Abstract
In vivo analyses of thymopoiesis in mice defective in signaling through Kit and γc or Kit and IL-7Rα demonstrate synergy and partial complementation of γc or IL-7–mediated signaling by the Kit signaling pathway. Our molecular analysis in T-lymphoid cells as well as in nonhematopoietic cells shows that Kit and IL-7R signaling pathways directly interact. KL-mediated activation of Kit induced strong tyrosine phosphorylation of γc and IL-7Rα in the absence of IL-7. Activated Kit formed a complex with either IL-7Rα or γc, and tyrosine phosphorylation of both subunits occurred independently of Jak3, suggesting that γc and IL-7Rα are each direct substrates of Kit. Kit activated Jak3 in an IL-7R–dependent manner. Moreover, deficient Stat5 activation of the Kit mutant YY567/569FF lacking intrinsic Src activation capacity was partially reconstituted in the presence of IL-7R and Jak3. Based on the molecular data, we propose a model of Kit-mediated functional activation of γc-containing receptors such as IL-7R, similar to the interaction between Kit and Epo-R. Such indirect activation of the Jak-Stat pathway induced by the interaction between an RTK and type I cytokine receptor could be the underlying mechanism for a context-specific signaling repertoire of a pleiotropic RTK-like Kit.
Introduction
The RTK Kit is expressed in hematopoietic progenitors, while its cognate ligand Kit ligand (KL, aka stem-cell factor) is expressed by stromal cells. Binding of KL to the extracellular domain of Kit and the subsequent activation of the Kit tyrosine kinase initiates several signaling pathways, including the PI-3K/Akt,1–4 Src family kinase (SFK),5–8 Jak/Stat,9–11 and the Ras-Raf-MAP kinase cascades.2,12 Loss of KL (steel locus [lsqb]Sl];) or Kit tyrosine kinase activity (dominant white spotting locus [W];) results in abnormalities of the hematopoietic, nervous, and reproductive systems.13–15 The hematopoietic phenotype caused by loss of Kit signaling includes profound anemia and an approximately 50% reduction in thymocyte numbers.16
In addition to the activation of “intrinsic” Kit signaling pathways, Kit has been shown to interact with type I cytokine receptors (eg, Epo-R17,18 and IL-3R19 ). The Kit/Epo-R crosstalk involves direct interaction between Kit and Epo-R leading to tyrosine phosphorylation of Epo-R by Kit in the absence of Epo. This interaction was postulated to be necessary for Kit to transduce survival and proliferation signals in certain cells. Although phosphorylation of tyrosine residues of the Epo-R cytoplasmic tail by the RTK Kit was demonstrated, the downstream mediators responsible for this synergism have not been identified.
Similar to the proposed synergy between Kit and Epo-R in erythropoiesis, analysis of T-cell development in mice defective in both Kit- and γc-dependent pathways reveal synergistic and partially complementary functions for these 2 distinct pathways.20,21 Unlike the homodimeric Kit and Epo-R, γc heterodimerizes with ligand-specific type I cytokine receptor subunits to form receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21.22,23 Both the RTK Kit and γc-containing receptors are involved in thymocyte development since loss of signaling events mediated by either Kit or γc results in reduction of thymic cellularity in mice. Among the cytokines binding to γc-containing receptors, IL-7 is the most important one for thymic development since loss of IL-7 signaling causes a severe reduction in thymic cellularity comparable with that caused by the loss of γc.24 Therefore, the complete γc-containing receptor responsible for the synergy in Kit and γc signaling in thymocyte development is likely to be the IL-7R, a heterodimer of γc and IL-7Rα. Downstream signaling events induced by IL-7–specific binding of IL-7R include activation of Jak3 and Stat5 as well as induction of PI3-K activity.25 In humans, loss of γc or IL-7Rα signaling causes severe combined immunodeficiency (SCID) marked by profound loss of thymopoiesis.26
Interestingly, comparison of murine knock-out models has revealed that the defect in T-cell generation caused by the IL-7Rα−/− genotype is more severe than that observed in IL-7−/− mice.24,27 Compared with IL-7−/− mice, IL-7Rα−/− mice exhibit decreased thymic cellularity and display a somewhat earlier block of differentiation of prothymocytes. This phenotypic difference suggests that another receptor pathway might partially complement the defect in IL-7 signaling by activation of IL-7R in absence of IL-7. Based on the previous results demonstrating that Kit can directly activate the Epo-R in the absence of Epo, Kit activation of IL-7R is a likely candidate for such complementation.
The parallels between synergistic actions of Kit and Epo-R in erythropoiesis and Kit- and γc-mediated pathways, most likely the IL-7R pathway, in thymic development prompted us to determine whether Kit and IL-7R directly interact, and to define changes in the repertoire of downstream signaling of Kit in the presence of IL-7R. Interactions between Kit and IL-7R would be expected to occur at immature stages of thymic differentiation, where both receptors are coexpressed (eg, CD4− CD8− CD3− [“triple-negative,” TN] thymocytes). The rarity of such cells precludes biochemical analyses of Kit/IL-7R interactions in primary thymocytes. To overcome those difficulties posed by the rarity of Kit-expressing thymocytes, reconstituted Jurkat T cells and nonhematopoietic 293T cells were used to characterize the molecular interactions between Kit and IL-7R.
Materials and methods
Reagents and antibodies
Recombinant KL was purchased from R&D Systems (Minneapolis, MN) and Biosource International (Camarillo, CA). Human IL-7 was obtained from R&D Systems. Polyclonal antibodies against Kit, IL-7R, γc, Jak3, Stat5, and ERK1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-specific antibodies against ERK1/2 (Tyr 204) and Stat5 (Tyr 694) were obtained from Santa Cruz Biotechnology and Cell Signaling Technology (Beverly, MA), respectively. The antiphosphotyrosine antibody (clone 4G10) was purchased from Upstate (Lake Placid, NY). Primers for polymerase chain reaction (PCR)–based mutagenesis were obtained from Invitrogen (Carlsbad, CA).
Expression constructs and mutagenesis
c-kit WT and mutant c-Kit constructs were described previously.28,29 The IL-7R cDNA was obtained by reverse-transcription (RT)–PCR using XhoI flanked primers and RNA from human thymocytes and subcloned into pCDNA 3.1 (Invitrogen). The γc cDNA, obtained by RT-PCR was described before,30 isolated by restriction with BamHI and SalI and subcloned into pCDNA 3.1 using BamHI and XhoI cloning sites. Lentiviral expression vectors for Kit and IL-7Rα were established by subcloning the cDNAs using appropriate restriction site into the pCCL-cppt-MNDU3-X-IRES-eGFP backbone (generously provided by Dr Donald Kohn, Childrens Hospital Los Angeles).
Tyrosine to phenylalanine mutations (Y/F) of the cytoplasmic tails of human IL-7Rα (tyrosines at positions 401, 449, and 456) and γc (positions 303, 325, 357, and 363) were introduced by site-directed mutagenesis (QuikChange; Stratagene, La Jolla, CA) using appropriate primers and were confirmed by restriction analysis and sequencing.
The murine Stat5b (pCDNA mStat5b) and the human Jak3 expression constructs (pME18S Jak3) were generously provided by Warren Leonard and John O'Shea (NIH, Bethesda, MD), respectively.
Cells, cell culture, transduction, and transfection methods
Jurkat T cells (clone E6–1) were obtained from ATCC (Manassas, VA) and cultured in RPMI (Irvine Scientific, Santa Ana, CA) supplemented with 10% FCS (Omega Scientific, Tarzana, CA). Concentrated lentiviral supernatants from 293T cells were used to infect Jurkat T cells for stable expression of Kit and IL-7Rα to generate Jurkat-K7 cells. High-expressing cells were established by cell sorting based on expression of eGFP and Kit/IL-7Rα receptors. Prior to stimulation with either IL-7 or KL, Jurkat-K7 cells were serum-starved for 6 hours in plain RPMI. 293T cells were cultured in DMEM with 10% FCS. To obtain transient expression of constructs, transfection was performed using DOTAP Liposomal Transfection Reagent (Roche, Indianapolis, IN) according to the manufacturer's recommendations. Cells were serum-starved in plain DMEM for 6 hours before stimulation. For Kit and IL-7R stimulation, cells were incubated with KL or IL-7 at concentrations of 500 ng/mL for the times as indicated at 37°C.
Immunoprecipitations and immunoblotting
Immunoprecipitations (IPs) and immunoblotting (IB) were performed as described before.28,29 In brief, cells (approximately 2-4 × 107) were harvested in cold PBS containing 1 mM sodium vanadate (Sigma, St Louis, MO), pelleted, and lysed in lysis buffer containing 10 mM Tris-HCl (pH 7.4), 5 mM EDTA, 130 mM NaCl, and 1% Triton (Sigma). Proteinase inhibitor cocktail tablets (Complete; Roche) were added according to the manufacturer's recommendations. After clarification by centrifugation and preclearing with protein A-Sepharose (Amersham/Pharmacia, Piscataway, NJ), antibody-protein complexes were collected with 30 μL protein A-Sepharose. In an immunoprecipitation experiment, 2 μg of antibody was used per IP. Lysates and bound fractions of precipitates were subjected to sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) (Hoefer; Amersham/Pharmacia) and Western blotting (WB) was performed on PVDF membranes (Immobilon-P; Millipore, Bedford, MA). Blots were incubated with primary antibodies as indicated. After washing, blots were incubated with appropriate HRP-coupled secondary antibodies (antimouse) or HRP-coupled to protein A (Amersham/Pharmacia) and developed using SuperSignal chemoluminescent substrates (Pierce Chemical, Rockford, IL). Individual experiments were performed 2 to 4 times and a representative experiment is shown.
Densitometric analysis of Western blot signals was performed using image analysis software (Kodak 1D; Eastman Kodak, New Haven, CT). Signal intensities of respective bands were measured using digital images of the blots, and relative phosphorylation was calculated by forming the ratio of the phosphorylation and the protein signals. Fold increase of relative phosphorylation was calculated by the ratio of the relative phosphorylation after stimulation and the respective value of unstimulated cells. We compared means using the paired t test to account for the correlation of measures within a sample across time or across conditions. In Figure 1C,D, log2 values of fold changes (from time point 0 minutes) were calculated and used for the t test analysis. In Figures 4C, 5C, and 6C, fold changes (from stimulated vs unstimulated cells) were calculated and used for the t test analysis (without converting them into log values). For such comparisons and intervals, the degrees of freedom (dfs) for the t statistic are quite small, 2 dfs for between time (Figure 1C,D) and 3 dfs for between condition (Figures 4C, 5C, and 6C). Thus, the corresponding critical values of the t statistic are large, and the standard error of the mean (SEM) is not by itself an appropriate measure of uncertainty. However, the calculation of P values using the appropriate dfs for t corrects for this problem. The t test also relies on normality of the measures. Graphic examination of residuals revealed no striking departures from normality.
Results
Establishment of Kit and IL-7R signaling in Jurkat T cells
Wild-type Jurkat T cells (Jurkat WT) endogenously express γc, lack detectable expression levels of Kit, and display only weak expression of IL-7Rα. To investigate the observed in vivo synergy of Kit and IL-7R pathways in a lymphohematopoietic background, we established Jurkat T cells stably expressing Kit and the IL-7Rα subunit (Jurkat-K7 cells) by lentivirally mediated gene transfer. Expression was verified both by flow cytometry and Western blotting (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article; and data not shown). Functionality of both Kit and IL-7R receptors was tested by stimulation of Jurkat-K7 cells with either KL or IL-7 for 5 or 10 minutes, respectively. KL stimulation of Jurkat-K7 cells led to a significant increase of tyrosine-phosphorylated proteins, whereas stimulation of Jurkat WT with KL had no effect on the overall tyrosine phosphorylation of proteins (Figure S1B). IL-7 stimulation of Jurkat WT or Jurkat-K7 cells resulted in detectable activation of Stat5 only in Jurkat-K7 cells (Figure S1C). Thus, both the Kit and IL-7R pathways are functional in Jurkat-K7 cells.
Tyrosine phosphorylation of γc and IL-7Rα by Kit
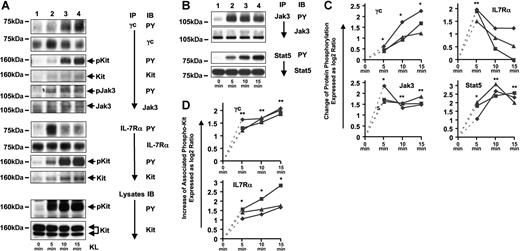
One of the first events in IL-7–mediated signaling is tyrosine phosphorylation of γc and IL-7Rα, the 2 subunits of IL-7R. To determine whether KL-mediated activation of Kit in Jurkat-K7 cells can induce an activated IL-7R complex in the absence of IL-7, we investigated tyrosine phosphorylation of γc and IL-7Rα in the course of Kit stimulation (0, 5, 10, and 15 minutes of KL). Kit stimulation resulted in an increase of tyrosine phosphorylation of both γc and IL-7Rα with somewhat different kinetics (Figure 1A,C): Phosphorylation of γc induced by Kit showed a continuous increase over 15 minutes, whereas phosphorylation of IL-7Rα showed a peak at 5 minutes of KL stimulation followed by a significant reduction of phosphorylation at the 10-minute time point. Analysis of the bound fraction of the anti-γc and anti–IL-7Rα immunoprecipitates by Western blotting revealed steadily increasing amounts of tyrosine-phosphorylated coprecipitating Kit protein over 15 minutes of KL stimulation (Figure 1A,D). Thus, both subunits of the IL-7R, γc and IL-7Rα, associated with activated Kit and became tyrosine phosphorylated after Kit stimulation. Another γc-associated protein that was tyrosine phosphorylated in response to KL was identified as Jak3 (Figure 1A).
KL stimulation of Jurkat-K7 cells results in the activation of IL-7R, Jak3, and Stat5. (A) γc and IL-7Rα are tyrosine phosphorylated by and associated with Kit after KL stimulation and γc-associated Jak3 is tyrosine phosphorylated in response to KL stimulation. Jurkat-K7 cells were stimulated with KL for the times indicated and γc and IL-7Rα were immunoprecipitated. Bound fractions of the γc IPs were analyzed for tyrosine phosphorylation and protein amounts of γc, γc-associated Kit, and γc-associated Jak3 (top panels). Bound fractions of the IL-7Rα IPs were analyzed for tyrosine phosphorylation of IL-7Rα, and IL-7Rα-associated Kit (middle panels). Lysates were analyzed for tyrosine-phosphorylated Kit and the presence of equal amounts of Kit protein at each time point of KL stimulation (bottom panels). (B) KL-induced tyrosine phosphorylation of Jak3 and Stat5. Immunoprecipitations of Jak3 and Stat5 were analyzed for tyrosine phosphorylation and protein amounts of Jak3 (top 2 panels) and Stat5 (bottom 2 panels). (C) Quantification of the relative change in KL-induced protein phosphorylation (γc, IL-7Rα, Jak3, and Stat5) between baseline (0 minutes) and 3 time points of KL stimulation (5, 10, and 15 minutes). Western blot signals on films from 3 independent experiments (■, ♦, ▴) were densitometrically evaluated as described in “Materials and methods, Immunoprecipitations and immunoblotting,” and fold changes were expressed as the log2 ratio. A log2 ratio of 1 is equal to a fold change of 2. The P values for the t tests (5, 10, 15 minutes versus 0 minute, respectively) are indicated by asterisks (*): *.01 ≤ P < .05; **.001 ≤ P < .01; ***P < .001. The connection from time point 0 (fold increase = 1, log2 1 = 0) to time point 5′ is indicated by a dashed line only for orientation as the 0′ value was used for normalization. Thus, mathematically it is not part of the graph anymore. (D) Quantification of the relative change in the amount of phospho-Kit associated with γc and IL-7Rα at 5, 10, and 15 minutes of KL stimulation. Data are displayed and statistically analyzed as in panel C.
KL stimulation of Jurkat-K7 cells results in the activation of IL-7R, Jak3, and Stat5. (A) γc and IL-7Rα are tyrosine phosphorylated by and associated with Kit after KL stimulation and γc-associated Jak3 is tyrosine phosphorylated in response to KL stimulation. Jurkat-K7 cells were stimulated with KL for the times indicated and γc and IL-7Rα were immunoprecipitated. Bound fractions of the γc IPs were analyzed for tyrosine phosphorylation and protein amounts of γc, γc-associated Kit, and γc-associated Jak3 (top panels). Bound fractions of the IL-7Rα IPs were analyzed for tyrosine phosphorylation of IL-7Rα, and IL-7Rα-associated Kit (middle panels). Lysates were analyzed for tyrosine-phosphorylated Kit and the presence of equal amounts of Kit protein at each time point of KL stimulation (bottom panels). (B) KL-induced tyrosine phosphorylation of Jak3 and Stat5. Immunoprecipitations of Jak3 and Stat5 were analyzed for tyrosine phosphorylation and protein amounts of Jak3 (top 2 panels) and Stat5 (bottom 2 panels). (C) Quantification of the relative change in KL-induced protein phosphorylation (γc, IL-7Rα, Jak3, and Stat5) between baseline (0 minutes) and 3 time points of KL stimulation (5, 10, and 15 minutes). Western blot signals on films from 3 independent experiments (■, ♦, ▴) were densitometrically evaluated as described in “Materials and methods, Immunoprecipitations and immunoblotting,” and fold changes were expressed as the log2 ratio. A log2 ratio of 1 is equal to a fold change of 2. The P values for the t tests (5, 10, 15 minutes versus 0 minute, respectively) are indicated by asterisks (*): *.01 ≤ P < .05; **.001 ≤ P < .01; ***P < .001. The connection from time point 0 (fold increase = 1, log2 1 = 0) to time point 5′ is indicated by a dashed line only for orientation as the 0′ value was used for normalization. Thus, mathematically it is not part of the graph anymore. (D) Quantification of the relative change in the amount of phospho-Kit associated with γc and IL-7Rα at 5, 10, and 15 minutes of KL stimulation. Data are displayed and statistically analyzed as in panel C.
Activation of Jak3 and Stat5 by Kit
Kit has been implicated in activation of the Jak/Stat signaling, although with contradictory results. Jak2 and Stat1 have been proposed as Jak-Stat intermediates directly activated by Kit.10,31 In contrast, IL-7–mediated activation of IL-7R results in activation of Jak3 and Jak3-dependent activation of Stat5.32 IL-7–mediated Stat5 activation requires association between IL-7Rα and Stat5 via the SH2 domain of Stat5. Unlike other Jak kinases that associate with many different receptors, Jak3 is thought to exclusively and constitutively associate with γc. This hypothesis is further consolidated by the observation that loss of γc or Jak3 results in nearly identical immunodeficient phenotypes.33,34 The observed tyrosine phosphorylation of γc-associated Jak3 (Figure 1A) suggested that Kit activates the Jak/Stat pathway in a γc-dependent manner. We directly analyzed whether Kit activation results in the activation of Jak3 and Stat5. Immunoprecipitation of Jak3 and Stat5 in a time-course experiment revealed that both Jak3 and Stat5 became increasingly tyrosine phosphorylated following KL stimulation of Jurkat-K7 cells (Figure 1B,C). Therefore, Kit signaling in the presence of IL-7R includes the activation of the IL-7R downstream targets Jak3 and Stat5. Since the only known upstream regulator of Jak3 is γc, we hypothesized that activation of Jak3 by Kit is indirect and γc dependent. In addition, activation of Stat5 might at least in part be indirect and require the presence of a type I cytokine receptor such as IL-7R.
Tyrosine phosphorylation of the IL-7 receptor complex by Kit in 293T cells
The experiments in Jurkat-K7 cells infer that Kit indirectly activates Jak3 and Stat5. However, endogenous expression of γc and Jak3 in Jurkat K7 cells precludes experiments in these cells to unveil the exact mechanism of Kit-mediated activation of Jak3 and Stat5. Therefore, we used a model of Kit/IL-7R interaction based on the nonhematopoietic epithelial kidney cell line 293T. Like COS1 or COS7 cells, 293T cells have previously been used to delineate signaling pathways of the IL-2 receptor, another γc-containing receptor.35–38 Separate expression of γc and IL-7Rα subunits as well as Jak3 and Stat5 enabled us to test the role of each of these components in Kit-mediated activation of IL-7R.
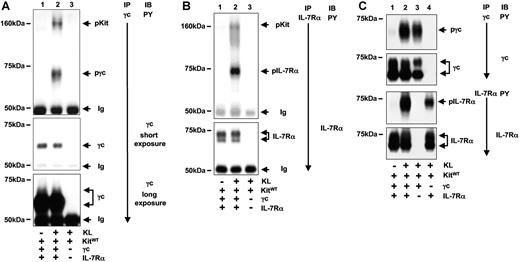
In 293T cells, we confirmed the result from Jurkat-K7 cells that activation of Kit resulted in tyrosine phosphorylation of IL-7R. After 5 minutes of KL stimulation, γc and IL-7Rα became strongly tyrosine phosphorylated (Figure 2A,B). Similar to the result in Jurkat K7 cells, γc and IL-7Rα were associated with activated Kit, suggesting that the tyrosine phosphorylation occurred as a result of direct interaction between Kit and γc and IL-7Rα. These results demonstrate that the interaction between the RTK Kit and a type I cytokine receptor is not a phenomenon specific only to hematopoietic cells and that reconstituted 293T cells represent a valid model to analyze the Kit/IL-7R interaction.
KL-induced activation of IL-7R in reconstituted 293T cells. (A) Tyrosine phosphorylation of γc and association of activated Kit with γc in response to KL. 293T cells were transiently transfected with constructs as indicated and stimulated with KL for 5 minutes. Immunoprecipitations of γc were analyzed with an anti-PY antibody detecting tyrosine-phosphorylated γc and γc-associated tyrosine-phosphorylated Kit (arrows, top panel, lane 2). The specificity of the protein association was verified by the control immunoprecipitation from KL-stimulated cells expressing Kit but lacking γc expression (lane 3). The blot was analyzed for the presence of γc protein (middle panel: short exposure; bottom panel: long exposure). (B) Tyrosine phosphorylation of IL-7Rα and association of IL-7R with activated Kit in response to KL. Analyses of IL-7Rα immunoprecipitates were performed as in panel A. (C) Independent tyrosine phosphorylation of γc and IL-7Rα by Kit. Kit was coexpressed with γc and IL-7Rα (lanes 1,2) or with γc or IL-7Rα alone (lanes 3 and 4, respectively). Immunoprecipitations against γc (top 2 panels) and IL-7Rα were performed and analyzed for tyrosine phosphorylation and presence of proteins as indicated.
KL-induced activation of IL-7R in reconstituted 293T cells. (A) Tyrosine phosphorylation of γc and association of activated Kit with γc in response to KL. 293T cells were transiently transfected with constructs as indicated and stimulated with KL for 5 minutes. Immunoprecipitations of γc were analyzed with an anti-PY antibody detecting tyrosine-phosphorylated γc and γc-associated tyrosine-phosphorylated Kit (arrows, top panel, lane 2). The specificity of the protein association was verified by the control immunoprecipitation from KL-stimulated cells expressing Kit but lacking γc expression (lane 3). The blot was analyzed for the presence of γc protein (middle panel: short exposure; bottom panel: long exposure). (B) Tyrosine phosphorylation of IL-7Rα and association of IL-7R with activated Kit in response to KL. Analyses of IL-7Rα immunoprecipitates were performed as in panel A. (C) Independent tyrosine phosphorylation of γc and IL-7Rα by Kit. Kit was coexpressed with γc and IL-7Rα (lanes 1,2) or with γc or IL-7Rα alone (lanes 3 and 4, respectively). Immunoprecipitations against γc (top 2 panels) and IL-7Rα were performed and analyzed for tyrosine phosphorylation and presence of proteins as indicated.
Independent tyrosine phosphorylation of γc and IL-7Rα during activation of Kit
Functional IL-7 signaling requires the presence of both subunits at the cell membrane and the formation of heterodimers between γc and IL-7Rα. We were interested if both of the IL-7R subunits are required for an interaction between Kit and IL-7R to occur. Following KL stimulation, tyrosine phosphorylation of γc and IL-7Rα occurred whether they were expressed as single subunits or coexpressed to form the complete IL-7R (Figure 2C). Although the tyrosine phosphorylation signal of either subunit was somewhat stronger when they were coexpressed, our results show that activation of Kit induces independent tyrosine phosphorylation of γc and IL-7Rα.
Kit-induced tyrosine phosphorylation of IL-7R is Jak3 independent
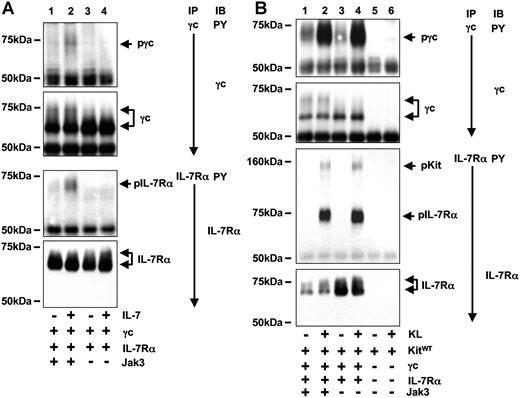
Heterodimerization of γc and IL-7Rα by IL-7 results in the activation of Jak3 and Jak1 leading to phosphorylation of tyrosine residues within the cytoplasmic tail of both subunits. Unlike Jurkat cells, 293T cells do not endogenously express Jak3. Therefore the 293T cell model offered the possibility to compare the role of Jak3 expression in IL-7– and Kit-dependent activation of IL-7R. As expected, IL-7–mediated tyrosine phosphorylation of γc and IL-7Rα was strictly dependent on expression of Jak3 in 293T cells (Figure 3A). However, KL-induced tyrosine phosphorylation of IL-7R was equally strong in the presence or absence of Jak3 (Figure 3B). Thus, unlike IL-7–mediated activation of IL-7R, which requires Jak3, Kit-induced activation of IL-7R was independent of Jak3.
In contrast to IL-7–mediated activation of IL-7R, Kit-mediated tyrosine phosphorylation of IL-7R is independent of Jak3. (A) IL-7–induced tyrosine phosphorylation of IL-7R is Jak3 dependent. 293T cells transiently expressing IL-7R and Jak3 (lanes 1,2) or IL-7R alone (lanes 3,4) were stimulated with IL-7 for 10 minutes and tyrosine phosphorylation and protein of γc (top 2 panels) and IL-7Rα (bottom 2 panels) were examined. Tyrosine phosphorylation of γc and IL-7Rα was detected only in IL-7–stimulated cells that express Jak3. (B) KL-induced tyrosine phosphorylation of IL-7R in absence of Jak3. 293T coexpressing Kit and IL-7R (lanes 1-4) in presence (lanes 1,2) or absence (lanes 3,4) of Jak3. Immunoprecipitations against γc (top 2 panels) and IL-7Rα were performed and analyzed for tyrosine phosphorylation and presence of proteins as shown. Specificity of IL-7Rα–associated activated Kit (arrow) was verified by immunoprecipitations from cells expressing only Kit (lanes 5,6).
In contrast to IL-7–mediated activation of IL-7R, Kit-mediated tyrosine phosphorylation of IL-7R is independent of Jak3. (A) IL-7–induced tyrosine phosphorylation of IL-7R is Jak3 dependent. 293T cells transiently expressing IL-7R and Jak3 (lanes 1,2) or IL-7R alone (lanes 3,4) were stimulated with IL-7 for 10 minutes and tyrosine phosphorylation and protein of γc (top 2 panels) and IL-7Rα (bottom 2 panels) were examined. Tyrosine phosphorylation of γc and IL-7Rα was detected only in IL-7–stimulated cells that express Jak3. (B) KL-induced tyrosine phosphorylation of IL-7R in absence of Jak3. 293T coexpressing Kit and IL-7R (lanes 1-4) in presence (lanes 1,2) or absence (lanes 3,4) of Jak3. Immunoprecipitations against γc (top 2 panels) and IL-7Rα were performed and analyzed for tyrosine phosphorylation and presence of proteins as shown. Specificity of IL-7Rα–associated activated Kit (arrow) was verified by immunoprecipitations from cells expressing only Kit (lanes 5,6).
Indirect activation of Jak3 by Kit
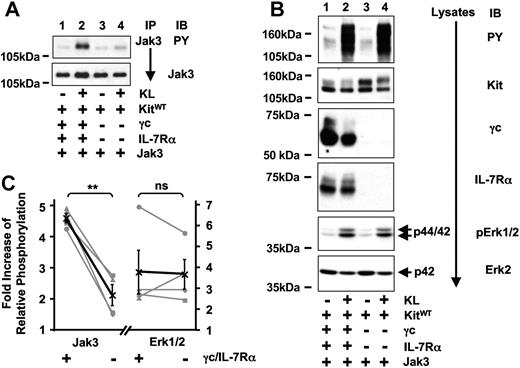
In Jurkat-K7 cells, KL stimulation resulted in the activation of Jak3, and Jak3 was detected as a γc-associated protein. The question of whether Kit activates Jak3 directly or in a IL-7R–dependent fashion was addressed in 293T cells by comparing tyrosine phosphorylation of Jak3 subsequent to activation of Kit in the presence or absence of IL-7R (Figure 4A,C). Compared with only marginal activation of Jak3 by Kit alone, there was strong Kit-mediated activation of Jak3 in cells coexpressing Kit and IL-7R. As an internal control, total tyrosine phosphorylation and activation of Erk1/2 after Kit-stimulation were analyzed (Figure 4B,C). Both were induced similarly as a result of Kit activation and were not significantly influenced by the presence of IL-7R. We therefore conclude that Kit-mediated activation of Jak3 is largely mediated indirectly by IL-7R comprised of γc and IL-7Rα. The marginal increase of Kit-induced Jak3 phosphorylation seen in the absence of IL-7R could be due to low endogenous expression of γc or another type I cytokine receptor activated by Kit.
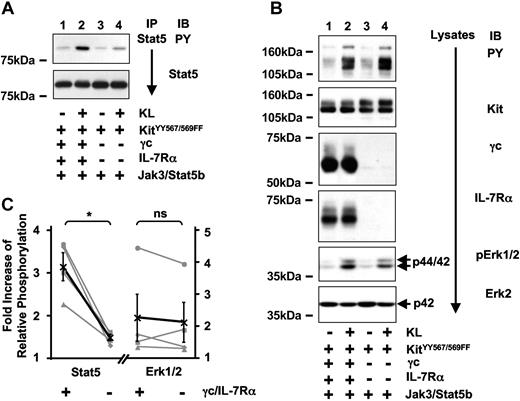
Kit-induced activation of Jak3 is dependent on IL-7R.(A) Kit-mediated activation of Jak3 is significantly stronger in the presence of IL-7R. 293T cells transiently coexpressing Kit and Jak3 in the presence (lanes 1,2) or absence (lanes 3,4) of IL-7R were stimulated with KL for 5 minutes, and tyrosine phosphorylation (top panel) and protein amounts of immunoprecipitated Jak3 (bottom panel) were compared between unstimulated (lanes 1,3) and stimulated (lanes 2,4) cells. (B) Kit-induced total tyrosine phosphorylation and activation of Erk1/2 is independent of IL-7R. Lysates were separated on SDS-PAGE and total tyrosine phosphorylation (first panel) as well as the amount of phosphorylated ERK1/2 were analyzed (fifth panel). Lysates were probed with antibodies as indicated to demonstrate Kit, γc, IL-7Rα (second to fourth panels), and endogenous ERK2 expression (bottom panel). (C) Quantification of 4 independent experiments analyzing KL-induced phosphorylation of Jak3 (right) and ERK1/2 (left graph) either in the presence (+) or absence (−) of γc and IL-7Rα. Results are expressed as fold increase of relative phosphorylation (see “Materials and methods” for details). Single experiments are shown in gray symbols (■, ♦, ▴, ●), the average is shown in black (X) and contains the error bars (standard error). P values were determined by paired t test analysis.
Kit-induced activation of Jak3 is dependent on IL-7R.(A) Kit-mediated activation of Jak3 is significantly stronger in the presence of IL-7R. 293T cells transiently coexpressing Kit and Jak3 in the presence (lanes 1,2) or absence (lanes 3,4) of IL-7R were stimulated with KL for 5 minutes, and tyrosine phosphorylation (top panel) and protein amounts of immunoprecipitated Jak3 (bottom panel) were compared between unstimulated (lanes 1,3) and stimulated (lanes 2,4) cells. (B) Kit-induced total tyrosine phosphorylation and activation of Erk1/2 is independent of IL-7R. Lysates were separated on SDS-PAGE and total tyrosine phosphorylation (first panel) as well as the amount of phosphorylated ERK1/2 were analyzed (fifth panel). Lysates were probed with antibodies as indicated to demonstrate Kit, γc, IL-7Rα (second to fourth panels), and endogenous ERK2 expression (bottom panel). (C) Quantification of 4 independent experiments analyzing KL-induced phosphorylation of Jak3 (right) and ERK1/2 (left graph) either in the presence (+) or absence (−) of γc and IL-7Rα. Results are expressed as fold increase of relative phosphorylation (see “Materials and methods” for details). Single experiments are shown in gray symbols (■, ♦, ▴, ●), the average is shown in black (X) and contains the error bars (standard error). P values were determined by paired t test analysis.
Partial reconstitution of Stat5 activation of the Kit mutant YY567/569FF by IL-7R
Using the reconstituted 293T cells, we confirmed the result from the Jurkat-K7 cells that IL-7 signaling induced activation of Stat5 and that IL-7–induced activation of Stat5 is dependent on Jak3 (Figure S2A). Kit activation in absence of IL-7R or Jak3 also led to Stat5 phosphorylation, leading to the conclusion that in 293T cells another signaling pathway exists that mediates activation of Stat5 by Kit (Figure S2B). By testing different tyrosine to phenylalanine mutants of Kit in 293T cells, we found that the Kit mutants KitY567F and KitY569F showed significantly reduced KL-induced activation of Stat5, and that the double-mutant KitYY567/569FF was unable to activate Stat5 (Figure S3). This mutant has previously shown to be deficient in the activation of SFKs.7 To reveal a possible synergy in the activation of Stat5 by Kit and IL-7R, we tested if the presence of IL-7R would have an impact on Stat5 activation by this Kit mutant. When Stat5 phosphorylation subsequent to KL-induced activation of KitYY567/569FF was analyzed, it was significantly higher when KitYY567/569FF was activated in the presence of IL-7R (Figure 5A,C). Presence of the IL-7R did not influence total tyrosine phosphorylation or Erk1/2 activation by KitYY567/569FF significantly (Figure 5B-C), suggesting that KitYY567/569FF-induced activation of Stat5, but not Erk1/2, occurs indirectly as a result of IL-7R transactivation. Although KitYY567/569FF was initially characterized as completely deficient in the activation of Stat5 when Jak3 was absent (Figure S3), there was marginal Stat5 phosphorylation induced by KitYY567/569FF even in the absence of IL-7R when Jak3 was present (Figure 5B, Jak3). Similar to the observation of marginal Kit-induced activation of Jak3 in the absence of IL-7R, we speculate that either low-level endogenous expression of γc or of another type I cytokine receptor could account for this effect.
Deficient Stat5 activation of KitYY567/569FF is partially reconstituted by Kit-induced transactivation of IL-7R. (A) Stat5 activation by KitYY567/569FF is partially reconstituted in the presence of IL-7R. Stat5 was immunoprecipitated from unstimulated (lanes 1,3) or KL-stimulated (lanes 2,4) 293T cells coexpressing KitYY567/569FF and Jak3 in the presence (lanes 1,2) and absence (lanes 3,4) of IL-7R and analyzed for tyrosine phosphorylation (top panel) and Stat5 protein (bottom panel). (B) Total tyrosine phosphorylation and activation of Erk1/2 induced by KitYY567/569FF are independent of IL-7R. Lysates were separated on SDS/PAGE and were analyzed for levels of total tyrosine phosphorylation, Kit, γc, IL-7Rα protein, phosphorylation of Erk1/2, and Erk2 protein. (C) Quantification of 4 independent experiments analyzing KitYY567/569FF-induced phosphorylation of Stat5 (left) and Erk1/2 (right graph) in presence (+) or absence (−) of γc and IL-7Rα. Data are displayed and statistically analyzed as in Figure 4C.
Deficient Stat5 activation of KitYY567/569FF is partially reconstituted by Kit-induced transactivation of IL-7R. (A) Stat5 activation by KitYY567/569FF is partially reconstituted in the presence of IL-7R. Stat5 was immunoprecipitated from unstimulated (lanes 1,3) or KL-stimulated (lanes 2,4) 293T cells coexpressing KitYY567/569FF and Jak3 in the presence (lanes 1,2) and absence (lanes 3,4) of IL-7R and analyzed for tyrosine phosphorylation (top panel) and Stat5 protein (bottom panel). (B) Total tyrosine phosphorylation and activation of Erk1/2 induced by KitYY567/569FF are independent of IL-7R. Lysates were separated on SDS/PAGE and were analyzed for levels of total tyrosine phosphorylation, Kit, γc, IL-7Rα protein, phosphorylation of Erk1/2, and Erk2 protein. (C) Quantification of 4 independent experiments analyzing KitYY567/569FF-induced phosphorylation of Stat5 (left) and Erk1/2 (right graph) in presence (+) or absence (−) of γc and IL-7Rα. Data are displayed and statistically analyzed as in Figure 4C.
Requirement for cytoplasmic tyrosines of IL-7R for the reconstitution of Stat5 activation by Kit
IL-7–mediated activation of Stat5 requires a tyrosine-phosphorylated docking site within the cytoplasmic tail of IL-7Rα for the SH2 domain of Stat5.39 Using mutants of γc and IL-7Rα where all cytoplasmic tyrosine residues were replaced by phenylalanine (IL-7RαY/F and γcY/F), we confirmed that Stat5 activation via IL-7 is abrogated in the absence of cytoplasmic tyrosine residues of IL-7R (Figure 6A,C). We then assessed whether tyrosine residues within IL-7R are also required for the indirect Stat5 activation through a Kit/IL-7R interaction. KL-inducible Stat5 phosphorylation was significantly higher when KitYY567/569FF was activated in the presence of the wild-type IL-7R complex containing cytoplasmic tyrosines (Figure 6B,C). Thus, like IL-7–mediated activation of Stat5, Stat5 activation through KitYY567/569FF in the presence of IL-7R is dependent on tyrosine residues within IL-7R.
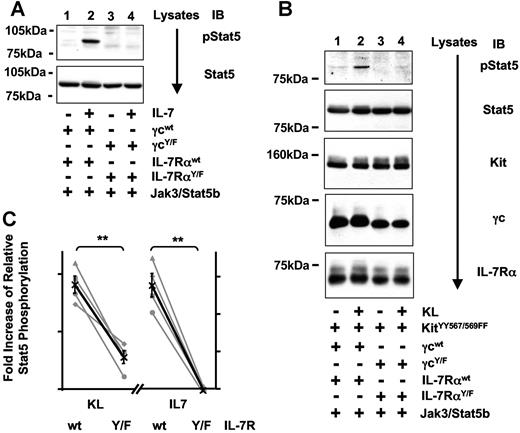
Partial reconstitution of KitYY567/569FF through transactivation of IL-7R requires tyrosine residues of IL-7R. (A) IL-7–mediated activation of Stat5 is dependent on tyrosine residues within IL-7R. Lysates of 293T cells coexpressing Jak3 and IL-7RWT (lanes 1,2) or a mutant IL-7R lacking all cytoplasmic tyrosine residues (γcY/F and IL-7RαY/F, lanes 3,4) were analyzed for phosphorylation of Stat5 on Tyr694 (top panel) and Stat5 protein (bottom panel). (B) Requirement for cytoplasmic tyrosines of IL-7R for KitYY567/569FF-induced Stat5 activation. Lysates from 293T cells coexpressing KitYY567/569FF and IL-7RWT (lanes 1,2) or the mutant IL-7R consisting of γcY/F and IL-7RαY/F (lanes 3,4) were analyzed for phosphorylation of Stat5 on Tyr694 Stat5, Kit, γc, and IL-7Rα protein levels. (C) Quantification of 4 independent experiments analyzing KitYY567/569FF-induced (left) or IL-7R-induced (right graph) phosphorylation of Stat5 in presence (wt) or absence (Y/F) of cytoplasmic tyrosine residues of IL-7R. Stat5 phosphorylation was determined by analyzing either lysates or immunoprecipitates of Stat5 for phosphorylation of Stat5 and protein levels of Stat5. Data are displayed and statistically analyzed as in Figure 4C.
Partial reconstitution of KitYY567/569FF through transactivation of IL-7R requires tyrosine residues of IL-7R. (A) IL-7–mediated activation of Stat5 is dependent on tyrosine residues within IL-7R. Lysates of 293T cells coexpressing Jak3 and IL-7RWT (lanes 1,2) or a mutant IL-7R lacking all cytoplasmic tyrosine residues (γcY/F and IL-7RαY/F, lanes 3,4) were analyzed for phosphorylation of Stat5 on Tyr694 (top panel) and Stat5 protein (bottom panel). (B) Requirement for cytoplasmic tyrosines of IL-7R for KitYY567/569FF-induced Stat5 activation. Lysates from 293T cells coexpressing KitYY567/569FF and IL-7RWT (lanes 1,2) or the mutant IL-7R consisting of γcY/F and IL-7RαY/F (lanes 3,4) were analyzed for phosphorylation of Stat5 on Tyr694 Stat5, Kit, γc, and IL-7Rα protein levels. (C) Quantification of 4 independent experiments analyzing KitYY567/569FF-induced (left) or IL-7R-induced (right graph) phosphorylation of Stat5 in presence (wt) or absence (Y/F) of cytoplasmic tyrosine residues of IL-7R. Stat5 phosphorylation was determined by analyzing either lysates or immunoprecipitates of Stat5 for phosphorylation of Stat5 and protein levels of Stat5. Data are displayed and statistically analyzed as in Figure 4C.
Discussion
Using Jurkat T cells and reconstituted 293T cells, we demonstrated that Kit signaling activates the IL-7R and its downstream pathways. KL stimulation of Kit led to tyrosine phosphorylation of IL-7R. Unlike IL-7–mediated signaling that requires the presence of both γc and IL-7Rα subunits, as well as Jak3, Kit induced tyrosine phosphorylation of both subunits in the absence of Jak3. Furthermore, Kit formed complexes with either γc or IL-7Rα, suggesting direct and independent association of Kit with γc or IL-7Rα. Thus, both subunits are likely to serve as independent substrates for the Kit receptor tyrosine kinase. KL stimulation in cells coexpressing Kit and IL-7R resulted in the activation of the IL-7R downstream targets Jak3 and Stat5. Kit-induced activation of Jak3 occurred indirectly via interaction with IL-7R, whereas Kit-mediated activation of Stat5 was transmitted via 2 distinct signaling pathways. One is a Kit-intrinsic, SFK-related pathway; the other is indirect and requires the interaction between Kit and IL-7R. Our results establish a model of Kit-induced activation of IL-7R signaling pathways via direct interaction between Kit and IL-7R.
Kit, γc, and IL-7Rα have each been shown to be necessary for thymopoiesis. Murine Kit loss-of-function mutants show depressed thymocyte numbers without affecting differentiation. Because the IL-7R consists of 2 subunits, γc and IL-7Rα, and is normally activated by its cognate ligand IL-7, the role of the IL-7R pathway in thymopoiesis can be analyzed via 3 genotypically distinct murine knock-out models for γc, IL-7Rα, and IL-7.24,27,40 These murine models show similar thymic phenotypes of both abnormal cellularity and blocked differentiation. The inhibition of thymopoiesis due to lack of IL-7Rα is more severe than that caused by lack of IL-7, with fewer thymocytes and an earlier block in differentiation.24,27 Our model of Kit-induced activation of IL-7R provides an explanation for the phenotypic difference between IL-7Rα and IL-7 knock-out mice: Kit can partially complement the lack of IL-7 via Kit-induced activation of IL-7R in the absence of IL-7. This complementary pathway would be absent in IL-7Rα–negative mice. Analyses of mice doubly mutated for Kit and γc revealed synergistic effects of Kit and γc signaling pathways in thymopoiesis.16,20 Consistent with the model of Kit-mediated activation of the IL-7R, the data from mice generated in our lab that are doubly mutated for Kit and either IL-7Rα or IL-7 show synergistic loss of thymopoiesis, which is the same whether IL-7Rα or IL-7 is lost (A. Toyama, T.J., K.W., manuscript in preparation). We hypothesize that direct interaction between and RTK and a type I cytokine receptor is the underlying principle not only for complementation but also for synergy of 2 distinct signaling pathways.
Direct interaction between Kit and IL-7R was indicated by coimmunoprecipitation between Kit and either the γc or IL-7Rα subunits of IL-7R. Investigations of the relationship between Kit and Epo-R signaling pathways in erythropoiesis have also demonstrated direct interaction between both receptors.17,18 Direct interaction between Kit and Epo-R was dependent on a 35-aa sequence in the intracytoplasmic domain of the Epo-R. By computational homology analysis (BLAST41 ), we found no significant homology between this Epo-R region and the cytoplasmic domains of IL-7Rα or γc (data not shown). Using binding assays with GST-fusion constructs of different regions of the intracellular domain of IL-7Rα, we were unable to identify a specific region required for interaction between Kit and IL-7Rα or γc (data not shown). Therefore, the whole cytoplasmic tail or additional transmembrane or even extracellular domains of IL-7R could be involved in mediating this interaction. In addition to direct protein-protein interactions, it is likely that a specific membrane environment is also necessary to establish the interaction between both receptors. In an independent study, we report that KL engagement of Kit results in the recruitment of Kit to membrane microdomains or lipid rafts.42 Interestingly, lipid rafts have also been implicated in the orchestration of Jak-Stat signaling, the most important downstream signaling pathway of γc-containing receptors.43 The hypothesis that lipid rafts are a specific membrane location that organizes Kit colocalization with type I cytokine receptors (eg, IL-7R or Epo-R) is currently under investigation.
Kit-mediated phosphorylation of IL-7R led to activation of a central downstream pathway of the IL-7R, namely Jak-Stat. Activation of Jak3 is thought to be restricted to γc-containing receptors including the IL-7R. Analyses of γc- or Jak3-negative mice as well as direct signaling analyses strongly support the idea of a physical and functional unit composed of γc and Jak3.34 In the present study, we introduce a novel mechanism for Jak3 activation via Kit-mediated activation of γc. Kit activation in Jurkat-K7 cells resulted in activation of Jak3 even in the absence of IL-7, and tyrosine-phosphorylated Jak3 was associated with γc. In 293T cells, we were able to verify that Kit-mediated activation of Jak3 was in fact largely γc dependent.
We hypothesized that Kit-mediated Stat5 activation in Jurkat-K7 cells is at least in part dependent on the IL-7R pathway. There is evidence that SFK-related signaling is involved in activation of Stat signaling.44 Use of the Kit mutant YY567/569FF incapable of activating SFKs enabled us to specifically analyze Stat5 activation through the interaction of Kit and IL-7R without interference of an SFK-related signaling pathway.7,45 The defect in Stat5 activation of KitYY567/569FF could partially be rescued when KitYY567/569FF was activated in the presence of IL-7R. Moreover, KitYY567/569FF-induced activation of Stat5 was dependent on tyrosine residues within IL-7R. This suggests that, by analogy to IL-7–mediated signaling, a tyrosine-phosphorylated docking site within the IL-7R cytoplasmic tail for the Stat5-SH2 domain is required for the indirect activation of Stat5 via Kit/IL-7R interaction.
The ability of Kit to interact with certain type I cytokine receptors raises questions about whether Jak-Stat activation downstream of Kit may largely depend on the receptor milieu that the cells express. Previous analyses of Jak-Stat pathways activated by Kit have been performed mainly in cells in which other type I cytokine receptors are coexpressed with Kit.9–11 For example, KL stimulation of Mo7e cells expressing endogenous Kit resulted in tyrosine phosphorylation of Jak2, Stat1, as well as Stat5, while Stat3 was serine phosphorylated in response to KL.9,10,31,46 In other cellular systems such as HCD57 or erythroid progenitor cells, these signaling effects of Kit were apparently absent.47 Apart from the possibility of methodological differences accounting for these apparently contradictory results, an alternative explanation for the discrepant analyses of Jak-Stat signaling downstream of Kit is that such signaling is indirectly activated in a cell-specific manner, depending on the type and expression level of interacting type I cytokine receptors. For example, Mo7e cells, which exhibit a broad range of Kit-induced activation of Stat species, namely Stat1, Stat3, and Stat5, also express a broad range of cytokine receptors and are known to respond proliferatively to IL-2, IL-3, IL-4, IL-6, IL-15, GM-CSF, IFN-α, IFN-β, INF-γ, NGF, TNF-α, and TPO.48–50
Our results with Kit and IL-7R, along with previous studies of Kit/Epo-R interactions, raise the possibility that Kit activates both “intrinsic” Kit-specific pathways as well as other signals that are mediated by interactions with type I cytokine receptors. Because such type I cytokine receptors can be expressed in a cell-type–specific manner or have cell-type–specific effects, the net effect of Kit interactions with these type I receptors may be additional cell-specific Kit signaling. Such context-specific Kit signals via interactions with type I cytokine receptors can explain the pleiotropic effects of Kit signaling in a wide variety of cell types (thymocytes, hematopoietic stem cells (HSCs), erythroid cells, myeloid progenitors, mast cells, neural progenitors, and germ cells). Context-specific Kit activation of Jak-Stat pathways can also explain complementarity and synergy observed between the Kit and IL-7R pathways for thymopoiesis in vivo. The partial complementarity between Kit and IL-7R can occur as a result of direct interaction of the receptors, leading to activation of IL-7R in the absence of the cognate ligand IL-7. Synergy might be mediated by alterations of Stat signals by dual receptor activation, for example, quantitative differences (more persistent tyrosine phosphorylation of Stat5) or qualitative changes (generation of different combinations of Stat homodimers or heterodimers51,52 ). The hypothesis that specificity of Kit signaling in lymphohematopoiesis is mediated by its interactions with γc-dependent receptors such as IL-7R is currently under investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the following grants: Childrens Hospital Los Angeles Research Institute Pilot and Feasibility Fellowship and Wright Foundation (T.J.), and NIH grants R01 AI50765, R01 HL54729, R01 HL70005, and P01 HL73104 (K.W.).
We thank Melissa Principale, Yenbou Liu, and DaPeng Yao for technical assistance, and Diane Linnekin and Ellen Rothenberg for helpful discussions.
National Institutes of Health
Authorship
Contribution: T.J. designed and performed experiments, analyzed data, and wrote the paper; S.S. performed experiments and analyzed data; S.G., P.S., and E.L. performed experiments; P.L. analyzed data; and K.W. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Jahn or Kenneth Weinberg, Division of Stem Cell Transplantation, Department of Pediatrics, Stanford School of Medicine, 300 Pasteur Dr, Stanford, CA 94305-5208; e-mail: tjahn@stanford.edu or kw@stanford.edu.