Abstract
Aurora kinases play an important role in chromosome alignment, segregation, and cytokinesis during mitosis. We have recently shown that hematopoietic malignant cells including those from acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) aberrantly expressed Aurora A and B kinases, and ZM447439, a potent inhibitor of Aurora kinases, effectively induced growth arrest and apoptosis of a variety of leukemia cells. The present study explored the effect of AZD1152, a highly selective inhibitor of Aurora B kinase, on various types of human leukemia cells. AZD1152 inhibited the proliferation of AML lines (HL-60, NB4, MOLM13), ALL line (PALL-2), biphenotypic leukemia (MV4-11), acute eosinophilic leukemia (EOL-1), and the blast crisis of chronic myeloid leukemia K562 cells with an IC50 ranging from 3 nM to 40 nM, as measured by thymidine uptake on day 2 of culture. These cells had 4N/8N DNA content followed by apoptosis, as measured by cell-cycle analysis and annexin V staining, respectively. Of note, AZD1152 synergistically enhanced the antiproliferative activity of vincristine, a tubulin depolymerizing agent, and daunorubicin, a topoisomerase II inhibitor, against the MOLM13 and PALL-2 cells in vitro. Furthermore, AZD1152 potentiated the action of vincristine and daunorubicin in a MOLM13 murine xenograft model. Taken together, AZD1152 is a promising new agent for treatment of individuals with leukemia. The combined administration of AZD1152 and conventional chemotherapeutic agent to patients with leukemia warrants further investigation.
Introduction
The Aurora family of serine/threonine kinases plays an important role in chromosome alignment, segregation, and cytokinesis during mitosis. The family consists of 3 members: Aurora A, B, and C, which share 67% to 76% amino acid sequence identity in their catalytic domains, but little similarity in their N-terminus.1 Aurora A localizes to spindle poles and has a crucial role in bipolar spindle formation.2 Aurora B is a chromosomal passenger protein and localizes at centromeres during prometaphase and subsequently relocates to midzone microtubules and midbodies during anaphase and telophase.1,3 Aurora B plays a role in chromosome alignment, kinetochore-microtubule biorientation, activation of the spindle assembly checkpoint, and cytokinesis in association with phosphorylation of histone H3 on Ser10.1,3 Aurora C is specifically expressed in the testis and plays a role in spermatogenesis.4 Its role in carcinogenesis remains unclear
Previous studies found that Aurora A and B were aberrantly expressed in a variety of solid tumors including prostate,5,6 colon,6 pancreas,7 breast,8 and thyroid cancers.9 In addition, increased levels of Aurora kinases correlated with advanced clinical stage in individuals with prostate cancer, as well as those with head and neck squamous cell carcinoma.5,10 We have recently shown that hematologic malignant cells including those from acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic myeloid leukemia (CML) aberrantly expressed Aurora A and B kinases.11 In addition, we have demonstrated that ZM447439, a novel and selective inhibitor of Aurora A and B kinases, effectively induced growth arrest and apoptosis of a variety of types of leukemia cells.11 Recent studies performed by other groups noted that the phenotype of HeLa cells after their exposure to ZM447439 was similar to those induced by knockdown of the Aurora B kinase gene.12
AZD1152 is a novel acetanilide-substituted pyrazole-aminoquinazoline prodrug that is converted rapidly to the active drug AZD1152 hydroxy-QPA (AZD1152-HQPA) in human plasma.13,14 AZD1152-HQPA is a specific inhibitor of the enzymatic activity of Aurora kinase, with selectivity for Aurora B (IC50 of 0.37 nM versus 1368 nM for Aurora B and A kinases, respectively); the inhibitor had even less activity against a panel of greater than 50 other serine-threonine and tyrosine kinases including FLT3, JAK2, and Abl.13,14 Preliminary studies showed that AZD1152 was active against a variety of solid tumors including colon, breast, and lung cancers.13,14 However, the effect of AZD1152 in hematologic malignancies remains to be fully elucidated. This study explored the effect of AZD1152 in a variety of types of leukemia, as well as tested its ability to enhance the activity of conventional anticancer agents in vitro as well as in vivo.
Materials and methods
Leukemia cells from patients as well as bone marrow mononuclear cells from healthy volunteers were freshly isolated with informed consent and approval by the Kochi University institutional review board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Reagents
AZD1152 and AZD1152-HQPA were provided by AstraZeneca (Macclesfield, United Kingdom). AZD1152-HQPA was dissolved in 100% dimethyl sulfoxide (DMSO; Burdick & Jackson, Muskegon, MI) to a stock concentration of 10 mM and stored at −80°C. AZD1152 was dissolved in 3 M Tris, pH 9.0, at a concentration of 2.5 mg/mL and used for our in vivo studies.
Cells
Colony-forming assay
The effects of AZD1152 on clonogenic growth of leukemia cells as well as normal bone marrow mononuclear cells were assessed by colony-forming assay using methylcellulose medium H4534 (StemCell Technologies, Vancouver, BC), as previously described.15
Thymidine uptake studies
Proliferation of leukemia cells was measured by tritiated thymidine uptake [3H-TdR] (Perkin Elmer, Boston, MA), as described.15
Cell-cycle analysis by flow cytometry
Cell-cycle analysis was performed on leukemia cells incubated with AZD1152-HQPA (1-10 nM) for 2 days at 5 × 105 cells/mL in 12-well plates (Flow Laboratories, Irvine, CA).15
Measurement of p-Histone H3 at Ser10 by flow cytometry
The Alexa Fluor 488–conjugated anti–p-histone H3 (Ser10; Cell Signaling Technology, Beverly, MA) was used to quantify the population of leukemia cells expressing the phosphorylated forms of histone H3. These experiments were performed using flow cytometry.
Apoptosis assays
The ability of AZD1152-HPQA to induce apoptosis of leukemia cells was measured by annexin V–FITC apoptosis detection kit according to the manufacturer's instructions (Pharmingen, San Diego, CA).
Immunoblotting
Immunoblotting was performed as described.15 Anti-PARP (Cell Signaling Technology) and ℒα-tubulin (Santa Cruz, Santa Cruz, CA) antibodies were used. Band intensities were measured by densitometry.
Mice
Female immune-deficient BALB/c nude mice at 4 weeks of age were purchased from JAPAN SLC (Shizuoka, Japan) and were maintained in pathogen-free conditions with irradiated chow. Animals were bilaterally, subcutaneously injected with 2 × 106 MOLM13 cells/tumor in 0.1 mL Matrigel (Collaborative Biomedical Products, Bedford, MA). When MOLM13 cells formed palpable tumors, mice were divided randomly into control (n=5) and treatment groups (n=5), and treatment was begun. AZD1152 (5 or 25 mg/kg) with or without vincristine (0.2 mg/kg) was given to mice by intraperitoneal injection 4 times a week or every another day, respectively. Daunorubicin (1 mg/kg) was given to mice by intraperitoneal injection 6 times during 2 weeks of treatment either alone or in combination with AZD1152 (5 mg/kg). The dose of these agents was determined by our preliminary studies (data not shown). Control diluent was given to the untreated control mice. Body weight and tumors were measured twice a week. Tumor sizes were calculated by the formula: a × b × c, where “a” is the length, “b” is the width, and “c” is the height in millimeters. At the end of the experiment, animals were killed by CO2 asphyxiation and tumor weights were measured after their careful resection. Tumor tissue was collected for analysis. The experiments were approved by the review board of Kochi University.
Data analysis
The combination index (CI) for growth inhibition elicited by AZD1152-HPQA and vincristine in leukemia cells was calculated using the median effect method of Chou and Talalay16 (Calcusyn Software, Biosoft, Cambridge, United Kingdom). CI values less than 1 indicate synergy, a CI of 1 indicates an additive effect, and a CI greater than 1 indicates antagonism between the 2 agents.
Statistical analysis
The nonparametric Mann-Whitney U test was performed to assess the difference of tumor volume or tumor weight between control and AZD1152-treated group. To assess the difference between 2 groups under multiple conditions, 1-way ANOVA followed by Bonferroni multiple comparison tests were performed using PRISM statistical analysis software (GraphPad Software, San Diego, CA).
Results
AZD1152-HQPA induced growth arrest of a variety of types of leukemia cells
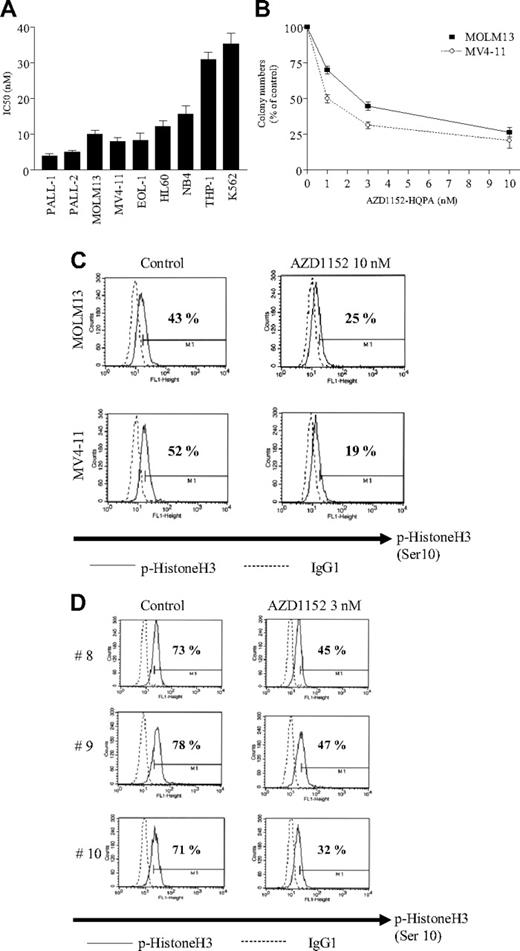
Leukemic cells were grown in liquid culture for 2 days in the presence of various concentrations of AZD1152-HQPA (1-100 nM). Growth inhibition was measured by thymidine uptake; the percent inhibition was graphed and the concentration of AZD1152-HQPA that induced 50% growth inhibition (IC50) of leukemia cells was determined (Figure 1). The growth of all cell lines was effectively inhibited in the presence of AZD1152-HQPA. For example, IC50s of Philadelphia chromosome–positive ALL PALL-2, acute monocytic leukemia MOLM13, and biphenotypic leukemia MV4-11 cells were approximately 5, 12, and 8 nM, respectively (Figure 1A). AZD1152-HQPA also inhibited the clonogenic growth of MOLM13 and MV4-11 cells with IC50s of 1 and 2.8 nM, respectively (Figure 1B).
AZD1152-HQPA inhibited the proliferation of leukemia cells. (A) 3H-thymidine uptake study. Cells from various types of human leukemias were cultured in the presence of various concentrations of AZD1152-HQPA (1-100 nM) for 48 hours. Proliferation of leukemia cells was measured by 3H-thymidine uptake (isotope added 6 hours before harvest), and the concentration that induced 50% growth inhibition (IC50) was calculated from dose-response curves. Results represent the mean plus or minus SD of 3 experiments performed in triplicate plate. (B) Clonogenic growth assay. MOLM13 or MV4-11 cells (1 × 105 cells/mL) were added 1:10 to methylcellulose medium H4534, containing 1% methylcellulose, 30% FCS, 1% BSA, 10−4 M mercaptoethanol, 2 mM l-glutamine, 50 ng/mL stem-cell factor, 10 ng/mL GM-CSF, and 10 ng/mL IL-3. Cells were placed in 24-well plates in a volume of 200 μL. Prior to this step, either AZD1152-HQPA (1-10 nM) or control diluent was placed into the wells. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2, and 10 days later colonies were counted. All experiments were done twice using triplicate plates per experimental point. AZD1152-HQPA inhibited phosphorylation of histone H3 (Ser10) in leukemia cells. Error bars represent SD. (C) MOLM13 or MV4-11 cells were exposed to AZD1152-HQPA (10 nM). After 24 hours, p-histone H3-expressing population (percentages are shown) was measured by FACScan (Becton Dickinson, Mountain View, CA). (D) Freshly isolated leukemia cells (case nos. 8-10, Table 1) were exposed to AZD1152-HQPA (3 nM). After 3 hours, p-histone H3–expressing population (percentages are shown) was measured by FACScan. Results represent one of the experiments performed twice in duplicate plate.
AZD1152-HQPA inhibited the proliferation of leukemia cells. (A) 3H-thymidine uptake study. Cells from various types of human leukemias were cultured in the presence of various concentrations of AZD1152-HQPA (1-100 nM) for 48 hours. Proliferation of leukemia cells was measured by 3H-thymidine uptake (isotope added 6 hours before harvest), and the concentration that induced 50% growth inhibition (IC50) was calculated from dose-response curves. Results represent the mean plus or minus SD of 3 experiments performed in triplicate plate. (B) Clonogenic growth assay. MOLM13 or MV4-11 cells (1 × 105 cells/mL) were added 1:10 to methylcellulose medium H4534, containing 1% methylcellulose, 30% FCS, 1% BSA, 10−4 M mercaptoethanol, 2 mM l-glutamine, 50 ng/mL stem-cell factor, 10 ng/mL GM-CSF, and 10 ng/mL IL-3. Cells were placed in 24-well plates in a volume of 200 μL. Prior to this step, either AZD1152-HQPA (1-10 nM) or control diluent was placed into the wells. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2, and 10 days later colonies were counted. All experiments were done twice using triplicate plates per experimental point. AZD1152-HQPA inhibited phosphorylation of histone H3 (Ser10) in leukemia cells. Error bars represent SD. (C) MOLM13 or MV4-11 cells were exposed to AZD1152-HQPA (10 nM). After 24 hours, p-histone H3-expressing population (percentages are shown) was measured by FACScan (Becton Dickinson, Mountain View, CA). (D) Freshly isolated leukemia cells (case nos. 8-10, Table 1) were exposed to AZD1152-HQPA (3 nM). After 3 hours, p-histone H3–expressing population (percentages are shown) was measured by FACScan. Results represent one of the experiments performed twice in duplicate plate.
We next examined antiproliferative effects of AZD1152-HQPA on freshly isolated leukemia cells from patients using a clonogenic assay. Aurora B transcripts were detectable in all cases, as measured by real-time polymerase chain reaction (PCR) (data not shown). Exposure of these cells to various concentrations of AZD1152-HQPA (0.3-10 nM) decreased colony-forming ability with IC50s less than 3 nM in all cases (Table 1). On the other hand, IC50s of AZD1152-HQPA (10 nM) for bone marrow mononuclear cells from healthy volunteers (n=3, data not shown) were more than 10 nM (data not shown).
Effect of AZD1152 on clonogenic growth of freshly isolated leukemia cells
| Pt no. . | Age/sex . | FAB . | WBC count, ×109/L . | % blast . | Genetic abnormalities . | Source . | IC50 . | Previous treatment . |
|---|---|---|---|---|---|---|---|---|
| 1 | 62/F | L2 | 6.4 | 60 | Normal | PB | 2 | Yes |
| 2 | 47/F | M4 | 4.4 | 70 | Normal | PB | 3 | Yes |
| 3 | 64/M | M2 | 32.1 | 26 | Complex | BM | 3 | No |
| 4 | 32/M | L2 | 77.1 | 54 | t(9;22)(q34;q11) | PB | 1 | Yes |
| 5 | 68/F | CMLbc | 12 | 70 | t(9;22)(q34;q11) | BM | 2 | Yes |
| 6 | 71/M | M2 | 6.3 | 17 | Complex | PB | 2 | Yes |
| 7 | 51/F | M7 | 4.4 | 63 | t(12;15)(p13;q15) | PB | 3 | Yes |
| 8 | 59/M | M1 | 7.9 | 81 | der(1;7)(q10;q10) | PB | 3 | Yes |
| 9 | 64/F | M2 | 141 | 100 | Normal | PB | 3 | Yes |
| 10 | 59/M | M1 | 8.8 | 92 | Complex | PB | 2 | Yes |
| Pt no. . | Age/sex . | FAB . | WBC count, ×109/L . | % blast . | Genetic abnormalities . | Source . | IC50 . | Previous treatment . |
|---|---|---|---|---|---|---|---|---|
| 1 | 62/F | L2 | 6.4 | 60 | Normal | PB | 2 | Yes |
| 2 | 47/F | M4 | 4.4 | 70 | Normal | PB | 3 | Yes |
| 3 | 64/M | M2 | 32.1 | 26 | Complex | BM | 3 | No |
| 4 | 32/M | L2 | 77.1 | 54 | t(9;22)(q34;q11) | PB | 1 | Yes |
| 5 | 68/F | CMLbc | 12 | 70 | t(9;22)(q34;q11) | BM | 2 | Yes |
| 6 | 71/M | M2 | 6.3 | 17 | Complex | PB | 2 | Yes |
| 7 | 51/F | M7 | 4.4 | 63 | t(12;15)(p13;q15) | PB | 3 | Yes |
| 8 | 59/M | M1 | 7.9 | 81 | der(1;7)(q10;q10) | PB | 3 | Yes |
| 9 | 64/F | M2 | 141 | 100 | Normal | PB | 3 | Yes |
| 10 | 59/M | M1 | 8.8 | 92 | Complex | PB | 2 | Yes |
Leukemia cells were freshly isolated from peripheral blood or bone marrow of patients and were cultured in methylcellulose medium in the presence of cytokines with/without various concentrations of AZD1152 (0.3-10 nM). After 14 days, colonies were counted. Concentration of AZD1152 that induced 50% inhibition of colony formation (IC50) was calculated from the dose-response curves.
Pt indicates patient; FAB, French-American-British (leukemia classification); WBC, white blood cell; CMLbc, blast crisis of chronic myeloid leukemia; F, female; PB, peripheral blood; M, male; and BM, bone marrow.
AZD1152-HQPA blocked phosphorylation of histone H3 in leukemia cells
Histone H3 is one of the substrates of Aurora B kinase.17 Phosphorylation of histone H3 on Ser10 is thought to play an important role in chromosome alignment during mitosis.17 We therefore examined whether AZD1152-HQPA inhibited phosphorylation of histone H3 (Ser10) in leukemia cells using flow cytometry. Approximately 43% or 52% of the population of either MOLM13 or MV4-11 cells, respectively, expressed the phosphorylated forms of histone H3; this population decreased to 25% or 19%, respectively, after their exposure to AZD1152-HQPA (10 nM) for 24 hours (Figure 1C). Also, AZD1152 (3 μM, 3 hours) significantly decreased expression of the phosphorylated forms of histone H3 in freshly isolated leukemia cells (Figure 1D; case nos. 8-10, Table 1), suggesting that AZD1152-HQPA effectively inhibited Aurora B kinase in these leukemia cells.
AZD1152-HQPA increased the population of cells with 4N/8N DNA content
Exposure of MOLM13 and PALL2 cells to AZD1152-HQPA prominently increased cell 4N/8N DNA content in a dose- and time-dependent manner (Figure 2), which was consistent with previous studies of ZM447439 (also an Aurora kinase inhibitor) in PALL-2 cells.11 Our observations suggested that cells exposed to AZD1152-HQPA exited mitosis and subsequently proceeded through S phase in the absence of cytokinesis (cell division).
Cell-cycle analysis. (A) MOLM13 or (B) PALL2 cells (5 × 105/mL) were plated in 24-well plates and cultured with various concentrations of AZD1152-HQPA (1-10 nM). After 24 or 48 hours, cells were harvested and cell-cycle distribution was analyzed by FACScan. Results represent 1 of the experiments performed 3 times in duplicate plate. Arrows indicate population with 2N, 4N, and 8N DNA content.
Cell-cycle analysis. (A) MOLM13 or (B) PALL2 cells (5 × 105/mL) were plated in 24-well plates and cultured with various concentrations of AZD1152-HQPA (1-10 nM). After 24 or 48 hours, cells were harvested and cell-cycle distribution was analyzed by FACScan. Results represent 1 of the experiments performed 3 times in duplicate plate. Arrows indicate population with 2N, 4N, and 8N DNA content.
AZD1152-HQPA induced apoptosis in leukemia cells
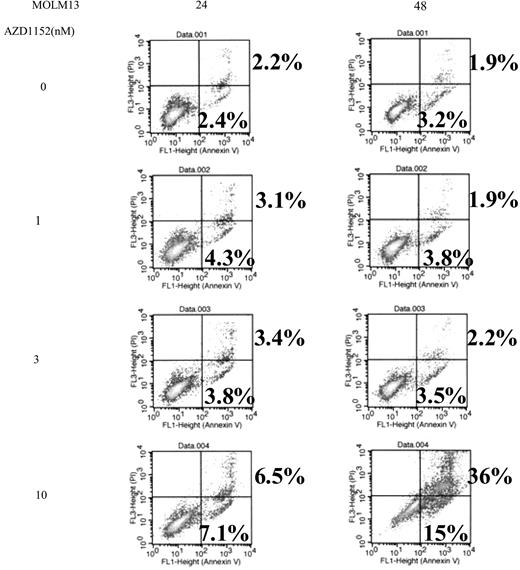
The ability of AZD1152 to induce apoptosis was assessed by measuring annexin V/propidium iodide (PI) staining in MOLM13 cells treated with AZD1152-HQPA (Figure 3). Annexin V+/PI− populations represent early apoptotic cells and annexin V+/PI+ populations represent late apoptotic/necrotic cells. Exposure of these cells to AZD1152-HQPA (1-10 nM) for 24 or 48 hours induced apoptosis in a dose-dependent manner (Figure 3); for example, exposure to either 3 or 10 nM AZD1152-HQPA induced either 6% or 50% of MOLM13 cells, respectively to become apoptotic at 48 hours (Figure 3).
Annexin V binding. MOLM13 cells (5 × 105/mL) were plated in 24-well plates and cultured with various concentrations of AZD1152-HQPA (1-10 nM). After 24 or 48 hours, cells were harvested, and annexin V binding and propidium iodide (PI) staining was analyzed by FACScan. Lower left quadrants show viable cells. Lower right quadrants show early apoptotic cells (annexin V+, PI−). Upper right quadrants show nonviable, late apoptotic/necrotic cells (annexin V+ and PI+). The numeric results represent the mean of triplicate plates, and a representative experiment is shown.
Annexin V binding. MOLM13 cells (5 × 105/mL) were plated in 24-well plates and cultured with various concentrations of AZD1152-HQPA (1-10 nM). After 24 or 48 hours, cells were harvested, and annexin V binding and propidium iodide (PI) staining was analyzed by FACScan. Lower left quadrants show viable cells. Lower right quadrants show early apoptotic cells (annexin V+, PI−). Upper right quadrants show nonviable, late apoptotic/necrotic cells (annexin V+ and PI+). The numeric results represent the mean of triplicate plates, and a representative experiment is shown.
AZD1152-HQPA potentiated the antiproliferative activity of vincristine directed against leukemia cells
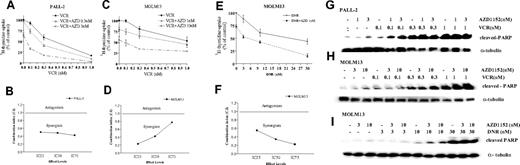
We examined the ability of AZD1152-HQPA to enhance the antiproliferative effects of conventional antileukemia agents, vincristine and daunorubicin. PALL-2 cells were cultured with various concentrations of AZD1152-HQPA (0.3-10 nM) and/or vincristine (0.1-1 μM) for 48 hours; proliferation was measured by 3H-thymidine uptake (Figure 4). Either AZD1152-HQPA (3 nM) or vincristine (0.3 μM) alone inhibited 3H-thymidine incorporation approximately 35% (Figure 4A). When cells were exposed to both compounds in combination, proliferation was inhibited by approximately 80% (Figure 4A), resulting in a CI value of less than 1 (synergistic antiproliferative effect, Figure 4B). AZD1152-HQPA also synergistically enhanced the antiproliferative activity of vincristine in MOLM13 cells (Figure 4C,D). Moreover, AZD1152-HQPA synergistically increased the growth inhibitory activity of daunorubicin, a topoisomerase II inhibitor, against MOLM13 cells (Figure 4E,F). Furthermore, the effect on cleavage of PARP, a feature characteristic of apoptosis, was examined in PALL-2 or MOLM13 cells after exposure to AZD1152-HQPA and/or vincristine. Vincristine alone increased levels of the cleaved form of PARP in a dose-dependent manner in PALL-2 and MOLM13 cells (Figure 4G,H). When the drug was combined with AZD1152-HQPA, the proportion of cleaved PARP was augmented (Figure 4G,H). Likewise, AZD1152-HQPA enhanced the ability of daunorubicin to cleave PARP in MOLM13 cells (Figure 4I).
AZD1152-HQPA potentiates effect of conventional chemotherapeutic agents. PALL-2 (A,B) or MOLM13 (C-F) cells were cultured with AZD1152-HQPA (1-10 nM) and/or either vincristine (VCR, 0.1-1 μM) or daunorubicin (DNR, 3-30 nM). After 2 days, cell proliferation was measured by 3H-thymidine uptake. The percent inhibition was graphed (A,C,E) and the concentration of each compound that induced 50%, 75%, or 90% growth inhibition (IC25, IC50, IC75) was determined (data not shown). The combination index (CI) of AZD1152-HQPA and VCR (B,D), or AZD1152-HQPA and daunorubicin (F) at various dose effects (IC25, IC50, IC75) was calculated using the median effect method. CI values less than 1 indicate synergy; CI of 1 indicates an additive effect; and CI greater than 1 demonstrates antagonism between the 2 agents. Western blot analysis of activated caspase (cleaved PARP) in PALL-2 (G) or MOLM13 (H-I). PALL-2 (G) or MOLM13 (H) cells were cultured with AZD1152-HQPA (1-10 nM) and/or VCR (0.1-1 μM). MOLM13 (I) cells were cultured with AZD1152-HQPA (1-10 nM) and/or DNR (3-30 nM). After 12 hours, cells were harvested, and proteins were extracted and subjected to Western blot analysis. The membranes were sequentially probed with anti-PARP and anti–α-tubulin antibodies. Results represent 1 of the 3 experiments performed independently. AZD indicates AZD1152-HQPA; VCR, vincristine; and DNR, daunorubicin. Error bars represent SD.
AZD1152-HQPA potentiates effect of conventional chemotherapeutic agents. PALL-2 (A,B) or MOLM13 (C-F) cells were cultured with AZD1152-HQPA (1-10 nM) and/or either vincristine (VCR, 0.1-1 μM) or daunorubicin (DNR, 3-30 nM). After 2 days, cell proliferation was measured by 3H-thymidine uptake. The percent inhibition was graphed (A,C,E) and the concentration of each compound that induced 50%, 75%, or 90% growth inhibition (IC25, IC50, IC75) was determined (data not shown). The combination index (CI) of AZD1152-HQPA and VCR (B,D), or AZD1152-HQPA and daunorubicin (F) at various dose effects (IC25, IC50, IC75) was calculated using the median effect method. CI values less than 1 indicate synergy; CI of 1 indicates an additive effect; and CI greater than 1 demonstrates antagonism between the 2 agents. Western blot analysis of activated caspase (cleaved PARP) in PALL-2 (G) or MOLM13 (H-I). PALL-2 (G) or MOLM13 (H) cells were cultured with AZD1152-HQPA (1-10 nM) and/or VCR (0.1-1 μM). MOLM13 (I) cells were cultured with AZD1152-HQPA (1-10 nM) and/or DNR (3-30 nM). After 12 hours, cells were harvested, and proteins were extracted and subjected to Western blot analysis. The membranes were sequentially probed with anti-PARP and anti–α-tubulin antibodies. Results represent 1 of the 3 experiments performed independently. AZD indicates AZD1152-HQPA; VCR, vincristine; and DNR, daunorubicin. Error bars represent SD.
AZD1152 inhibited the proliferation of MOLM13 xenografts in vivo
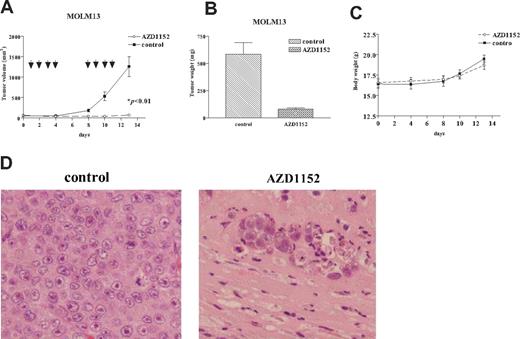
We evaluated the ability of AZD1152 to inhibit the growth of human MOLM13 cells growing as xenografts using an immunodeficient murine model. Tumor volumes were measured twice per week (Figure 5A), and tumor weights were determined at autopsy (Figure 5B). AZD1152 (25 mg/kg) markedly suppressed the growth and weights of AZD1152-treated tumors. As shown in Figure 5A, the mean volume of MOLM13 tumors in the mice who received AZD1152 (71 ± 20 mm3) was significantly decreased compared with control mice (1261 ± 200 mm3) (P < .01). In addition, the difference of mean tumor weights between these 2 groups (78 ± 16 mg, AZD1152 treated; 583 ± 150 mg, control diluent) was significant (Figure 5B, P < .01). None of the mice showed signs of wasting (Figure 5C) or other toxicity (data not shown). The tumors and organs of the mice were fixed, stained, and viewed by light microscopy. The tumors from control mice showed typical histologic appearance of infiltrating leukemia cells (Figure 5D). Tumors from mice treated with AZD1152 showed necrotic tissue with infiltration of phagocytic cells (Figure 5D). No leukemia cells were detected.
Effect of AZD1152 on the proliferation of MOLM13 cells in a murine xenograft model. MOLM13 cells were injected bilaterally subcutaneously into BALB/c nude mice, forming 2 tumors/mouse. After MOLM13 cells formed palpable tumors, mice were randomized into 2 groups (n=5) and treatment was initiated. AZD1152 (25 mg/kg per mouse) were administered to mice by intraperitoneal injection 4 times a week for 2 weeks. Control mice (n=5) were given control diluent. Tumor volumes were measured every week. Each point represents the mean ± SD of 10 tumors. (B) Tumor weights at autopsy. After 2 weeks of treatment, tumors were removed and weighed. Results represent mean ± SD of tumor weights. Statistical significance was determined by Mann-Whitney U test. Bars indicate SD. (C) Body weight of mice was measured twice a week during treatment. (D) Hematoxylin-eosin staining. Stained sections were examined and photographed with an Olympus BX50 (Olympus, Tokyo, Japan). MOLM13 tumors from mice treated with AZD1152 (right panel) showed extensive tumor necrosis with phagocytes (original magnification × 200). Diluent control displayed sheets of leukemia cells (left panel).
Effect of AZD1152 on the proliferation of MOLM13 cells in a murine xenograft model. MOLM13 cells were injected bilaterally subcutaneously into BALB/c nude mice, forming 2 tumors/mouse. After MOLM13 cells formed palpable tumors, mice were randomized into 2 groups (n=5) and treatment was initiated. AZD1152 (25 mg/kg per mouse) were administered to mice by intraperitoneal injection 4 times a week for 2 weeks. Control mice (n=5) were given control diluent. Tumor volumes were measured every week. Each point represents the mean ± SD of 10 tumors. (B) Tumor weights at autopsy. After 2 weeks of treatment, tumors were removed and weighed. Results represent mean ± SD of tumor weights. Statistical significance was determined by Mann-Whitney U test. Bars indicate SD. (C) Body weight of mice was measured twice a week during treatment. (D) Hematoxylin-eosin staining. Stained sections were examined and photographed with an Olympus BX50 (Olympus, Tokyo, Japan). MOLM13 tumors from mice treated with AZD1152 (right panel) showed extensive tumor necrosis with phagocytes (original magnification × 200). Diluent control displayed sheets of leukemia cells (left panel).
AZD1152 enhanced the ability of vincristine or daunorubicin to inhibit the proliferation of human MOLM13 leukemic xenografts in vivo
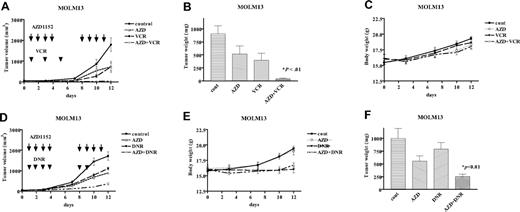
We further evaluated the antigrowth activity of AZD1152 combined with vincristine in vivo (Figure 6). AZD1152 (5 mg/kg) or vincristine (0.2 mg/kg) alone inhibited the proliferation of MOLM13 xenografts by approximately 50% compared with control tumors (Figure 6A). When mice were treated with both compounds, tumor growth was almost completely inhibited (Figure 6A), resulting in decreased weight of MOLM13 tumors at autopsy (Figure 6B). The mice treated with the combination of AZD1152 and vincristine did not show signs of wasting (Figure 6C). Similarly, AZD1152 enhanced the action of daunorubicin in MOLM13 xenograft model (Figure 6D,E), although daunorubicin (1 mg/kg × 6) was toxic to these nude mice (Figure 6F).
Effect of coadministration of AZD1152 and either vincristine or daunorubicin on the proliferation of MOLM13 cells in murine xenograft model. (A,D) MOLM13 cells were injected bilaterally subcutaneously into BALB/c nude mice, forming 2 tumors/mouse. When MOLM13 tumors were palpable, mice were randomized into 4 groups (n=5) and treatment was initiated. AZD1152 (5 mg/kg) was administered to mice by intraperitoneal injection 4 times a week for 2 weeks. VCR (0.2 mg/kg) was given every another day during the first week of therapy. DNR (1 mg/kg) was given to mice by intraperitoneal injection 6 times during 2 weeks of treatment. Tumor volumes were measured twice a week. Each point represents the mean ± SD of 10 tumors. (B,E) Tumor weights at autopsy. After 2 weeks of treatment, tumors were removed and weighed. Results represent mean plus or minus SD of tumor weights. Statistical significance was determined by one-way ANOVA followed by Bonferroni multiple comparison tests. Bars indicate SD. (C,F) Body weight. Body weight of mice was measured twice a week during treatment. AZD indicates AZD1152; VCR, vincristine; DNR, daunorubicin; and cont, diluent control.
Effect of coadministration of AZD1152 and either vincristine or daunorubicin on the proliferation of MOLM13 cells in murine xenograft model. (A,D) MOLM13 cells were injected bilaterally subcutaneously into BALB/c nude mice, forming 2 tumors/mouse. When MOLM13 tumors were palpable, mice were randomized into 4 groups (n=5) and treatment was initiated. AZD1152 (5 mg/kg) was administered to mice by intraperitoneal injection 4 times a week for 2 weeks. VCR (0.2 mg/kg) was given every another day during the first week of therapy. DNR (1 mg/kg) was given to mice by intraperitoneal injection 6 times during 2 weeks of treatment. Tumor volumes were measured twice a week. Each point represents the mean ± SD of 10 tumors. (B,E) Tumor weights at autopsy. After 2 weeks of treatment, tumors were removed and weighed. Results represent mean plus or minus SD of tumor weights. Statistical significance was determined by one-way ANOVA followed by Bonferroni multiple comparison tests. Bars indicate SD. (C,F) Body weight. Body weight of mice was measured twice a week during treatment. AZD indicates AZD1152; VCR, vincristine; DNR, daunorubicin; and cont, diluent control.
Discussion
This study found that AZD1152, a novel and selective Aurora B kinase inhibitor, slowed or prevented the growth of a variety of leukemia subtypes in vitro with IC50s ranging from 3 to 40 nM. IC50s of ZM334739, another Aurora kinase inhibitor, against these same leukemia cells ranged from 3 to 30 μM in our previous studies,11 suggesting that AZD1152 was approximately 100 times more potent than ZM447439. Mode of action of AZD1152 in leukemia cells appeared to be almost identical to that of ZM447439; both compounds induced accumulation of cells with a 4N/8N DNA content, followed by apoptosis. Both were more active against PALL-1 and -2 cells, possessing wild-type p53, compared with other leukemia cell lines. The p53-dependent postmitotic checkpoint may determine cell fate after exposure to Aurora kinase inhibitors.
Of note, AZD1152 enhanced the antiproliferative effect of vincristine, a tubulin depolymerizing agent, in vitro as well as in vivo. The cells treated with Aurora kinase inhibitor entered and exited mitosis without cell division, and then proceeded to a second S phase.12 On the other hand, vincristine inhibits mitosis; it binds to the beta-tubulin subunit of the alpha/beta-tubulin heterodimer, and inhibits polymerization of microtubules, resulting in failure of mitosis.18 The mode of action in mitosis is different between each agent. This could explain the molecular mechanisms by which combination of both compounds produced a synergistic inhibition of growth. The combination of the Aurora kinase inhibitor and tubulin depolymerizing agent, such as docetaxel and paclitaxel, should be explored in future experiments.
AZD1152 effectively inhibited clonogenic growth of leukemia cells from patients with Ph+ ALL and blast crisis of CML (sample nos. 4 and 5, Table 1), although AZD1152 did not inhibit ABL kinase activity in K562 CML cells (Ikezoe et al, unpublished data, January 2007). Both patients had relapsed after initial therapy with imatinib followed by allogenic bone marrow transplantation. In vitro experiments showed that freshly isolated leukemia cells from these patients were resistant to growth inhibition after culture with imatinib (data not shown). AZD1152 may be useful for treatment of individuals with imatinib-resistant CML or ALL. Recent studies showed that the pan–Aurora kinase inhibitor MK-0457 (formerly VX-680) decreased the ABL kinase activity induced by the T351I-BCR/ABL mutation, which mediates clinical resistance to imatinib, nilotinib, and dasatinib.19 In addition, 3 patients with Ph+ ALL or CML possessing T315I BCR/ABL mutation achieved clinical responses after treatment with MK-0457.19 MK-0457 might inhibit the proliferation of CML cells possessing T315I BCR/ABL mutation, via inhibition of Aurora kinases. MK-0457 possesses many “off-target” kinases including FLT3 and c-KIT, and is being tested in clinical trials for the individuals with relapsed or refractory leukemias.20
Other Aurora kinase inhibitors have been developed, which includes MLN8054. MLN8054 is relatively selective for Aurora A (IC50 of 34 nM versus 5700 nM for Aurora A and B kinases, respectively). Preclinical studies with MLN8054 showed the potent antitumor activity in human lung cancer xenograft models.21 MLN8054 is undergoing evaluation in clinical trials for patients with advanced solid tumors.
AZD1152 is currently being studied in phase 1 clinical trials. Preliminary results suggest that the maximum tolerated dose of AZD1152, given as a 2-hour weekly infusion, is 200 mg. Neutropenia was the dose-limiting toxicity. No significant anemia, thrombocytopenia, or neuropathy was observed. A melanoma, nasopharyngeal carcinoma and adenocystic carcinoma patient achieved prolonged stable disease lasting more than 25 weeks.22,23
Taken together, AZD1152 is a promising new agent for treatment of individuals with leukemia. Combined administration of AZD1152 and other chemotherapeutic agents, such as vincristine, should be further investigated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Public Trust of Hraguchi Cancer Research Memorial Foundation, AstraZeneca Research Grant, and the Fund for Academic Research from Kochi University. H.P.K. is supported by National Institutes of Health (NIH) grants as well as by the Inger Fund and the Parker Hughes Trust.
We wish to thank Dr Kirsten Mundt (AstraZeneca Pharmaceuticals, United Kingdom) for providing AZD1152.
Authorship
Contribution: T.I. contributed to the concept and design, interpreted and analyzed the data, and wrote the article; J.Y. and C.N. performed the experiments; T.T., A.T., Y.K., N.K., K.B., K.T., and H.T. provided clinical samples; H.P.K. provided critical revision and intellectual content; and A.Y. provided important intellectual content and gave final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takayuki Ikezoe, Department of Hematology and Respiratory Medicine, Kochi University, Nankoku, Kochi 783-8505, Japan; e-mail: ikezoet@kochi-u.ac.jp.