Abstract
Multipotent mesenchymal stromal cells (MSCs) inhibit proliferation, helper, and effector functions in most if not all peripheral blood mononuclear cell (PBMC) subpopulations in vitro. The molecular mechanism is widely thought to imply tryptophan degradation by the interferon-γ (IFNγ)–induced expression of indoleamine 2,3-dioxygenase (IDO). However, IDO inhibitors were not able to restore proliferation of PBMCs in each case. Moreover, human MSCs with an IFNγ receptor 1 (R1) defect inhibited proliferation of HLA-mismatched PBMCs to a similar extent as control MSCs. In contrast to healthy MSCs, IFNγR1-deficient MSCs showed no detectable mRNA for IDO—neither in the absence nor in the presence of recombinant human IFNγ, nor in coculture with HLA-mismatched PBMCs. Based on gene expression profiling, we were able to show that insulin-like growth factor (IGF)–binding proteins contribute to the inhibitory mechanism of MSCs. Taken together, human MSCs exert important immunomodulatory functions in the absence of IFNγR1 signaling and IDO, partially accounted for by IGF-binding proteins.
Introduction
Human multipotent mesenchymal stromal cells (MSCs) can be readily expanded from bone marrow aspirates by ex vivo culture and characterized by immunophenotype and plasticity.1 These cells maintain plasticity for classical mesenchymal tissues and others to a lesser extent.2 Moreover, expanded MSCs display immunomodulatory properties (ie, inhibition of antigen presentation, maturation of dendritic cells, cytotoxicity of natural killer [NK] cells, and proliferation of peripheral blood mononuclear cells [PBMCs]) in response to polyclonal stimulation or in alloresponses.3–5 These immunoregulatory features of MSCs led to their clinical application in pediatric and adult patients.6–8 However, the identity of soluble factors necessary for the inhibition exerted by MSCs remains unclear. Particularly, the type of mitogens used for stimulation of PBMCs may have consequences for the predominant inhibitory pathway engaged by MSC-derived factors.9 Among others, interferon-γ (IFNγ) has been proposed as a prime candidate for initiation of MSC-mediated immune regulation.9–12 A well-known IFNγ-inducible gene in this context is indoleamine 2,3-dioxygenase (IDO).13 IDO initiates degradation of the essential amino acid tryptophan and, in concert with other enzymes, might possibly give rise to metabolites of tryptophan, which inhibit PBMC activation. Blocking this enzyme in cocultures of MSCs and HLA-mismatched PBMCs almost completely abrogated the inhibitory effects of MSCs on the proliferating allogeneic PBMCs.11,12 However, other researchers could not find an effect of IDO in their experimental system.14 Moreover, IFNγ itself has also been shown to turn MSCs into antigen-presenting cells.15 Some antiproliferative effects are mediated by insulin-like growth factor–binding proteins (IGFBPs), in particular IGFBP3.16 This family of proteins has been involved in growth inhibition in several experimental settings relevant for the system studied here.17,18 In order to address the relevance of IFNγ-induced IDO expression for the inhibitory function of human MSCs in alloresponses, we analyzed human IFNγ receptor 1 (R1)—deficient MSCs
Materials and methods
These studies were approved by the Institutional Review Board (IRB) of the University Hospital Tübingen, Germany.
Cell culture
After informed consent was obtained in accordance with the Declaration of Helsinki, cryoconserved bone marrow aspirates from a boy with a frameshift mutation in the IFNγ-R1 subunit of the IFNγ receptor were thawed. Control specimens of MSCs were derived from excess material of standard bone marrow biopsies in children treated for leukemia (IRB approval 241/2005V). MSC cultures were established as reported earlier.19 The deletion of a T at position 523 in the IFNγ-R1 subunit was verified by conventional sequence analysis of a reverse transcriptase–polymerase chain reaction (RT-PCR) product (data not shown). Plasticity assays and flow cytometry were carried out as described elsewhere (data not shown).20
Proliferation assays
HLA-mismatched PBMCs were obtained from healthy volunteers (IRB approval 279/2003V). PBMCs were stained with CFSE and analyzed by flow cytometry after the incubation times indicated according to standard protocols.19 The PBMCs were stimulated with IL-2 and OKT3 as described previously.19 A total of 75 000 PBMCs were added per well of a 96-well plate (Greiner, Frickenhausen, Germany) already containing 10 000, 20 000, or 30 000 MSCs where indicated. A total of 200 U/mL rhIFNγ (R&D Systems, Minneapolis, MN) or 1 mM 1-methyltryptophan (Sigma, München, Germany) was added in specified samples.
RT-PCR
Isolation of RNA from MSCs and conversion to cDNA were performed as described previously.19 PCR amplification was done using the following primer: β-actin forward: CAGTGGGGATGTCTTCATAA, reverse: AGTCCGCCTAGAAGCA; IDO forward: ATCACCATGGCATATGTGTGGG, reverse: GTGAAACACTTGAAGGGCTTTCTC.
IGFBP affinity chromatography
Briefly, 1 mg rhIGF (kindly provided by Kabi Pharmacia, Erlangen, Germany) was immobilized on a 1 mL High-Trap NHS-activated sepharose column (Amersham, Uppsala, Sweden). Using these columns, media conditioned by cocultures of wild-type MSCs (MSCwt) and PBMCs as well as MSCIFNγR1− and PBMCs was depleted from free IGF-binding proteins. Reduction of IGFBP was monitored by radioimmunoassay (RIA) for IGFBP2 and IGFBP3.
Results and discussion
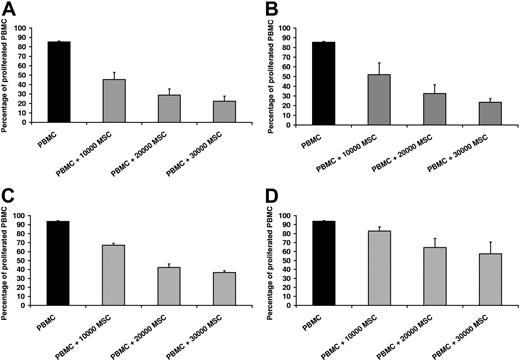
Functional characterization of a protein in vivo is frequently based on knockout experiments in animal models. In the human system, naturally occurring mutations can provide similar information. Hence, we established MSCs from a bone marrow aspirate of a boy with defective IFNγ receptor using standard culture conditions.19 We reconfirmed a frameshift mutation (523delT) in subunit 1 of the receptor by PCR sequencing (data not shown). The clinical course with disseminated atypical mycobacteriosis in our patient correlated with this dominant mutation.21,22 In vitro, MSCIFNγR1− did not up-regulate activation markers such as HLA-DR in response to IFNγ, in contrast to what is known for MSCwt.15 MSCIFNγR1− showed the same morphology, growth behavior, immunophenotype, and differentiation potential into adipocytes and osteoblasts as MSCwt (data not shown). Immunologic functions of MSCs are typically assessed by the capacity to inhibit proliferation of PBMCs after polyclonal stimulation or in a mixed-lymphocyte reaction. Therefore, MSCwt and MSCIFNγR1− were cocultured with HLA-mismatched PBMCs in the presence of IL-2 and OKT3 as polyclonal stimulators. Based on the literature, one would have expected a stronger inhibition of PBMC proliferation due to IFNγ-induced up-regulation of IDO in MSCwt in contrast to MSCIFNγR1−.12 Interestingly, PBMCs were inhibited by both MSCwt and MSCIFNγR1− in a cell-number–dependent fashion (Figure 1A,B). In contrast to the anticipated outcome, PBMCs cocultured with MSCs proliferated even more vigorously in the presence of exogenous IFNγ (Figure 1C,D). This was due to a direct stimulation of PBMCs by rhIFNγ (data not shown).
MSCIFNγR1− and MSCwt inhibit proliferation of PBMCs to a similar extent. (A) Proliferation of HLA-mismatched PBMCs in the absence (■) or presence (▩) of MSCwt; representative result of 5 independent experiments. MSCs inhibited PBMC proliferation in a dose-dependent manner. (B) PBMCs were inhibited by MSCs with IFNγR1 defect to a similar extent as by MSCwt (representative of n = 5). (C) Proliferation of HLA-mismatched PBMCs in the absence (■) or presence (▨) of MSCwt, when human rIFNγ was added to the cultures. PBMCs cocultured with MSCwt proliferated more vigorously in presence of rhIFNγ (representative of n = 5). (D) This effect mediated by rhIFNγ was even stronger when PBMCs were cocultured with MSCIFNγR1− (representative of n = 3). Data are shown as means of triplicates plus or minus standard deviation (±SD) of one representative experiment.
MSCIFNγR1− and MSCwt inhibit proliferation of PBMCs to a similar extent. (A) Proliferation of HLA-mismatched PBMCs in the absence (■) or presence (▩) of MSCwt; representative result of 5 independent experiments. MSCs inhibited PBMC proliferation in a dose-dependent manner. (B) PBMCs were inhibited by MSCs with IFNγR1 defect to a similar extent as by MSCwt (representative of n = 5). (C) Proliferation of HLA-mismatched PBMCs in the absence (■) or presence (▨) of MSCwt, when human rIFNγ was added to the cultures. PBMCs cocultured with MSCwt proliferated more vigorously in presence of rhIFNγ (representative of n = 5). (D) This effect mediated by rhIFNγ was even stronger when PBMCs were cocultured with MSCIFNγR1− (representative of n = 3). Data are shown as means of triplicates plus or minus standard deviation (±SD) of one representative experiment.
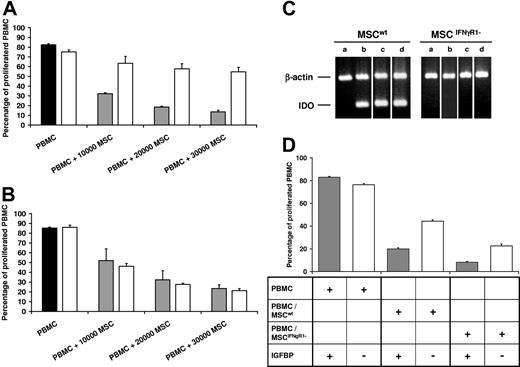
In a second set of experiments we addressed the role of IDO, which is an IFNγ–responsive gene and known to induce tolerance in vivo. Interestingly, addition of the IDO inhibitor 1-methyltryptophan partially restored the proliferation rate of PBMCs in the presence of IL-2 and OKT3 as well as MSCwt, whereas 1-methyltryptophan had no effect in the cocultures of PBMCs with MSCIFNγR1− (Figure 2A,B). This result indicated that IDO was involved in the inhibition of PBMCs by MSCwt, but not by MSCIFNγR1−. Subsequently, we studied expression of IDO by RT-PCR in MSCwt and MSCIFNγR1−, which were cocultured in a standard proliferation assay with allogeneic PBMCs either in direct cell-cell contact or separated by a semipermeable membrane. Figure 2C shows that IDO expression was indeed completely absent in MSCIFNγR1− in the presence of allogeneic PBMCs and IL-2 and OKT3. Expectedly, exogenously added IFNγ did not elicit IDO transcription in these cells either. Nevertheless, MSCIFNγR1− did inhibit proliferation of these allogeneic PBMCs. In conclusion, MSCs can exert immunomodulatory effects in the absence of normal IFNγ receptor function and independently of IDO. We compared the gene expression profile of MSCwt and MSCIFNγR1− using the Affymetrix cDNA chips (Affymetrix, Santa Clara, CA). Among other candidate molecules with minor functional effects in our hands, IGFBPs were expressed by both MSCwt and MSCIFNγR1−. Conditioned media from cocultures of PBMCs with MSCwt or MSCIFNγR1−, respectively, was depleted from free IGFBPs by affinity chromatography using immobilized human recombinant IGF. Depletion was monitored exemplarily for IGFBP2 and IGFBP3 by RIA (data not shown). Figure 2D indicates that conditioned media was sufficient to inhibit PBMC proliferation. Depletion of free IGFBPs reduced the inhibitory effect. These results indicate that the IGF/IGFBP system, among other molecules, contribute to the inhibitory effect of MSCs on PBMCs.
MSCwt and MSCIFNγR1- inhibit proliferation of PBMCs in the absence of IDO—contribution of IGFBPs. Proliferation of HLA-mismatched PBMCs in the absence (■) or presence (▩) of MSCwt compared with PBMCs in the presence of 1 mM of the IDO inhibitor 1-methyltryptophan (□). (A) 1-methyltryptophan partially restored the proliferation of PBMCs cocultured with MSCwt (representative of n = 5). (B) By contrast, 1-methyltryptophan had no effect on cocultures of MSCIFNγR1− and PBMCs (representative of n = 3). (C) RT-PCR of RNA isolated from MSCs. IDO was not constitutively expressed (lane a) in either MSCwt or MSCIFNγR1−. IDO was induced in MSCwt when they were cocultured with PBMCs in the presence of IL-2 and OKT3 (lane b, separated by a semipermeable membrane; lane c, in direct cell-cell contact) or when IFNγ was added (lane d) (representative of n = 3). (D) IL-2/OKT3-induced proliferation of PBMCs in media conditioned by PBMCs alone, by cocultures of MSCwt/PBMCs or by MSCIFNγR1−/PBMCs, respectively, for 3 days. Media conditioned by MSCwt/PBMCs and by MSCIFNγR1−/PBMCs inhibited proliferation of PBMCs (▩). Inhibition was partially reverted by depletion of free IGFBP from the media of both cocultures, MSCwt/PBMCs and MSCIFNγR1−/PBMCs (□) (representative of n = 3). Data are shown as means of triplicates (±SD) of one representative experiment.
MSCwt and MSCIFNγR1- inhibit proliferation of PBMCs in the absence of IDO—contribution of IGFBPs. Proliferation of HLA-mismatched PBMCs in the absence (■) or presence (▩) of MSCwt compared with PBMCs in the presence of 1 mM of the IDO inhibitor 1-methyltryptophan (□). (A) 1-methyltryptophan partially restored the proliferation of PBMCs cocultured with MSCwt (representative of n = 5). (B) By contrast, 1-methyltryptophan had no effect on cocultures of MSCIFNγR1− and PBMCs (representative of n = 3). (C) RT-PCR of RNA isolated from MSCs. IDO was not constitutively expressed (lane a) in either MSCwt or MSCIFNγR1−. IDO was induced in MSCwt when they were cocultured with PBMCs in the presence of IL-2 and OKT3 (lane b, separated by a semipermeable membrane; lane c, in direct cell-cell contact) or when IFNγ was added (lane d) (representative of n = 3). (D) IL-2/OKT3-induced proliferation of PBMCs in media conditioned by PBMCs alone, by cocultures of MSCwt/PBMCs or by MSCIFNγR1−/PBMCs, respectively, for 3 days. Media conditioned by MSCwt/PBMCs and by MSCIFNγR1−/PBMCs inhibited proliferation of PBMCs (▩). Inhibition was partially reverted by depletion of free IGFBP from the media of both cocultures, MSCwt/PBMCs and MSCIFNγR1−/PBMCs (□) (representative of n = 3). Data are shown as means of triplicates (±SD) of one representative experiment.
MSCs have elicited great clinical interest as a tool for immune modulation based on the in vitro data. However, in vivo data from mice clearly demonstrated that these effects do not prevent rejection of MHC-mismatched MSCs in an immunocompetent host.23 Despite the expression of IDO, which was found to be involved in induction of tolerance, MSCs were unable to achieve a long-term engraftment.12,13 We and others have demonstrated that clinical application of MSCs can be successful in some, but not all patients who developed severe graft-versus-host disease (GVHD) or hemophagocytosis following allogeneic stem-cell transplantation.7,8 As there are experimental settings where MSCs in the presence of IFNγ do not inhibit, but rather stimulate PBMC proliferation, clearly a detailed understanding of the molecular mechanisms would allow a more promising application in patients.15 Obviously, IFNγ plays an ambiguous role in various in vitro settings and may be different in vivo. Human MSCs defective in the IFNγ receptor were able to inhibit PBMC proliferation. This is striking evidence that IFNγR1-mediated effects of IFNγ are dispensable for the inhibitory effect of human MSCs on the proliferation of PBMCs.
The role of IDO and the exact nature of the inhibitory mechanism imposed by IDO beyond depletion of the essential amino acid tryptophan in the current setting is unclear. The rejection of erythropoietin-producing MSCs in the immunocompetent mouse model did already argue against a significant role in MSC-induced inhibition of PBMCs, but in vitro evidence from knock-down or IDO-deficient MSCs of human origin was missing.23 In our experiments, we were able to show that the immunomodulatory effects of human MSCs in vitro were independent of detectable IDO transcription. Taken together, inhibition of PBMC proliferation by human MSCs in vitro is independent of functional IFNγ receptor and IDO expression. From expression profiling and functional analyses we obtained evidence that the IGF/IGFBP system is involved in the inhibitory mechanism of human MSCs. The IGF/IGFBP system is known to play a regulatory role in lymphocyte proliferation, where IGF induces proliferation, and IGFBP may inhibit this effect. This effect and others that may play a minor role for human MSCs in vitro may be more important in vivo. Further experiments are under way to analyze the role of IGFs and individual IGFBPs in greater detail.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful for experienced technical support by L. Dannecker and M. Pechan.
This work was supported in part by Deutsche José Carreras Leukämie-Stiftung (DJCLS 05120), Bauer Stiftung zur Förderung von Forschung und Wissenschaft, Förderverein für krebskranke Kinder Tübingen, and DFG-Graduiertenkolleg 794.
Authorship
Contribution: F.G. designed and performed research; B.S. designed and performed research; S.V. performed research; E.K. collected data; W.F. contributed vital new reagents; R.H. wrote the paper; and I.M. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ingo Müller, University Children's Hospital, Hoppe-Seyler-St 1, 72076 Tübingen, Germany; e-mail: ingo.mueller@med.uni-tuebingen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal