Abstract
Incorporation of apoptosis-inducing agents into current therapeutic regimens is an attractive strategy to improve treatment for drug-resistant leukemia. We tested the potential of arsenic trioxide (ATO) to restore the response to dexamethasone in glucocorticoid (GC)–resistant acute lymphoblastic leukemia (ALL). Low-dose ATO markedly increased in vitro GC sensitivity of ALL cells from T-cell and precursor B-cell ALL patients with poor in vivo response to prednisone. In GC-resistant cell lines, this effect was mediated, at least in part, by inhibition of Akt and affecting downstream Akt targets such as Bad, a proapoptotic Bcl-2 family member, and the X-linked inhibitor of apoptosis protein (XIAP). Combination of ATO and dexamethasone resulted in increased Bad and rapid down-regulation of XIAP, while levels of the antiapoptotic regulator Mcl-1 remained unchanged. Expression of dominant-active Akt, reduction of Bad expression by RNA interference, or overexpression of XIAP abrogated the sensitizing effect of ATO. The inhibitory effect of XIAP overexpression was reduced when the Akt phosphorylation site was mutated (XIAP-S87A). These data suggest that the combination of ATO and glucocorticoids could be advantageous in GC-resistant ALL and reveal additional targets for the evaluation of new antileukemic agents.
Introduction
Poor response to 7-day monotherapy with prednisone is one of the strongest predictors of adverse outcome for the treatment of childhood acute lymphoblastic leukemia (ALL).1 Despite intensive research efforts, the mechanisms underlying glucocorticoid (GC) resistance in ALL are still poorly understood. GC resistance is unlikely to be GC-receptor (GR)–mediated because mutations of the GR are uncommon and alterations in GR expression or function have not been detected in primary leukemia samples from GC-resistant patients.2–4
Because most agents used in ALL treatment, including glucocorticoids, induce programmed cell death (apoptosis), mechanisms that lead to an increased antiapoptotic state in leukemic cells may constitute an alternative explanation for GC resistance in ALL. There is increasing evidence for abnormal apoptotic signaling in GC-resistant ALL, involving members of the Bcl-2 family of regulators of the intrinsic apoptotic pathway.5 The antiapoptotic Bcl-2 family member Mcl-1 was overexpressed in primary ALL cells as part of the gene expression signature associated with in vitro GC resistance.6,7 Expression levels of the proapoptotic Bcl-2 family protein Bim were increased after exposure to GC in sensitive cells.8–11 Interestingly, induction of Bim was markedly attenuated in response to prednisone in GC-resistant primary ALL cells that were amplified by xenotransplantation.2 Deletion of Bim or Puma conferred GC resistance of normal lymphoid tissues in mice.12 These studies suggest that an imbalance between proapoptotic and antiapoptotic regulators may be involved in the resistance mechanisms. In agreement with this hypothesis, overexpression of Bcl-2 or Mcl-1 rendered murine lymphoid cells resistant to dexamethasone.13
An imbalance that favors an antiapoptotic state in leukemic cells may result from constitutive activation of certain prosurvival pathways. Alterations of the phosphoinositide 3-kinase (PI3K)/Akt pathway, a central integrator of survival signals, were frequently detected in human tumors,14 including adult lymphoma and ALL cell lines.15–17 Akt is able to modulate a number of apoptotic regulators directly. Phosphorylation of the Bcl-2 family member Bad by Akt was shown to induce Bad sequestration by protein 14-3-3, thereby blocking its proapoptotic function.18 At the postmitochondrial level, Akt phosphorylation stabilized levels of XIAP, a member of the inhibitor of apoptosis protein (IAP) family that inhibits caspases-3, -7, and -9.19 Response of lymphoma cells to the PI3K/Akt inhibitor LY294002 correlated with a decrease in XIAP protein levels,17 suggesting that XIAP may be a relevant target in lymphoma. An important downstream effector of Akt is mTOR (mammalian target of rapamycin).20 Inhibition of mTOR with rapamycin sensitized multiple myeloma and ALL cells to dexamethasone,21,22 underscoring the importance of this pathway. Interestingly, the effect of rapamycin in ALL cell lines was associated with a marked decrease of the antiapoptotic regulator Mcl-1.21
Among therapeutic agents with a potential to inhibit Akt, arsenic trioxide (ATO) is of interest for several reasons. The safety profile of ATO for the effective treatment of acute promyelocytic leukemia (APL) is well established.23–25 ATO was shown to bear relevant cytotoxic activity on T-ALL cell lines,26,27 which may involve a broad range of mechanisms.28 Depending on the type of malignancy studied, induction of oxidative stress and reactive oxygen species production by ATO was associated with a perturbation of the NF-κB29,30 or the PI3K/Akt15 pathways. In ALL cell lines, down-regulation of Akt by RNA interference increased the cytotoxic effect of ATO, indicating that inhibition of this pathway by ATO may be relevant for its effect on lymphoid malignancies.15 Given the broad range of mechanisms by which ATO could interfere with apoptosis resistance, we decided to evaluate this agent in combination with dexamethasone in GC-resistant ALL. Here we provide evidence that low-dose ATO treatment acts as a GC sensitizer in ALL and that this effect is dependent on inhibition of Akt. These data are consistent with a role for XIAP and Bad, 2 direct targets of Akt, in ATO-induced GC sensitization.
Patients, materials, and methods
Cell culture
CEM-C7-14 and CEM-C1-15 were kindly provided by Dr E. B. Thompson (University of Texas Medical Branch, Galveston, TX). We will refer to these lines as CEM-C7 and CEM-C1 in the text. The CEM, MOLT-4 (no. CRL-1582; ATCC, Manassas, VA), and Jurkat (no. TIB-152; ATCC) cell lines were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, and 100 IU/mL penicillin/streptomycin (both from Invitrogen, Carlsbad, CA).
Reagents
ATO was obtained from Sigma (Buchs, Switzerland); PD98059, from VWR international (Dietikon, Switzerland); and dexamethasone, from Mepha Pharma (Aesch, Switzerland). Rabbit antibodies were from Cell Signaling Technology (Danvers, MA): anti-PARP (1:1000), anti-Bim (1:1000), anti-Bid (1:1000), anti-XIAP (1:1000), anti-Akt (1:1000), anti-pAkt (pSer473, 1:1000), anti-Bad (1:1000), rabbit anti-pBad (pSer136, 1:1000), anti–Bcl-XL (1:1000), anti-PUMA (1:1000), and anti-cIAP1 (1:1000). Rabbit anti-cIAP2 (1:700) and antisurvivin were from R&D systems (Minneapolis, MN). Rabbit antiactin (1:3000) and rabbit anti–Mcl-1 (1:3000) were from Sigma, and mouse anti–Bcl-2 was from Dako (Glostrup, Denmark).
Expression vectors, siRNA, and transfection
Full-length human XIAP cDNA was subcloned into the expression vector pEGFP-C1. Mutagenesis was performed by polymerase chain reaction (PCR) on a subcloned XIAP fragment. For XIAP-S87A, we used the primers 5′-CACAGGAAAGTAGCCCCAAATTGCAG-3′ and 5′-AATTTGGGGCTACTTTCCTGTGTCTTCC-3′; for the XIAP-S87E construct, the primers 5′-CACAGGAAAGTAGAGCCAAATTGCAG-3′ and 5′-AATTTGGCTCTACTTTCCTGTGTCTTCC-3′ were used. The pEGFP-XIAP constructs and a myristoylated/palmitylated-HA-Akt construct31 were transfected using nucleofection (Amaxa, Cologne, Germany). Transfection efficiency was controlled using the pEGFP plasmid (Clontech, Mountain View, CA) where appropriate. For nucleofection, 30 nM Bad siRNA (Bad nucleotides 817-837 and 1033-1051, NM_004322; Silencer siRNA library, cat no. 81 820; Ambion, Austin, TX) was used (solution v and Amaxa program T-016).
Patient samples
Primary ALL cells were recovered in small portions from banked frozen presentation samples on dry ice using a sterile scalpel and allowed to recover in RPMI supplemented with 10% FCS, 5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL sodium selenite (Sigma) for 4 hours. Cell viability as determined by trypan blue exclusion after thawing was between 50% and 85%. Cells (5 × 104) were incubated in 100 μL medium in round-bottom 96-well plates. Patients were enrolled in the ongoing ALL-BFM 2000 protocol and had given informed consent in accordance with the Declaration of Helsinki. Approval was obtained from the institutional review board of the Medical School Hannover for these studies. This IRB is the central IRB for all participating centers in trial ALL-BFM 2000. In addition, the studies have been approved by the local IRBs of each participating institution, including the University Children's Hospital Zurich. According to ALL-BFM criteria, prednisone good-response (PGR) was defined as the reduction of leukemic blasts in the peripheral blood to lower than 1000 per microliter on day 8 after 7 days of monotherapy with prednisone. Minimal residual disease (MRD) was measured after induction therapy on treatment day 33 and after induction at week 12. MRD high risk is defined as a persistent high MRD load at week 12 (10−3 or greater).
Treatment, MTT assay, and determination of apoptosis
Experiments were performed in 96-well plates with 2 × 104 cells per well. Viability of cells was assessed using the cell proliferation kit (MTT) (Roche Applied Sciences, Rotkreuz, Switzerland). The minimal absorbance of control wells was OD 0.8. For primary patient samples, the trypan blue dye exclusion assay was used to determine the effect of treatment on cell viability. Annexin-V stainings were performed with FITC-labeled annexin-V and 1 mg/mL propidium iodide (BD Biosciences, San Jose, CA). Briefly, treated 105 cells were washed in cold PBS and then resuspended in 100 μL binding buffer (10 mM HEPES/NaOH, pH 7.4, and 140 mM NaCl2). Annexin-V–FITC and PI (5 μL each) were added and incubated at 4° in the dark for 30 minutes before flow cytometry analysis (Beckman Coulter Cytomics FC500; Hialeah, FL). Caspase activity was detected by CaspGLOW Red Active Caspase-3 Staining Kit (Alexis Platform, Lausen, Switzerland) according to the manufacturer's instructions. Briefly, 1 μL specific substrate for caspase-3 (Red-DEVD-FMK) was added to 105 treated cells in 300 μL of medium for 1 hour at 37°C and enzymatic cleavage was analyzed by flow cytometry.
All experiments were performed in triplicate and repeated at least 3 times. Data analysis was done with the GraphPad prism software (San Diego, CA) and statistical analysis using the Student t test.
Determination of cellular reactive oxygen species (ROS)
The generation of ROS was analyzed using 2′-dichlorofluorescein diacetate (DCFDA; Sigma). Cells (2 × 104) were incubated with ATO for 5 hours. After incubation with 20 μM DCFDA for 4 hours at 37°C, signals were analyzed by fluorometry using filters for excitation at 485/20 nm and emission at 530/35 nm. The results were normalized to total protein expression levels and are shown as arbitrary fluorescent units.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis
For Western blotting, whole-cell extracts were prepared from 1 × 106 cells using radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-Cl, pH 6.8, 100 mM NaCl, 1% Triton-X-100, 0.1% SDS) supplemented with complete mini protease inhibitor cocktail (Roche Applied Sciences) and 1 mM sodium orthovanadate (Sigma) for 20 minutes on ice. For detection of primary antibodies, we used horseradish peroxidase–labeled goat antirabbit or antimouse antibodies from Pierce Biotechnology (Rockford, IL). Signal detection was performed using the chemiluminescence substrate from Pierce Biotechnology, directly scanned with the ChemiGenius imaging system (SynGene, Cambridge, United Kingdom) and quantified with the GeneTools 3.1 image analysis software (SynGene).
Results
ATO augments the response to dexamethasone in glucocorticoid-resistant acute lymphoblastic cell lines
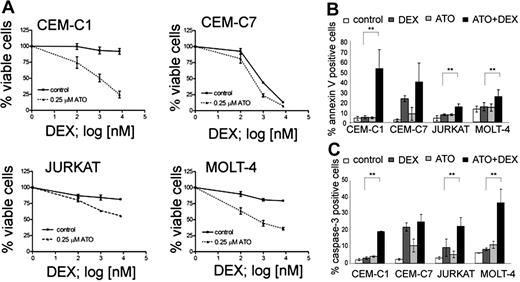
In order to evaluate the potential of ATO for the treatment of glucocorticoid (GC)–resistant ALL, we selected a panel of T-ALL cell lines that show different sensitivity to GC and established dose-response curves for ATO and dexamethasone (Table 1). As expected from previous reports,15,27,32 the GC-sensitive CEM-C7, and the GC-resistant CEM-C1, MOLT-4, and Jurkat cell lines were sensitive to ATO with IC50 values of 2.0, 1.8, 1.2, and 0.76 μM, respectively. This concentration range was safely achieved in the peripheral blood of adult APL patients.33 Next, we performed combination experiments to evaluate the sensitizing effect of ATO on dexamethasone. A concentration of 0.25 μM ATO was sufficient to resensitize all 3 GC-resistant lines (CEM-C1, Jurkat, and MOLT-4) to dexamethasone (Figure 1A). At this concentration, ATO alone induced cell death in less than 5% of cells compared with untreated controls. In contrast, the response to dexamethasone could be augmented only slightly in GC-sensitive CEM-C7 cells (Figure 1A). We verified that increased cell death was indeed due to apoptosis induction by using flow cytometry to detect increased phosphatidylserine exposure and caspase activation in apoptotic cells (Figure 1B,C). Single-agent treatment with dexamethasone resulted in increased annexin-V positivity and caspase activation in the GC-sensitive CEM-C7 cells, but only slightly if at all in GC-resistant cells. Combination treatment of dexamethasone with 0.25 μM ATO increased annexin-V positivity and caspase activation compared with controls with either ATO alone or dexamethasone alone in GC-resistant cells, and to a lesser extent in GC-sensitive cells (CEM-C7). The amount of PI-positive cells was consistently low (between 0.3% and 4.5% of all the cells) with the exception of CEM-C1 cells treated with the combination of ATO and Dex, where 11.5% ± 0.85% of the cells were PI positive (Table S2). To verify that the effect of ATO was independent of the p53 status in ALL cell lines, we used the MDM2 antagonist nutlin-3, which induces apoptosis by displacing p53 from MDM2 only when p53 function is intact34 (Table 1; Figure S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). We found GC-resistant T-ALL cells that are responsive to ATO with (CEM, Jurkat) and without (MOLT-4) functional evidence for a p53 mutation. We also evaluated the effect of ATO in combination with agents that are commonly used for ALL induction chemotherapy. All 4 T-ALL cell lines were sensitive to asparaginase, vincristine, and daunorubicin and their drug-response profile was not altered by combination with ATO (Figure S1). To evaluate if glucocorticoid receptor (GR)–mediated transactivation could also contribute to GC sensitization by ATO, we performed combinatorial treatment experiments after transfection of a GR-responsive luciferase reporter (Figure S3). As expected, dexamethasone treatment resulted in increased transactivation of the GR-responsive reporter gene in GC-resistant cells, indicating the presence of functional GR in these cells. ATO did not influence reporter transactivation, either alone or in combination with dexamethasone. These results indicate that low-dose ATO can resensitize GC-resistant ALL cells to dexamethasone via GR-independent mechanisms.
Characteristics of the T- ALL cell lines included in this study
| Characteristics . | Cell line . | |||
|---|---|---|---|---|
| CEM-C7 . | CEM-C1 . | Jurkat . | MOLT-4 . | |
| IC50 DEX, μM | 0.8 | >10 | > 10 | 10 |
| IC50 PRED, μM | 1.8 | >100 | > 100 | > 100 |
| ATO, μM | 2.0 | 1.8 | 1.2 | 0.76 |
| GR status | L753F het | L753F het | R477H het | ? |
| p53 status | het mut | het mut | het mut | no mutation |
| Nutlin assay | no effect | no effect | no effect | inhibition |
| Characteristics . | Cell line . | |||
|---|---|---|---|---|
| CEM-C7 . | CEM-C1 . | Jurkat . | MOLT-4 . | |
| IC50 DEX, μM | 0.8 | >10 | > 10 | 10 |
| IC50 PRED, μM | 1.8 | >100 | > 100 | > 100 |
| ATO, μM | 2.0 | 1.8 | 1.2 | 0.76 |
| GR status | L753F het | L753F het | R477H het | ? |
| p53 status | het mut | het mut | het mut | no mutation |
| Nutlin assay | no effect | no effect | no effect | inhibition |
IC50s were calculated using Graphpad Prism software with triplicate values from MTT assays. The GR mutation R477H was also found in families with generalized resistance to GC, mutation L753F was found in the patient samples from which the CEM line was derived, and probably is not relevant for GC-resistance in CEM-C1. p53 mutations as reported in the literature. We determined the dose response curves for nutlin-3 with the MTT assay.
het indicates heterozygous; mut, mutation; DEX, dexamethasone; and PRED, prednisone.
? indicates mutation status unknown.
ATO sensitizes resistant T-ALL cell lines to dexamethasone treatment. (A) Response to increasing concentrations of dexamethasone (DEX) after 72-hour incubation with or without 0.25 μM ATO. Cell viability was assessed using the MTT assay. (B) Apoptosis rates were determined using annexin-V/propidium iodide stainings. Cells were incubated for 48 hours (CEM-C1, CEM-C7, and MOLT-4) or 72 hours (Jurkat) with control, 0.25 μM ATO, 7.6 μM Dex (or 0.1 μM for CEM-C7), and the appropriate combination and analyzed by flow cytometry. (C) Induction of caspase-3 activity was assessed by flow cytometry. For panel A, the measurements were normalized to untreated controls, and for all experiments, values of 3 experiments are shown as mean plus or minus SD; **P < .05 (Student t test).
ATO sensitizes resistant T-ALL cell lines to dexamethasone treatment. (A) Response to increasing concentrations of dexamethasone (DEX) after 72-hour incubation with or without 0.25 μM ATO. Cell viability was assessed using the MTT assay. (B) Apoptosis rates were determined using annexin-V/propidium iodide stainings. Cells were incubated for 48 hours (CEM-C1, CEM-C7, and MOLT-4) or 72 hours (Jurkat) with control, 0.25 μM ATO, 7.6 μM Dex (or 0.1 μM for CEM-C7), and the appropriate combination and analyzed by flow cytometry. (C) Induction of caspase-3 activity was assessed by flow cytometry. For panel A, the measurements were normalized to untreated controls, and for all experiments, values of 3 experiments are shown as mean plus or minus SD; **P < .05 (Student t test).
Low-dose ATO generates oxidative stress and, in combination with dexamethasone, a reduction in Akt phosphorylation with concomitant effects on Akt targets Bad and XIAP
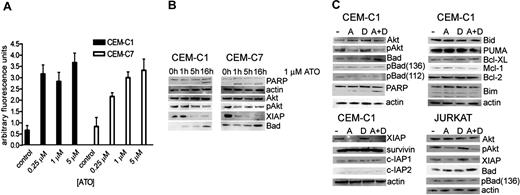
Given that induction of intracellular ROSs is one of the putative mechanisms of ATO-induced apoptosis in cancer cells, we determined the impact of different ATO concentrations on ROS generation (Figure 2A). Cells were treated with ATO concentrations of 0.25, 1, and 5 μM, and ROS production was measured by fluorometry. In GC-resistant cell lines, a 5-fold increase of ROSs was detected both at subtoxic (0.25 μM) and cytotoxic (1-5 μM) ATO concentrations. Since increased oxidative stress is commonly associated with activated oncogenic signaling, we focused next on the evaluation of key regulators of survival pathways. We first evaluated Akt, based on reports of high levels of phospho-Akt in lymphoblastic cell lines. Exposure to 1 μM ATO led to a decrease of phospho-ser 473 Akt, the activated phosphorylated form of Akt, within 10 hours of treatment, whereas the overall Akt levels remained unchanged (Figure 2B). We next focused on known Akt targets, including Bad, a proapoptotic member of the Bcl-2 family of intrinsic apoptosis pathway regulators,18 and the X-linked inhibitor of apoptosis protein XIAP, a potent inhibitor of caspases.19 Exposure to 1 μM ATO correlated with an increase in Bad protein levels after 16 hours of treatment. Simultaneously, XIAP levels were considerably decreased.
ATO treatment induces ROS production and dephosphorylation of Akt, which is associated with an increase of Bad and decrease of XIAP. (A) ROS generation in CEM-C1 and -C7 was quantified by fluorometry with varying concentrations of ATO as indicated. Values of 3 experiments are shown as mean (−SD); P equals .05 (Student t test). (B) ATO as single agent. CEM-C1 and -C7 cells were incubated for different times with 1 μM ATO. Western blot analysis using whole-cell extracts from a representative experiment is shown. Antiactin was used for loading control. (C) Combination for 5 hours of 0.25 μM ATO with 1 μM DEX in CEM-C1 and Jurkat cells; A indicates ATO; D, DEX.
ATO treatment induces ROS production and dephosphorylation of Akt, which is associated with an increase of Bad and decrease of XIAP. (A) ROS generation in CEM-C1 and -C7 was quantified by fluorometry with varying concentrations of ATO as indicated. Values of 3 experiments are shown as mean (−SD); P equals .05 (Student t test). (B) ATO as single agent. CEM-C1 and -C7 cells were incubated for different times with 1 μM ATO. Western blot analysis using whole-cell extracts from a representative experiment is shown. Antiactin was used for loading control. (C) Combination for 5 hours of 0.25 μM ATO with 1 μM DEX in CEM-C1 and Jurkat cells; A indicates ATO; D, DEX.
If modulation of Akt, Bad, and XIAP were relevant for the synergistic effect observed with low-dose ATO and dexamethasone, then the combination treatment should have similar effects on these targets as ATO alone. Combination treatment resulted in a decrease of Akt phosphorylation and in an increase of Bad compared with low-dose ATO treatment and similar to the effect obtained with 1 μM ATO alone (Figure 2C). Detection of PARP cleavage as a measure of apoptosis appeared more pronounced in the combination treatment when compared with the single agents. A sharp decrease of XIAP levels occurred with 0.25 μM ATO, without detectable difference when dexamethasone was added. Also included in our panel were a number of proapoptotic and antiapoptotic regulators. As shown in Figure 2C, levels of the proapoptotic BH3-only proteins PUMA, Bim, and Bid as well as the antiapoptotic regulators Bcl-2, Bcl-XL, and Mcl-1 were unchanged after exposure to ATO and dexamethasone. These results indicate that inhibition of Akt is a possible mechanism of the sensitizing effect of low-dose ATO on dexamethasone. Modulation of Bad and XIAP may be relevant for this effect, although the effect on XIAP could also be mediated by another ATO-dependent mechanism. To verify whether other IAP family members were affected, we analyzed the expression levels of survivin, c-IAP1, and c-IAP2 after treatment with either ATO alone, dexamethasone alone, or the combination of both. As shown in Figure 2C, expression levels of these IAP family proteins were unchanged suggesting that down-regulation of XIAP is a specific effect.
Transient expression of dominant-active myrAkt decreases GC response in sensitive cells and interferes with the GC-sensitizing effect of ATO
To verify the relevance of Akt inactivation for the GC-sensitizing effect of ATO, we used a dominant-active myristoylated form of Akt.31 We transiently expressed myrAkt for 24 hours in CEM-C1 cells and analyzed the effect on cell viability following treatment with dexamethasone or the combination of ATO at 0.25 μM and varying concentrations of dexamethasone of 0.1, 1, and 7.6 μM for 48 hours. Transient expression of myrAkt conferred complete resistance to dexamethasone treatment and inhibited the sensitizing effect of ATO to dexamethasone, rendering CEM-C1 resistant to the combination treatment (Figure 3A). One would expect that myrAkt expression would also alter the expression of the Akt targets XIAP and Bad in this experiment. Indeed, myrAkt expression rescued XIAP protein levels, and Bad phosphorylation was increased compared with mock-transfected cells (Figure 3B). XIAP down-regulation was inhibited and Bad phosphorylation at Ser136 was maintained at control levels when myrAkt-overexpressing cells were incubated with the combination of ATO and dexamethasone. These data indicate that Akt activity protects XIAP from degradation induced by low-dose ATO and dexamethasone as well as maintains Bad in its inactive phosphorylated state.
Transient expression of myrAkt impairs the sensitizing effect of ATO on dexamethasone-mediated cell death. (A) Dominant-active myrAkt was transiently expressed in CEM-C1 cells. MyrAkt rendered cells completely resistant to dexamethasone treatment and reduced the effect of 0.25 μM ATO on the cytotoxic effect of increasing DEX concentrations (0.1 μM, 1.0 μM, 7.6 μM). Values from triplicate MTT assays are shown as mean plus or minus SD. (B) myrAkt expression in CEM-C1 cells enhances the levels of Ser136 p-Bad and restores XIAP. Cells were treated as indicated 24 hours after transfection. Whole-cell extracts were obtained after 5 hours of drug exposure and analyzed by Western blot.
Transient expression of myrAkt impairs the sensitizing effect of ATO on dexamethasone-mediated cell death. (A) Dominant-active myrAkt was transiently expressed in CEM-C1 cells. MyrAkt rendered cells completely resistant to dexamethasone treatment and reduced the effect of 0.25 μM ATO on the cytotoxic effect of increasing DEX concentrations (0.1 μM, 1.0 μM, 7.6 μM). Values from triplicate MTT assays are shown as mean plus or minus SD. (B) myrAkt expression in CEM-C1 cells enhances the levels of Ser136 p-Bad and restores XIAP. Cells were treated as indicated 24 hours after transfection. Whole-cell extracts were obtained after 5 hours of drug exposure and analyzed by Western blot.
Since the members of the IAP family are transcriptionally regulated by NF-κB,35 we also evaluated the effect of ATO on NF-κB activation. Cells were stimulated with ATO, dexamethasone, or in combination, and the effect on nuclear translocation of NF-κB was analyzed. We could not detect any decrease in the nuclear translocation of the p65 subunit of NF-κB in this experiment (Figure S3). Collectively, our results suggest that a pathway involving Akt, XIAP, and Bad is affected as a result of exposure to ATO and dexamethasone.
Down-regulation of Bad and overexpression of XIAP confer resistance to ATO-induced GC sensitization
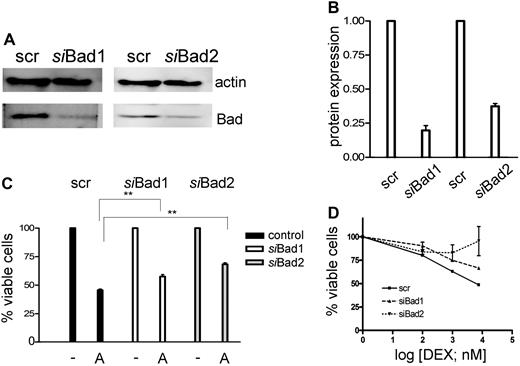
To demonstrate that inactivation of Bad may play a role in GC resistance, we knocked down Bad levels by RNA interference in CEM-C1 cells (Figure 4A). A significant decrease of Bad was observed with 2 different specific siRNAs, but not with a scrambled control (Figure 4B), which correlated with a reduction of ATO-mediated cytotoxicity at 1-μM concentration (Figure 4C). As expected, down-regulation of Bad affected the sensitizing effect of low-dose ATO in combination with dexamethasone, whereby one of the specific siRNAs almost completely rescued the GC-resistance phenotype (Figure 4D). These results are consistent with the modulation of a pathway involving Bad in GC sensitization, but imply that additional mechanisms account for the full effect of ATO treatment.
Down-regulation of Bad using siRNA decreases the sensitivity of the cells to the combination treatment of ATO and dexamethasone. (A) CEM-C1 cells were transfected with 2 distinct siRNAs directed against Bad. Down-regulation of Bad was assessed after 48 hours by Western blot. (B) Signal quantification by densitometry. (C) siBad treatment of CEM cells decreased the cytotoxic effect of 1 μM ATO as single agent and (D) of the combination of low-dose ATO (0.25 μM) with DEX. A scrambled siRNA was used for control. Triplicate results of the MTT assay are shown as mean plus or minus SD; **P < .05 (Student t test).
Down-regulation of Bad using siRNA decreases the sensitivity of the cells to the combination treatment of ATO and dexamethasone. (A) CEM-C1 cells were transfected with 2 distinct siRNAs directed against Bad. Down-regulation of Bad was assessed after 48 hours by Western blot. (B) Signal quantification by densitometry. (C) siBad treatment of CEM cells decreased the cytotoxic effect of 1 μM ATO as single agent and (D) of the combination of low-dose ATO (0.25 μM) with DEX. A scrambled siRNA was used for control. Triplicate results of the MTT assay are shown as mean plus or minus SD; **P < .05 (Student t test).
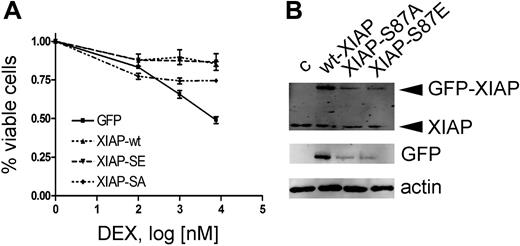
Rapid down-regulation of XIAP due to loss of the stabilizing phosphorylation on serine residue 87 could represent such a mechanism. We therefore investigated the effect of XIAP overexpression on dexamethasone sensitization. To evaluate the relevance of the Akt phosphorylation site, we mutated serine 87 to either glutamine (XIAP-S87E, gain of function) or alanine (XIAP-S87A, loss of function) as reported previously.19 As anticipated, overexpression of XIAP rendered CEM-C1 cells resistant to ATO-induced GC-mediated cell death (Figure 5A). Despite overexpression levels at 25% of wild-type XIAP, XIAP-S87E induced GC resistance to a similar extent (Figure 5B). In contrast, XIAP-S87A expression was clearly less effective at rescuing cells from ATO-induced GC-sensitization at expression levels that were comparable to the S87E mutant. The correlation of decreased XIAP levels with inhibition of Akt phosphorylation could be explained by an effect on XIAP protein turnover. Indeed, the dominant-active mutant XIAP-S87E, which is less susceptible to proteasomal mediated degradation, was able to restore the resistance phenotype at much lower expression levels compared with the wild-type protein (Figure 5A,B). Collectively, our results show that dephosphorylation of Bad and destabilization of XIAP levels, as a consequence of Akt inhibition, are important for the GC-sensitizing effect of ATO.
Resistance to ATO-mediated GC sensitization by XIAP overexpression requires the XIAP-S87 phosphorylation site. (A) Response to increasing concentrations of DEX after 72-hour incubation in combination with 0.25 μM ATO, after transient transfection of different XIAP constructs as indicated and described in “Patients, materials, and methods.” Triplicate results of the MTT assay are shown as mean plus or minus SD. (B) Western blot with specific anti-XIAP antibody (top panel) and an anti-GFP antibody (bottom panel).
Resistance to ATO-mediated GC sensitization by XIAP overexpression requires the XIAP-S87 phosphorylation site. (A) Response to increasing concentrations of DEX after 72-hour incubation in combination with 0.25 μM ATO, after transient transfection of different XIAP constructs as indicated and described in “Patients, materials, and methods.” Triplicate results of the MTT assay are shown as mean plus or minus SD. (B) Western blot with specific anti-XIAP antibody (top panel) and an anti-GFP antibody (bottom panel).
Combination of ATO with the mTOR inhibitor rapamycin is not synergistic
Having established Akt as important mediator in the GC sensitization, and given that mTOR is also a potential target of Akt, we asked whether intrapathway inhibition of Akt and mTOR by combination of rapamycin with ATO increases the GC sensitization effect of ATO (Figure S4). As reported recently,21 rapamycin augmented the GC sensitivity of resistant CEM cells, an effect that was shown to be associated with decreased Mcl-1 levels. Indeed, 10 nM rapamycin sensitized CEM-C1 to dexamethasone with a comparable efficiency with the results achieved with 0.25 μM ATO. However, combination of the 2 agents was not synergistic. Thus, the 2 approaches appear to be equivalent but not complementary in the CEM cell-line model of GC resistance.
ATO improves glucocorticoid sensitivity of primary leukemic cells from ALL patients with poor in vivo response to prednisone
In order to validate the results obtained in cell lines, we selected pretreatment samples from 7 ALL patients at disease presentation based on their prednisone response.1 We included 2 patients with poor initial response to prednisone (PPR) and persistence of minimal residual disease after induction chemotherapy (MRD-HR), 2 patients with a PPR but low or no detectable MRD at week 12 (non–MRD-HR), and 3 patients with good response to prednisone (PGR) (Table 2). We first determined the response to ATO as a single agent in vitro (Figure 6A). At low concentration, ATO was not cytotoxic for normal bone marrow leukocytes. The IC50 values for ATO-sensitive leukemia cells (3 PPR, 1 PGR) were comparable with the responses obtained in cell lines. Low-dose ATO treatment clearly increased the dexamethasone sensitivity of T-ALL and pre-B-ALL cells from prednisone poor-responder patients (Figure 6B, Pt 1-4), but not in cells obtained from the prednisone good-responder patients (Figure 6B, Pt 5-7). This response was observed in both T-ALL (Pt 1-2) and precursor B-ALL (Pt 3-4), similar to the observations made in cell lines. These results indicate that ATO sensitization to dexamethasone does occur in primary cells from GC-resistant patients and suggest that combination therapy with ATO might be beneficial for GC-resistant patients.
Patients characteristics
| Characteristic . | Patient no. . | ||||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | |
| Age, y | 9 | 2 | 13 | 13 | 3 | 3 | 2 |
| Subtype | T | T | pc-B | pc-B | T | pc-B | pc-B |
| Blast, d0 | 207 | 338 | 70 | 69 | 34 | 0.1 | 69 |
| Blast, d8 | 127 | 1.8 | 5.7 | 1.8 | 0.7 | 0 | 0 |
| Pred. resp* | poor | poor | poor | poor | good | good | good |
| BM blast d0 | 92% | 95% | n/a | 99% | 87% | 98% | 96% |
| MRD† | HR | non-HR | HR | non-HR | non-HR | non-HR | non-HR |
| Characteristic . | Patient no. . | ||||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | |
| Age, y | 9 | 2 | 13 | 13 | 3 | 3 | 2 |
| Subtype | T | T | pc-B | pc-B | T | pc-B | pc-B |
| Blast, d0 | 207 | 338 | 70 | 69 | 34 | 0.1 | 69 |
| Blast, d8 | 127 | 1.8 | 5.7 | 1.8 | 0.7 | 0 | 0 |
| Pred. resp* | poor | poor | poor | poor | good | good | good |
| BM blast d0 | 92% | 95% | n/a | 99% | 87% | 98% | 96% |
| MRD† | HR | non-HR | HR | non-HR | non-HR | non-HR | non-HR |
Blast in Giga/L
T indicates T-ALL; pc-B, precursor-B-ALL; Pred. resp, prednisone response; BM, bone marrow; HR, high risk and n/a, not available.
Prednisone poor response is defined as more then 1000 blasts/μL at day 8 of prednisone therapy.
HR MRD is defined as an MRD load of 10−3 or greater at week 12 of treatment.
ATO sensitizes primary leukemia cells from prednisone poor-responder patients to dexamethasone. (A) Effect of ATO as single agent on 7 ALL patients who are described in Table 2. No cytotoxic effect could be detected in bone marrow leukocytes from a healthy donor at 0.33 μM ATO. (B) Response to increasing concentrations of DEX with or without 0.33 μM ATO on ALL cells from prednisone poor responders with T-ALL (Pt 1-2), pre-B-ALL (Pt 3-4), from prednisone good responders with T-ALL (Pt 5) and pre-B-ALL (Pt 6-7).
ATO sensitizes primary leukemia cells from prednisone poor-responder patients to dexamethasone. (A) Effect of ATO as single agent on 7 ALL patients who are described in Table 2. No cytotoxic effect could be detected in bone marrow leukocytes from a healthy donor at 0.33 μM ATO. (B) Response to increasing concentrations of DEX with or without 0.33 μM ATO on ALL cells from prednisone poor responders with T-ALL (Pt 1-2), pre-B-ALL (Pt 3-4), from prednisone good responders with T-ALL (Pt 5) and pre-B-ALL (Pt 6-7).
Discussion
We report here that subtoxic doses of arsenic trioxide (ATO) can restore the sensitivity to dexamethasone in glucocorticoid (GC)–resistant T and precursor B acute lymphoblastic leukemia cells and provide evidence for the Akt/XIAP pathway as candidate target for combinatorial therapy in GC resistance.
Characteristic profiles of activated survival signals can be detected in leukemia.36,37 One of the key signaling nodes that is commonly activated in human tumors is Akt.14 High levels of endogenous phospho-Akt in ALL cell lines correlated with an increased sensitivity to the PI3K/Akt inhibitor LY294002,17 suggesting that this pathway may be important for the survival of leukemia cells. Constitutive activation of prosurvival pathways could contribute to drug resistance by promoting the resistance to apoptosis. Recent gene expression profiling data suggest that Akt might indeed play a role in GC-resistant ALL. Gene set enrichment analysis revealed that among gene sets derived from the BioCarta pathway database (San Diego, CA), the Akt pathway was the most highly enriched set in GC-resistant pre-B-ALL samples.21 Because different oncogenic events may result in activation of the Akt pathway,14 approaches to target this pathway may be more widely applicable and not dependent on the presence of a particular mutation. Based on these considerations, we postulated that the broad range of biologic activities of ATO, including its potential to inhibit the Akt pathway,15 could offer an advantage in combination therapy of GC-resistant ALL.
The recent report that single-agent ATO treatment did not induce a clinical response in 11 adult patients with relapsed and refractory ALL38 should not preclude further evaluation of this agent in combination therapy in ALL. For example, combination of ATO with the tyrosine kinase inhibitor imatinib resulted in more efficient induction of apoptosis in chronic myelogenous leukemia (CML).39 We have shown here that subtoxic concentrations of ATO (0.25 μM) increase the sensitivity to dexamethasone effectively in GC-resistant cells, which is reflected by a level of ROS production comparable with levels achieved at higher cytotoxic ATO doses (1-5 μM). This is relevant because toxicity on normal hematopoietic tissues was not relevant using ATO concentrations below 0.5 μM.40,41 Thus, lower doses of ATO may be sufficient to achieve clinically relevant drug-sensitization effects.
Different pathways were altered by ATO treatment depending on the type of hematologic malignancy studied, such as the MAPK pathway in AML cells and the NF-κB pathway in myelodysplastic syndrome.30,42 This may reflect context-dependent differences in signal transduction pathway activation and illustrates the potential advantage to use an agent with such a broad target range. We found evidence mostly for an implication of Akt and downstream Akt targets in GC sensitization by ATO, but recognize that we have examined only a subset of downstream effectors that have been previously implicated in the response to ATO.28 Among other possible mechanisms that we evaluated, we could not detect changes in Erk phosphorylation or find evidence for NF-κB inhibition. In our hands, ATO treatment also did not result in nuclear translocation of the apoptosis-inducing factor (AIF) (data not shown).
Our results show that ATO inhibits Akt activation and influences 2 important regulators of apoptosis, the proapoptotic Bcl-2 family member Bad and a member of the inhibitor of apoptosis protein (IAP) family, XIAP. Down-regulation of Bad by RNA interference decreased ATO-mediated GC sensitivity suggesting that part of the ATO effect is mediated through interference with the intrinsic apoptotic pathway via Bad. We also provide data for a complementary mechanism at the postmitochondrial level via regulation of XIAP, which strongly regulates apoptosis by direct binding and inactivation of the caspases-3, -7, and -9. XIAP was reported to be stabilized by Akt phosphorylation, resulting in a decrease of ubiquitin-mediated protein degradation.19 Consistent with the hypothesis that Akt inhibition could influence XIAP protein stability, we observed a sharp decrease of XIAP after ATO treatment. Accordingly, overexpression of dominant-active myrAkt restored the XIAP levels, which correlated with GC resistance. Furthermore, the presence of the Akt phosphorylation site on XIAP was required for a complete reversion of the GC sensitization by ATO. Down-regulation of XIAP levels could also be due in part to inhibition of translation resulting from a decrease of mTOR activity, since mTOR is a target of Akt and a decrease of XIAP translation was reported as part of the synergistic effect of rapamycin and dexamethasone combination in multiple myeloma.22 However, this would not explain why other IAPs, survivin, cIAP1, and cIAP2, were not affected by ATO treatment. Nevertheless, our experiments identified the Bcl-2 family member Bad and XIAP as candidate targets for combination therapy in drug-resistant ALL and provide a rationale for the evaluation of more specific small molecule inhibitors that target apoptotic regulators in GC-resistant ALL.43,44
Given the crosstalk between mTOR and Akt,45 the ATO effect could also be mediated via Akt-induced mTOR inhibition. Indeed, recent data show that the mTOR inhibitor rapamycin increased the sensitivity of CCRF-CEM cells to GC.21 This effect was associated with down-regulation of the antiapoptotic regulator Mcl-1. However, we could not detect any effect of ATO-mediated Akt inhibition on Mcl-1 levels, implying that either indirect inhibition of mTOR was not relevant for the ATO effect or that Mcl-1 down-regulation may not be a direct effect of mTOR inhibition. The combination of ATO and rapamycin did not increase the sensitivity of CEM cells to GC. This suggests that the 2 agents could have overlapping effects. However, rapamycin was shown to revert the resistance phenotype conferred by myrAkt overexpression in GC-sensitive lymphoid cells only partially,21 indicating that part of the resistance mechanism is independent of mTOR signaling. Our results also indicate that down-regulation of Mcl-1 is not essential for GC sensitization, supporting the concept that Mcl-1–independent mechanisms are important. Among other Bcl-2 family members that could be involved in GC resistance, a failure of Bim induction has been described to occur in GC-resistant ALL cells.2 In our experiments, an increase in Bim isoform levels was not detected in ATO-mediated GC sensitization.
Experiments with primary cells are indispensable to model drug resistance in pre-B-ALL, due to the lack of representative cell lines. We did detect a GC-sensitizing effect using low-dose ATO consistently in GC-resistant samples, but not in GC-sensitive samples, both from T-ALL and precursor B-ALL patients. This observation strongly suggests that GC-resistant ALL cells may be addicted to prosurvival pathways that can be influenced by ATO treatment. Improved preclinical models of de novo drug-resistant ALL will be required in order to define the profiles of pathway activation that may predict resistance to different drugs in MRD-HR ALL and to test appropriate agents in combination therapy. Our data indicate that combination of low-dose ATO with glucocorticoids may be a valuable and affordable approach to improve the treatment of GC-resistant ALL.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant of the Fondation pour la Recherche Cancer de l' Enfant, by a grant from the United Bank of Switzerland in the name of an anonymous donor, and by grants of Cancerfonden Sverige and Minerva Institute Helsinki.
We thank Rainer Lanz for providing the MMTV-LUC reporter construct; Ivan Maillard, Charles Roberts, and Gregory Melroe for critical reading of the paper; and David Betts and Marco Thali for assistance with patient samples.
Authorship
Contribution: B.C.B., B.W.S., and J.-P.B. designed the experiments and analyzed the data; B.C.B, L.B., and R.M. performed the experiments; J.-P.B. drafted and wrote the paper; F.K.N, G.C., M.S., D.L., and R.M. contributed essential reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Pierre Bourquin, Department of Oncology, University Childrens Hospital Zurich, Steinwiesstrasse 75, CH-8032 Zurich; e-mail: jean-pierre.bourquin@kispi.uzh.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal