections for multiple comparisons.
The effect of epinephrine on SS RBC adhesion was even greater than that observed with IBMX and forskolin. Epinephrine in general induced both time-dependent adhesion of SS RBCs to venular endothelium and led to the permanent obstruction of small-diameter (9 to 25 μm) vessels (Figure 3A; Videos S5Video 6. Epinephrine induced time-dependent adhesion of SS RBCs, 27 minutes after infusion of SS RBCs (MOV, 1.31 MB)–S7). SS RBC adhesion was also observed in much larger vessels (up to 83 μm in diameter) (Video S8). In some vessels of up to 183 μm in diameter, there were actually occasional transient stoppages in blood flow with reversal of direction, indicating shifts in network flow dynamics (Video S9). Vaso-occlusion occurred most frequently where vessels curved and at junctions, although it was also observed in straight nonjunctional venular segments. In contrast, sham-treated normal RBCs showed almost no adhesion to vessel walls, and epinephrine-stimulated adhesion of normal RBCs was all but absent (Figure 3B).
The effect of epinephrine on murine sickle RBC adhesion was also tested in nude mice. Sham-treated murine SS RBCs adhered weakly to postcapillary vessels (Figure 3C). However, epinephrine up-regulated adhesion of murine SS RBCs to endothelium but without promotion of frank vaso-occlusion. In contrast, sham- and epinephrine-treated normal murine RBCs completely failed to adhere to endothelium (Figure 3D).
Human RBC adhesion was quantified as percent venular length occupied by SS RBCs. The percentage of venular length occupied by SS RBCs significantly increased when cells were treated either with IBMX + forskolin or with epinephrine for vessels up to 25 μm in diameter (P < .05) (Figure 3E). The percentage of venular length occupied by SS RBCs also increased significantly in vessels with a diameter larger than 25 μm (P < .05) when cells were treated either with IBMX + forskolin or with epinephrine (Figure 3E). As a result of epinephrine-stimulated SS RBC adhesion and vaso-occlusion, blood flow rates were dramatically decreased, with microvessel red cell fluxes of 19 700 ± 9000 and 6600 ± 1500 RBCs per minute in vessels less than 50 μm in diameter for sham-treated and epinephrine-treated cells, respectively (P < .001). The percentage of vessel length occupied by human SS RBCs was also plotted as a function of venular diameter. The percent vessel length occupied by SS RBCs was inversely related to the vessel diameter in both treatment groups (animals infused with sham- or epinephrine-treated SS RBCs). For a venule diameter of 8 μm, percent vascular wall occupied by sham-treated SS RBCs was significantly lower than for epinephrine-treated SS RBCs (8% ± 0.6% and 38.7% ± 1.89%, respectively; P < .001). Percent vascular wall occupied by sham-treated SS RBCs decreased and became null when venular diameter reached 33 μm. However, despite the decrease in percent vascular length occupied by adherent epinephrine-treated SS RBCs in relation to increased vascular diameter, the percent venular length occupied by epinephrine-treated SS RBCs at a venular diameter of 33 μm stayed significantly high when compared with sham-treated cells (P < .001) (Figure 3F).
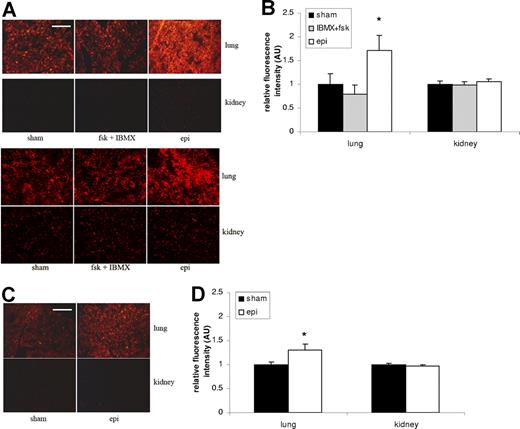
Epinephrine induces human RBC organ sequestration
We examined whether exposure of SS RBCs to epinephrine influenced the trapping of RBCs in organs typically affected and damaged in SCD. Trapping of sham-treated SS and normal RBCs was detected in the lung but only weakly in the kidney (Figure 4). Epinephrine significantly increased trapping in the lung of both SS RBCs (1.7-fold) (Figure 4A-B) and normal RBCs, although more modestly (1.3-fold) (Figure 4C-D) compared with sham-treated SS (P < .05) and normal (P < .05) RBCs, respectively. However, neither IBMX + forskolin nor epinephrine increased SS RBC sequestration in the kidney compared with sham-treated cells.
Epinephrine increases SS RBC organ sequestration. (A,B) SS RBCs were sham treated, fsk + IBMX treated, or epi treated. Sham-treated SS RBCs were detected to some degree in the lung but only minimally in the kidney. Treatment with fsk + IBMX did not significantly increase SS RBC trapping in the lung or kidney, and epi treatment led to significant increases in RBCs trapping only in the lung. Error bars show SEM of 3 different experiments. *P < .05 compared with sham-treated. The 2 parts in panel A represent 2 different experiments with similar results. (C,D) Normal RBCs were sham treated or epi treated. Epi treatment only had a significant effect on trapping of normal RBCs in the lung compared with sham-treated cells. Error bars show SEM of 3 different experiments. *P < .05 compared with sham-treated. Scale bar = 100 μm.
Epinephrine increases SS RBC organ sequestration. (A,B) SS RBCs were sham treated, fsk + IBMX treated, or epi treated. Sham-treated SS RBCs were detected to some degree in the lung but only minimally in the kidney. Treatment with fsk + IBMX did not significantly increase SS RBC trapping in the lung or kidney, and epi treatment led to significant increases in RBCs trapping only in the lung. Error bars show SEM of 3 different experiments. *P < .05 compared with sham-treated. The 2 parts in panel A represent 2 different experiments with similar results. (C,D) Normal RBCs were sham treated or epi treated. Epi treatment only had a significant effect on trapping of normal RBCs in the lung compared with sham-treated cells. Error bars show SEM of 3 different experiments. *P < .05 compared with sham-treated. Scale bar = 100 μm.
Epinephrine-stimulated SS RBC adhesion and organ sequestration is β2-AR mediated
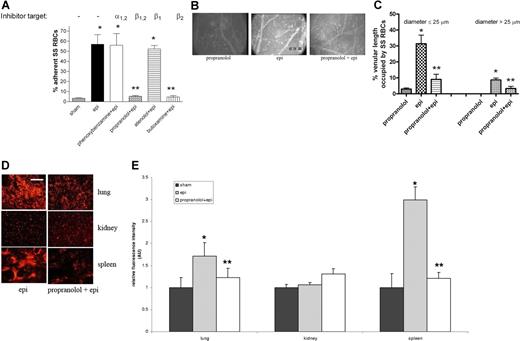
SS RBCs expressed significantly more β-ARs (20.63 ± 1.8 fmol/mg protein; n = 15) than did normal RBCs (10.83 ± 0.8 fmol/mg protein; n = 5) (P < .01), and adhesion of epinephrine-treated SS RBCs to HUVECs in vitro was significantly blocked by the β1,2- and β2-AR antagonists propranolol and butoxamine, respectively (Figure 5A), while phenoxybenzamine and atenolol, α- and β1-AR antagonists, respectively, did not inhibit adhesion. Thus, epinephrine appears to enhance SS RBC adhesion via stimulation of β2-ARs.
Effect of propranolol on epinephrine-induced SS RBC adhesion and organ sequestration. (A) β- and β2-AR antagonists abolish epi-induced SS RBC adhesion to HUVECs in vitro at a shear stress of 2 dynes/cm2. SS RBCs were sham treated, epi treated, or pretreated with phenoxybenzamine, propranolol, atenolol, or butoxamine followed by treatment with epi (n = 3 each). Error bars show SEM of 3 different experiments. *P < .01 compared with sham-treated; **P < .001 compared with epi-treated. (B) In vitro propranolol treatment had no effect on SS RBC adhesion (n = 5) in vivo, whereas epi dramatically increased SS RBC adhesion and vaso-occlusion to venule walls (n = 5). Propranolol treatment of SS RBCs significantly reduced subsequent epi-stimulated adhesion and stasis (n = 5). (C) Effect of propranolol on percent venular length occupied by SS RBCs. Video frames showing more than 30 vessel segments were used to quantify the length of venules occupied by SS RBCs in animals infused with SS RBCs treated as described for panel A (n = 5 for each treatment). The values were averaged among groups of animals to represent the mean percent venular length occupied by SS RBCs. Error bars show SEM. *P < .01 compared with propranolol-treated RBCs for vessels up to 25 μm in diameter, and P < .001 for vessels more than 25 μm in diameter. **P < .01 compared with epi-treated RBCs regardless of the vessel diameter. (D-E) SS RBCs were epi treated or propranolol + epi treated. Epi-treated SS RBCs were extensively trapped in the lung and spleen. Propranolol significantly reduced epi-treated SS RBC trapping in the lung and spleen. SS RBC trapping in the kidney was similar for all cell preparations. Error bars show SEM of 5 different experiments. *P < .05 compared with sham-treated (not pictured); **P < .05 compared with epi-treated. Scale bar = 100 μm.
Effect of propranolol on epinephrine-induced SS RBC adhesion and organ sequestration. (A) β- and β2-AR antagonists abolish epi-induced SS RBC adhesion to HUVECs in vitro at a shear stress of 2 dynes/cm2. SS RBCs were sham treated, epi treated, or pretreated with phenoxybenzamine, propranolol, atenolol, or butoxamine followed by treatment with epi (n = 3 each). Error bars show SEM of 3 different experiments. *P < .01 compared with sham-treated; **P < .001 compared with epi-treated. (B) In vitro propranolol treatment had no effect on SS RBC adhesion (n = 5) in vivo, whereas epi dramatically increased SS RBC adhesion and vaso-occlusion to venule walls (n = 5). Propranolol treatment of SS RBCs significantly reduced subsequent epi-stimulated adhesion and stasis (n = 5). (C) Effect of propranolol on percent venular length occupied by SS RBCs. Video frames showing more than 30 vessel segments were used to quantify the length of venules occupied by SS RBCs in animals infused with SS RBCs treated as described for panel A (n = 5 for each treatment). The values were averaged among groups of animals to represent the mean percent venular length occupied by SS RBCs. Error bars show SEM. *P < .01 compared with propranolol-treated RBCs for vessels up to 25 μm in diameter, and P < .001 for vessels more than 25 μm in diameter. **P < .01 compared with epi-treated RBCs regardless of the vessel diameter. (D-E) SS RBCs were epi treated or propranolol + epi treated. Epi-treated SS RBCs were extensively trapped in the lung and spleen. Propranolol significantly reduced epi-treated SS RBC trapping in the lung and spleen. SS RBC trapping in the kidney was similar for all cell preparations. Error bars show SEM of 5 different experiments. *P < .05 compared with sham-treated (not pictured); **P < .05 compared with epi-treated. Scale bar = 100 μm.
β-ARs are also involved in epinephrine-stimulated SS RBC adhesion in vivo. Propranolol-treated SS RBCs behaved similarly to sham-treated SS RBCs, showing minimal adhesion (Figure 5B). As with the results shown in Figure 3, epinephrine alone induced pronounced SS RBC adhesion, with obstruction of small-diameter venules. SS RBC treatment in vitro with propranolol followed by epinephrine all but eliminated RBC adhesion to postcapillary vessels, and obstruction of vessels was dramatically reduced (Figure 5B). As a result of β-AR blockade by propranolol, circulation of SS RBCs significantly improved, with fluxes of 18 800 (± 7900), 3500 (± 1400), and 16 700 (± 5000) RBCs per minutes in vessels less than 50 μm in diameter for the propranolol-treated, epinephrine-treated, and propranolol + epinephrine–treated cells, respectively (P < .001 for both propranolol-treated versus epinephrine-treated and epinephrine-treated versus propranolol + epinephrine–treated). Propranolol also induced a significant decrease in percentage venular length occupied by epinephrine-treated SS RBCs from 31% (± 5.46%) to 9% (± 3.2%) for vessels up to 25 μm in diameter (P < .01 for epinephrine-treated versus propranolol + epinephrine–treated) and from 9% (± 1.2%) to 3% (± 1.3%) for vessels more than 25 μm in diameter (P < .01 for epi-nephrine-treated versus propranolol + epinephrine–treated) (Figure 5C).
In addition, SS RBC trapping in the lung and spleen significantly decreased when SS RBCs were treated in vitro with propranolol followed by epinephrine compared with organ sequestration of SS RBCs treated with epinephrine alone (P < .05) (Figure 5D-E). SS RBC trapping in kidney did not vary significantly after epinephrine or propranolol treatment of RBCs.
Epinephrine-stimulated human SS RBCs adhere via LW glycoprotein and activate murine leukocyte adhesion
We attempted to block vaso-occlusion induced by epinephrine-stimulated SS RBCs using sLW and sCD44. Intravenous administration of 500 μg sCD44 failed to inhibit epinephrine-stimulated SS RBC adhesion and vaso-occlusion (Figure 6A). In contrast, infusion of sLW (500 μg) abrogated adhesion of subsequently injected epinephrine-stimulated SS RBCs to postcapillary venules and markedly reduced small-diameter vessel obstruction (Figure 6A). sLW at 100 or 250 μg failed to significantly inhibit epinephrine-stimulated SS RBC adhesion and vaso-occlusion (data not shown). However, 500 μg sLW induced a significant decrease by 69% ± 3.2% in percentage venular length occupied by epinephrine-stimulated SS RBCs for vessels up to 25 μm in diameter (P < .001 for sCD44 versus sLW) (Figure 6B). Similarly, when vessel diameter was more than 25 μm, sLW reduced the percentage of venular length occupied by epinephrine-treated SS RBCs by 79% ± 6.44% (P < .01 for sCD44 versus sLW). sLW also significantly improved circulation of epinephrine-treated SS RBCs, with RBC fluxes of 4800 ± 2000 and 18 600 ± 6100 RBCs per minute in vessels less than 50 μm in diameter for sCD44- and sLW-treated animals, respectively (P < .001). Our data thus strongly suggest that SS RBC adhesion to endothelium and subsequent vaso-occlusion are mediated at least in part through activated LW, supporting the pathophysiological significance of LW in vivo.
Epinephrine-activated SS RBCs adhere through LW and induce activation and adhesion of murine leukocytes. (A-B) Epinephrine-induced SS RBC adhesion and vaso-occlusion are mediated via LW. (A) Inhibition of SS RBC adhesion with soluble proteins was performed as described in “Material and methods.” Sham-treated SS RBCs showed little adhesion to vessel walls, whereas epi-treated SS RBCs showed marked adhesion to postcapillary venules, with intermittent vaso-occlusion. Infusion of sCD44 (as a negative control) 30 minutes prior to intravenous administration of epi-treated SS RBCs (n = 5) had no effect on epi-treated SS RBC adhesion to postcapillary venules, whereas infusion of sLW 30 minutes prior to injection of epi-treated SS RBCs (n = 5) markedly inhibited adhesion to postcapillary vessels. (B) Involvement of LW in SS RBC adhesion to endothelium and vaso-occlusion. Animals were infused with sham- or epi-treated SS RBCs 30 minutes after intravenous administration of sCD44 or sLW (n = 5 for each treatment). Thirty segments of venules were analyzed to quantify the length of venules occupied by SS RBCs. The values were averaged among groups of animals to represent the mean percent venular length occupied by SS RBCs. Error bars show SEM of 5 different experiments. *P < .001 compared with sCD44/epi-treated (animals/cells) for vessels up to 25 μm in diameter, and P < .05 for vessels more than 25 μm in diameter. (C) Epi-activated human SS RBCs induce adhesion of murine leukocytes to endothelium. Fluorescently labeled (red) sham- (n = 3) and epi-treated SS RBCs (n = 3) were infused 30 minutes after injection of rat FITC-labeled antimouse CD45 or LFA-1 antibodies. In the presence of epi-treated human SS RBCs, murine leukocytes adhered to postcapillary endothelium, and this adhesion was blocked by antibody against mouse LFA-1. Arrows indicate identical areas visualized with filters for red and green fluorescence to show where human SS RBCs and murine leukocytes are adherent to endothelium. In some areas, both murine leukocytes and human SS RBCs were adherent, whereas in other areas only one adherent cell type was present. Top panels show adhesion of human SS RBCs, bottom panels show adhesion of murine leukocytes.
Epinephrine-activated SS RBCs adhere through LW and induce activation and adhesion of murine leukocytes. (A-B) Epinephrine-induced SS RBC adhesion and vaso-occlusion are mediated via LW. (A) Inhibition of SS RBC adhesion with soluble proteins was performed as described in “Material and methods.” Sham-treated SS RBCs showed little adhesion to vessel walls, whereas epi-treated SS RBCs showed marked adhesion to postcapillary venules, with intermittent vaso-occlusion. Infusion of sCD44 (as a negative control) 30 minutes prior to intravenous administration of epi-treated SS RBCs (n = 5) had no effect on epi-treated SS RBC adhesion to postcapillary venules, whereas infusion of sLW 30 minutes prior to injection of epi-treated SS RBCs (n = 5) markedly inhibited adhesion to postcapillary vessels. (B) Involvement of LW in SS RBC adhesion to endothelium and vaso-occlusion. Animals were infused with sham- or epi-treated SS RBCs 30 minutes after intravenous administration of sCD44 or sLW (n = 5 for each treatment). Thirty segments of venules were analyzed to quantify the length of venules occupied by SS RBCs. The values were averaged among groups of animals to represent the mean percent venular length occupied by SS RBCs. Error bars show SEM of 5 different experiments. *P < .001 compared with sCD44/epi-treated (animals/cells) for vessels up to 25 μm in diameter, and P < .05 for vessels more than 25 μm in diameter. (C) Epi-activated human SS RBCs induce adhesion of murine leukocytes to endothelium. Fluorescently labeled (red) sham- (n = 3) and epi-treated SS RBCs (n = 3) were infused 30 minutes after injection of rat FITC-labeled antimouse CD45 or LFA-1 antibodies. In the presence of epi-treated human SS RBCs, murine leukocytes adhered to postcapillary endothelium, and this adhesion was blocked by antibody against mouse LFA-1. Arrows indicate identical areas visualized with filters for red and green fluorescence to show where human SS RBCs and murine leukocytes are adherent to endothelium. In some areas, both murine leukocytes and human SS RBCs were adherent, whereas in other areas only one adherent cell type was present. Top panels show adhesion of human SS RBCs, bottom panels show adhesion of murine leukocytes.
Model of SS RBC activation by epinephrine. We previously demonstrated that stimulation of ARs by epi affects downstream events via AC through activation of Gαs protein complex. Increased intracellular cAMP as a result of activation of AC leads to activation of PKA, which acts as a downstream effector to up-regulate SS RBC adhesion mediated by activation of LW, which becomes phosphorylated and preferentially recognizes the endothelial αvβ3 integrin. We now propose that epinephrine acts via stimulation of RBC β2-ARs and leads to activation of LW to interact with both endothelial cells as well as leukocytes.
Model of SS RBC activation by epinephrine. We previously demonstrated that stimulation of ARs by epi affects downstream events via AC through activation of Gαs protein complex. Increased intracellular cAMP as a result of activation of AC leads to activation of PKA, which acts as a downstream effector to up-regulate SS RBC adhesion mediated by activation of LW, which becomes phosphorylated and preferentially recognizes the endothelial αvβ3 integrin. We now propose that epinephrine acts via stimulation of RBC β2-ARs and leads to activation of LW to interact with both endothelial cells as well as leukocytes.
In addition, infusion of epinephrine-treated human SS RBCs modestly activated murine leukocyte adhesion to endothelium. After injection of FITC-labeled antimurine CD45 (a panleukocyte marker) antibody prior to epinephrine-treated SS RBC infusion, we saw small numbers of murine leukocytes adherent to endothelium following RBC infusion, a phenomenon not observed when sham-treated SS RBCs were infused (Figure 6C). However, adhesion of activated SS RBCs was not exclusively limited to sites of leukocyte adhesion. Furthermore, leukocyte adhesion was blocked with FITC-labeled antibody against murine LFA-1 without prevention of SS RBC adhesion.
Discussion
The sequence of events leading to often suddenly symptomatic vaso-occlusion in humans with SCD is still poorly understood. Our data demonstrate that LW activation by epinephrine via stimulation of β2-AR signaling in SS but not normal RBCs can result in pathophysiologic consequences in vivo. Higher levels of β2-AR expression on SS RBCs, compared with the levels of receptor expression on normal RBCs, in addition to differential regulation of one or more steps in the β2-AR signaling pathway in SS versus normal RBCs in response to epinephrine, (Figure 7)11,28 may account for adhesion of SS but not normal RBCs to endothelium. Epinephrine clearly not only caused SS RBC adhesion and vascular blockade but also enhanced RBC trapping in organs typically affected in SCD, including the spleen and lungs. In humans the frequency of symptomatic vaso-occlusive events is low when the percentage of SS RBCs is below 20% to 30%. However, epinephrine-treated human SS RBCs promoted vaso-occlusion in our experiments even though the percentage of infused cells never exceeded 10% of the total circulating RBCs. Murine sickle RBCs also responded to epinephrine stimulation by increased adhesion in vitro and in nude mice, but without promotion of frank vaso-occlusion. This was possibly due to the lower percentage of infused murine RBCs (≤ 5%) versus human RBCs, in combination with their small size relative to human RBCs and the relatively lower adhesion of sickle murine RBCs compared with human SS RBCs (Figure 1).
We observed RBC adhesion and vaso-occlusion predominantly in small postcapillary venules, where a combination of the diameter constraint, low RBC velocities, increased contact time between RBCs and endothelium caused by low or intermittent flow,29 and perhaps endothelial differences compared with arterioles could allow for optimal adhesion to endothelium of epinephrine-activated SS RBCs at low shear stress.30 Because only human SS RBCs but not normal human RBCs adhered to both human11 and murine endothelium in vitro (Figure 1) and in vivo (Figure 3), interactions between activated human SS RBCs and murine endothelium in nude mice were not due to nonspecific binding. We also recognize that the characteristics of endothelial cells and microcirculatory beds vary among different tissues; it remains to be determined whether SS RBC adhesion is equally avid in all tissues.
Our model represents an appropriate system for identifying the effect of stress hormones on SS RBC adhesion and vaso-occlusion in vivo, because it allows for the manipulation of RBCs exclusively, while still allowing study of adhesion and vaso-occlusion in an intact vasculature, in the context of physiologic blood flow and shear stresses, in the presence of all normal blood components, and where vessels were noninstrumented and contralateral to the window chamber. In fact, the normal survival of sham-treated SS RBCs coinfused with epinephrine-treated SS RBCs (Figure 2C) demonstrated that adhesion of SS RBCs is not due to activation of the endothelium by epinephrine in our model. Of note, SS RBC adhesion and vaso-occlusion were as striking as adhesion and vaso-occlusion observed in other models using proinflammatory cytokines such as platelet-activating factor (PAF)31 and tumor necrosis factor-α (TNF-α).27,32,33
Numerous mechanisms of RBC adhesion to endothelium have been demonstrated in in vitro and ex vivo models, but there are only limited data regarding the contribution of specific factors initiating or inducing those interactions in vivo. Human SS RBCs bind in vitro to multiple endothelial adhesion molecules, including selectins34 and integrins,11,31 as well as to membrane-bound intermediary proteins such as thrombospondin.35 The interaction of RBC sialyl Lewis moieties with selectins has been proposed to slow RBC transit and cause rolling behavior in small vessels,36 while interactions with αvβ3 may cause stable adhesion.31 Our studies specifically suggest a pathological role for both epinephrine through stimulation of β2-ARs on SS RBCs and RBC LW—the counterreceptor for αvβ3 integrin11,31 —in mediating not only SS RBC adhesion to both cultured murine ECs and murine postcapillary vessels but vaso-occlusive events as well. Human SS RBCs have been previously shown to adhere to both human and rodent αvβ3,11,31 and murine ECs also express αvβ3.37,38 Indeed, Kaul et al have demonstrated that peptides based on αv-binding domains of erythrocyte LW inhibit SS RBC–endothelial interactions and vaso-occlusion in an ex vivo rodent microcirculation.39 Thus, LW-mediated SS RBC adhesion in vivo also likely occurs via interactions with murine endothelial αvβ3 integrin.
Studies using Fisher-344 or nude rats receiving implants with dorsal skin fold or cranial window chambers, respectively, have shown undetectable levels of leukocyte-endothelial interactions in dermal or pial vasculature under baseline conditions.40,41 Such interactions occurred only after induction of an inflammatory reaction42 or application of an inflammatory mediator.43 Similarly, we observed no evidence of an inflammatory response to dorsal skin–window chamber implantation, because we did not detect adherent murine leukocytes in postcapillary endothelium when human RBCs were not infused (data not shown). Furthermore, frequent blood flow stagnation observed in some of the vessels in mice infused with epinephrine-treated, but not with sham-treated SS RBCs or sham- or epinephrine-treated normal RBCs, was likely a result of vascular occlusion rather than window chamber implantation, which was relatively remote.
Turhan et al demonstrated in sickle mice that murine SS RBCs can bind to adherent leukocytes in inflamed cremasteric vessels, producing vaso-occlusion.27 We observed adhesion of small numbers of murine leukocytes to endothelium but only when epinephrine-activated, not sham-treated, human SS RBCs were infused into animals (Figure 6C). Activated SS RBC adhesion to endothelium was not leukocyte dependent, however, because the sites of adhesion of SS RBCs and leukocytes were often different. This unexpected observation generates yet another possible paradigm in the pathogenesis of sickle cell vaso-occlusion in which activation of SS RBCs promotes not only RBC adhesion but activation of leukocyte adhesion to endothelium as well. Because LW is known to bind to leukocyte β2 integrins, SS RBCs may also interact via LW with leukocytes adherent to endothelium.44,–46 It is also possible that adhesion of activated SS RBCs induces endothelium injury and activation in vivo, which in turn increases leukocyte adhesion. Activation of endothelial cells as a result of contact with SS RBCs has been previously demonstrated in vitro.47
Our data identify activated LW as a potential target for development of antiadhesive reagents designed to prevent vaso-occlusion. Our previous in vitro findings are now supported by our in vivo studies showing that LW is an activatable adhesion molecule critically involved in RBC adhesion to ECs. Finally, our data provide evidence for a mechanism that might explain the intriguing but heretofore poorly documented temporal association of vaso-occlusion with a broad array of physiological stresses.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants HL58939, HL070769, and HL63409 (M.J.T.) and grant HL07057 (R.Z.) from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH); grant CA40355 (M.W.D.) from the National Cancer Institute, NIH; grant DK065040 (R.Z.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH; support from the Howard Hughes Medical Institute (B.J.M.); and grant GM007171 (B.J.M.) from the National Institute of General Medical Sciences, NIH.
We thank Dr Jean-Pierre Cartron (Paris, France) for providing the LW cDNA from which we derived our sLW construct.
National Institutes of Health
Authorship
Contribution: R.Z. designed and performed most of the experiments, analyzed and interpreted data, performed statistical analysis, and drafted the manuscript; B.J.M. performed most of the dorsal skin window chamber implantations and some intravital miscroscopy; E.J.W. helped in analyzing and interpreting data and in drafting the manuscript; M.B. performed molecular biology experiments and prepared soluble LW and CD44; K.X. assisted with studies of leukocyte adhesion; S.S. performed some of the dorsal skin window chamber implantations; M.D. performed assays of adrenergic receptor expression; M.W.D. helped in the design and interpretation of the in vivo work; and M.J.T. supervised the research study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marilyn J. Telen, Box 2615, Duke University Medical Center, Durham, NC 27710; e-mail:telen002@mc.duke.edu.

![Figure 2. Immune interactions and survival of human RBCs in nude mice. (A) Anesthetized nude mice were infused with Dil (rhodamine)–labeled sham-treated or epi-treated normal RBCs. Blood samples collected 40 minutes after infusion were analyzed for murine immunoglobulin, which was detected using FITC-labeled antimurine immunoglobulin, bound to human RBCs. Insignificant murine immunoglobulin was bound to circulating sham- or epi-treated human normal RBCs compared with noninfused cells. One representative experiment is shown (n = 3). (B,C) Anesthetized mice were injected with a 1:1 mixture of Dil-labeled sham- (■) and DiO-labeled epi-treated (□) RBCs ([B] normal, [C] SS RBCs; n = 3 for each). Error bars show SEM of 3 different experiments. The percentages of sham- and epi-treated normal RBCs circulating in the bloodstream of animals at all times were not significantly different. A significantly greater percentage of sham-treated than epi-treated SS RBCs was retained in the circulation at all times (P < .001). (D,E) Normal RBCs were sham treated or epi treated. Sham-treated normal RBCs were detected to some degree in the spleen. Epi treatment did not lead to a significant increase in splenic trapping of normal RBCs. (F,G) Epi treatment had a significant effect on trapping of SS RBCs in the spleen compared with sham-treated cells. *P < .05 compared with sham-treated. Scale bar for panels D and F = 100 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2006-11-056101/2/m_zh80200708140002.jpeg?Expires=1763589408&Signature=Y9~Y55UmMVHu5aWJn-3TsQbAFtntAjJFjs9SmYLCctoBjsnJAv1dnf-qilxAU84VvzBTaeim~YYFjNG3BMUy19s7VXpKDrGlclhSGiZC-m1ta~TJ4gAOdy4TGVWz~dK5tmKKc2qLmCtNqEqX2Ec3aCHnxANosRuPZXTxTA-z8ss-i3P7aWGBvoRKtMqOSjos7~drhx5v6hy90RFo01ktk7JYLEvdniHVSlkPNl8LLPYCGSXD6sAyQCwWm82LvPWoBEwBCH0XDwIxb2k57yzlfwiaChBIRIG~-8t2lfqtDVNP~r~N05xxcDwyAe~0t8ab3Y1XUZcMGr-823tQ17AVTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal