Abstract
ShcA is an important mediator of Ras/MAPK activation in PTK-regulated pathways triggered by surface receptors. This function is subserved by the constitutively expressed p52-kDa isoform. Besides activating Ras, p52Shc couples the TCR to Rho GTPases, and thereby participates in actin cytoskeleton remodeling in T cells. Here we have addressed the potential involvement of p52Shc in T-cell chemotaxis and the role of the phosphorylatable tyrosine residues, YY239/240 and Y317, in this process. We show that CXCR4 engagement by the homeostatic chemokine, SDF-1α, results in p52Shc phosphorylation and its assembly into a complex that includes Lck, ZAP-70, and Vav. This process was found to be both Lck and Gi dependent. Expression of p52Shc mutants lacking YY239/240 or Y317, or p52Shc deficiency, resulted in a profound impairment in CXCR4 signaling and SDF-1α–dependent chemotaxis, underscoring a crucial role of p52Shc as an early component of the CXCR4 signaling cascade. p52Shc was also found to be required for ligand-dependent CXCR4 internalization independently of tyrosine phosphorylation. Remarkably, CXCR4 engagement promoted phosphorylation of the ζ chain of the TCR/CD3 complex, which was found to be essential for CXCR4 signaling, as well as for SDF-1α–dependent receptor endocytosis and chemotaxis, indicating that CXCR4 signals by transactivating the TCR.
Introduction
By controlling leukocyte trafficking and homing to specific microenvironments, chemokines and their receptors represent a strategic element in the regulation of immune responses.1 Initially implicated in directing transmigration of neutrophils and other cellular components of the innate immune system to inflamed tissues, chemokines have been found to coordinate temporally and topologically the directional migration and positioning of leukocytes within lymphoid organs and tissues not only in inflammation, but also in homeostatic conditions, thereby participating both in hematopoietic cell differentiation in central lymphoid organs and in the initiation of adaptive immune responses in peripheral lymphoid organs. Although the distinction has become less clear-cut, chemokines have been classified on the basis of their primary function as inflammatory and homeostatic. Among the latter is SDF-1α/CXCL12, which interacts with CXCR4. In the hematopoietic system, CXCR4 controls stem-cell migration to the bone marrow and retention of B-cell and granulocytic precursors within this microenvironment, thereby permitting their differentiation. Furthermore, SDF-1α is a potent chemoattractant for mature T cells, monocytes, and neutrophils.2 CXCR4 has also been implicated in brain and heart development and angiogenesis, as well as in some pathological conditions, including tumor metastasis and joint infiltration by inflammatory CD4+ cells in rheumatoid arthritis.3
Like other chemokine receptors, CXCR4 is a 7-spanning transmembrane receptor coupled to a pertussis toxin (PTX)–sensitive heterotrimeric Gi protein, which, by inhibiting the activity of adenylate cyclase, modulates the levels of intracellular cAMP.4 This second messenger represents the link to PI-3 kinase,5,6 which not only is implicated in cell motility by promoting reorganization of the actin cytoskeleton, but also regulates cell survival by controlling the activity of the serine/threonine kinase Akt/PKB.7 Interestingly, CXCR4 has been shown to also activate 2 protein tyrosine kinase (PTK)–dependent pathways. The first is initiated by a Src kinase that interacts with a specific cytosolic loop of CXCR4,8 and involves additional PTKs of the Syk, Tec, and FAK families, which promote the formation of a multimolecular complex leading both to changes in the transcriptional profile of the cell through activation of MAP kinases and to actin remodeling through the activation of Ras-related GTPases, including RhoA, Rac, and Rap1.6,9–13 The second, Gi-independent, pathway is mediated by Jak2/STAT3.14,15 While their interrelationships are as yet poorly defined, both of these pathways are required for chemotaxis. Signaling by CXCR4 occurs in lipid rafts, where the receptor is recruited in response to ligand binding,16 an event that also results in receptor homodimerization.14 Transactivation of receptor PTKs by CXCR4 has been demonstrated,17 suggesting that an indirect mechanism of activation of the PTK-dependent pathways may also be operational.
The Shc adaptor is an important mediator of PTK-regulated pathways triggered by surface receptors. Shc is expressed as 3 isoforms encoded by the same gene locus, of which the largest, of 66 kDa, has been implicated in apoptotic responses to oxidative stress,18 while the other 2, of 52 and 46 kDa, act as molecular adaptors that couple surface receptors to Ras activation.19 p52Shc has been identified as a component of signaling pathways initiated by a number of surface receptors in T cells, including the T-cell antigen receptor, the CD4 coreceptor, the IL-2 and IL-4 receptors, and integrins.19 Besides activating the Ras/MAPK pathway, p52Shc contributes to the activation of other small Ras-related GTPases. Of particular note is its capacity to promote remodeling of the actin cytoskeleton through activation of Rho GTPases.20 Here we have addressed the potential involvement of p52Shc in T-cell chemotaxis. Our data identify p52Shc as an early and essential component in the CXCR4 PTK-dependent signaling cascade that controls T-cell chemotaxis.
Materials and methods
Cells, antibodies, GST fusion proteins, and reagents
Cell lines included the T-lymphoma Jurkat line as well as stable Jurkat transfectants expressing either HA-tagged wild-type p52Shc or mutants thereof carrying Tyr→Phe substitutions either at positions 239/240 (Shc2F) or at position 317 (Shc1F), which have been previously described.20 Cells also included the Lck-defective Jurkat variant JCaM1,21 the Shc-deficient Jurkat variant JSL1,22 and the TCR-deficient Jurkat T-cell line E6.157,23 as well as previously described Jurkat T-cell transfectants expressing constitutively active mutants of either Fyn (F528) or Lck (F505).24 Furthermore, stable JSL1 transfectants were generated using expression constructs encoding either HA-tagged p52Shc, or p52/p46Shc, or p46Shc. The latter 2 were obtained by site-directed mutagenesis of ATG1 and ATG1 + ATG2 (to TTG), respectively, of a construct containing an approximately 8-kb EcoRI-BamHI fragment of the genomic Shc locus spanning promoter 1, exon 1, promoter 2, and exons 1 to 6, fused in frame with the portion of Shc cDNA containing all remaining exons (L.L., P.G.P., unpublished results, 2002). Human peripheral blood mononuclear cells were purified from whole blood by density gradient centrifugation on Ficoll-Paque (Amersham Biosciences, Arlington Heights, IL) and subsequently depleted of monocytes by adherence. Peripheral blood T cells were transiently transfected using the Amaxa nucleofector device (Amaxa Biosystems, Cologne, Germany) and the conditions for T-cell transfection recommended by the manufacturer. Endotoxin-free plasmid DNA was purified using a kit from Qiagen (Hilden, Germany).
Phosphospecific antibodies recognizing the phosphorylated active forms of Shc, ZAP-70, and Erk1/2 were from Cell Signaling Technology (Beverly, MA); anti–phospho-CD3ζ and anti–phospho-Jak2 were from Santa Cruz Biotechnology (Santa Cruz, CA) and anti–phospho-Vav, from Biosource (Camarillo, CA). Anti-Erk2, anti-HA, anti-CD3ζ, and anti-Shc mAbs were from Santa Cruz Biotechnology; anti-Grb2 and anti-Lck mAbs, from BD Transduction Lab (Heidelberg, Germany); antiactin, from Chemicon (Temecula, CA), anti-phosphotyrosine, anti-Vav, and anti–ZAP-70 mAbs, and anti-Lck, anti-LAT, anti-Itk, and anti-Shc polyclonal antibodies from Upstate Biotechnology (Boston, MA). Anti-CXCR4 antibodies were kindly provided by J. Hoxie, Leukosite (Cambridge, MA), and the MRC AIDS Reagent Project. IgG antibodies from OKT3 (anti-CD3; American Type Culture Collection, Manassas, VA) hybridoma supernatants were purified on Mabtrap (Amersham Biosciences) and titrated by flow cytometry. Secondary peroxidase-labeled antibodies were from Amersham Biosciences and secondary fluorochrome-labeled antibodies, from Dako (Glostrup, Denmark). SDF-1α, AG490, and PP2 were purchased from Sigma Aldrich (Milan, Italy). Pertussis toxin (PTX) was kindly provided by R. Rappuoli (Novartis Vaccines, Siena, Italy). Recombinant GST-ZAP-70 was a kind gift of S. Plyte (Pfizer Italia, Nerviano, Italy). A glutathione-Sepharose 4B–immobilized GST fusion containing the Shc CH1 domain was described previously.25 A kit for Jak2 RNA interference was purchased from Santa Cruz Biotechnology. siRNA was transfected by electroporation and assays carried out after 48 hours.
Activations, immunoprecipitations, immunoblots, in vitro binding, and kinase assays
Cells were starved overnight at 37°C in RPMI 0.5% BCS and for 2 hours in RPMI containing 1% BSA. Activations with 10 to 500 ng/mL SDF-1α (10 ng/mL for cell lysate; 500 ng/mL for immunoprecipitates due to the higher concentration of cells during activation) were carried out at 37°C in RPMI containing 1% BSA for the indicated times, identified on the basis of a time course analysis (Figure S1, available on the Blood website; see the supplemental materials link at the top of the online article). When required, cells were pretreated at 37°C overnight with 500 ng/mL PTX and for 2 hours with 100 μM AG490 or 20 μM PP2 in RPMI containing 1% BSA before activation. Activations by cross-linking of mouse mAbs to TCR/CD3 in solution were carried out as described25 by addition of anti-CD3 mAb and secondary antibodies, and incubation for 5 minutes at 37°C. Cells (2 × 106 cells/sample for analysis of total cell lysates) were lysed in 1% (vol/vol) Triton X-100 in 20 mM Tris-HCl, pH 8, 150 mM NaCl (in the presence of 0.2 mg/mL Na orthovanadate, 1 μg/mL pepstatin, leupeptin, and aprotinin, and 10 mM phenyl methyl sulfonyl fluoride), resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. Alternatively, postnuclear supernatants from 2.5 to 5 × 107 cells/sample were immunoprecipitated using the appropriate polyclonal antibodies and protein A Sepharose (Amersham Biosciences). Immunoblots were carried out using peroxidase-labeled secondary antibodies and a chemiluminescence detection kit (Pierce, Rockford, IL). In vitro autophosphorylation assays of Lck-specific immunoprecipitates were carried out in 20 μL of 20 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 10 mM MnCl2, 10 μCi (0.37 MBq) γ-[32P]ATP, at room temperature for 10 minutes. The activity of Lck was also evaluated using 12.5 μg/sample enolase as substrate and 50 μM unlabeled ATP. The reaction products were subjected to SDS-PAGE, transferred to nitrocellulose, and exposed to a Phosphorimager (Molecular Dynamics, Sunnyvale, CA). The filters were subsequently probed with anti-Lck mAb as immunoprecipitation control. For in vitro binding assays, cell lysis was carried out using 3% Triton X-100 to disrupt preexisting molecular interactions. Postnuclear supernatants were adjusted to 1% Triton X-100, precleared for 30 minutes at 4°C with agarose-bound GST, and subsequently incubated for 2 hours at 4°C with the agarose-bound GST-CH1 fusion protein. The GST-CH1 fusion protein was phosphorylated in vitro in 20 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, 50 μM ATP for 20 minutes at 37°C using 125 ng GST-ZAP-70/reaction. The effectiveness of phosphorylation was checked by immunoblot with antiphosphotyrosine antibodies.
Flow cytometry, chemotaxis assays, and calcium flux analysis
Flow cytometry was carried out using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). F-actin was analyzed by flow cytometry on cells permeabilized and stained with TRITC-labeled phalloidin as described.26 Chemotaxis assays were carried out using 24-well Transwell chambers with 5-μm pore size polycarbonate membranes (Corning Life Sciences, Schiphol-Rijk, the Netherlands) essentially as described.15 Filters were soaked overnight in 0.2% BSA in HBSS w/o Ca2+/Mg2+. The chemotaxis medium (500 μL RPMI containing 1% BSA) with or without SDF-1α (10 ng/mL) was placed in the lower chamber, and 100 μL cell suspension (5 × 105 cells/sample) in chemotaxis medium was placed in the upper chamber. After 2-hour incubation at 37°C in humidified air with 5% CO2, the upper chamber was emptied, filters were removed, and the contents of the lower chamber were recovered. After 2 washes in PBS, cells in the lower chamber were counted by flow cytometry. The migration index was calculated by determining the ratio of migrated cells in treated versus untreated samples. The average number of cells that had migrated to the lower chamber in the SDF-1α–stimulated samples was approximately 30%. Spontaneous migration was consistently approximately 0.5%. Analysis of Ca2+ in Fura-2–loaded cells was carried out as described27 in 140 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 0.2 mM EGTA, 15 mM Hepes, pH 7.4, in the absence of added Ca2+. Fluorescence was measured with a Varian Cary Eclipse fluorescence spectrophotometer (Palo Alto, CA) (excitation wavelengths: 340 nm and 380 nm; emission: 510 nm) equipped with magnetic stirring and temperature control. At the end of each incubation, digitonin (50 μg/mL) and EGTA (20 mM) were added in order to measure maximal (Rmax) and minimal (Rmin) ratio (340/380) fluorescence values, respectively.
CXCR4 internalization
Cells were incubated on ice with 100 ng/mL SDF-1α for 45 minutes (time 0) in RPMI containing 1% BSA and subsequently washed and transferred to 37°C. After incubation for the indicated times, cells were washed, and CXCR4 surface expression was analyzed by flow cytometry after indirect labeling with anti-CXCR4 antibody followed by FITC-labeled secondary antibodies. The levels of surface binding at time 0 were determined on a sample taken before transfer to 37°C.
Results
p52Shc is phosphorylated in response to CXCR4 engagement by SDF-1α and forms a complex with Lck, ZAP-70, and Vav
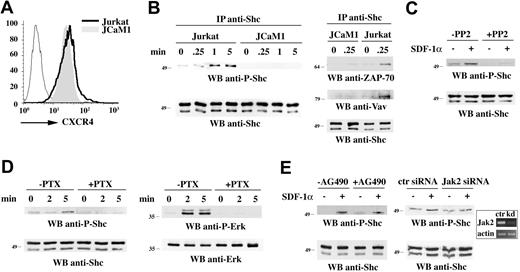
The adaptor function of Shc requires its phosphorylation on 3 specific tyrosine residues, YY239/240 and Y317 in human p52Shc, localized within the central collagen homology (CH1) domain. Following phosphorylation, these tyrosines become docking sites for Grb2/Sos, thereby promoting guanine nucleotide exchange on Ras.19 The capacity of CXCR4 to induce Shc phosphorylation was analyzed in Jurkat T cells treated with SDF-1α. As shown in Figure 1A, SDF-1α induced the rapid and robust phosphorylation of p52Shc, which reached a plateau at 1 minute. Similar results were obtained on human peripheral blood lymphocytes (Figure 1A). In agreement with a recent report,28 coimmunoprecipitation experiments revealed a constitutive interaction of p52Shc with the protein tyrosine kinase (PTK) Lck, which in some experiments was slightly enhanced in response to stimulation with SDF-1α (Figure 1B). p52Shc phosphorylation was accompanied by its transient association with the PTK ZAP-70, as well as with the Vav guanine nucleotide exchanger (Figure 1B).
p52Shc is phosphorylated in response to SDF-1α and forms a complex with Lck, ZAP-70, and Vav. (A) Immunoblot analysis with anti–phospho-Shc antibodies of Shc-specific immunoprecipitates from lysates of Jurkat T cells (left) or freshly purified human PBLs (right) either untreated or treated for the indicated times with SDF-1α. A control anti-Shc blot of the stripped filter is shown (bottom). (B) Immunoblot analysis with anti-Lck, anti–ZAP-70, and anti-Vav antibodies of Shc-specific immunoprecipitates from lysates of Jurkat T cells treated as above. A control anti-Shc blot is shown (bottom). (C) Immunoblot analysis with anti-Vav, anti–ZAP-70, and anti-Grb2 antibodies of the proteins recovered from in vitro binding assays of Jurkat T-cell lysates using a CH1-GST fusion protein. The CH1-GST protein was either unphosphorylated or phosphorylated in vitro using recombinant ZAP-70. An antiphosphotyrosine blot of the stripped filter is shown as phosphorylation control. A Coomassie staining of the input GST fusion is also shown. (D, top) Immunoblot analysis with anti-Shc antibodies of postnuclear supernatants of Jurkat T-cell lines stably transfected with either empty vector (ctr) or HA-tagged wild-type p52Shc (Shc) or p52Shc mutants lacking either YY239/240 (Shc2F) or Y317 (Shc1F). (D, bottom) Flow cytometric analysis of CXCR4 surface expression on the Jurkat transfectants. (E,F) Immunoblot analysis with anti-Lck (E) or anti–ZAP-70 and anti-Vav (F) antibodies of HA-specific immunoprecipitates from lysates of the Jurkat T-cell transfectants treated for 1 minute with SDF-1α. Control anti-Shc blots of the stripped filters are shown below. The migration of molecular mass markers is indicated.
p52Shc is phosphorylated in response to SDF-1α and forms a complex with Lck, ZAP-70, and Vav. (A) Immunoblot analysis with anti–phospho-Shc antibodies of Shc-specific immunoprecipitates from lysates of Jurkat T cells (left) or freshly purified human PBLs (right) either untreated or treated for the indicated times with SDF-1α. A control anti-Shc blot of the stripped filter is shown (bottom). (B) Immunoblot analysis with anti-Lck, anti–ZAP-70, and anti-Vav antibodies of Shc-specific immunoprecipitates from lysates of Jurkat T cells treated as above. A control anti-Shc blot is shown (bottom). (C) Immunoblot analysis with anti-Vav, anti–ZAP-70, and anti-Grb2 antibodies of the proteins recovered from in vitro binding assays of Jurkat T-cell lysates using a CH1-GST fusion protein. The CH1-GST protein was either unphosphorylated or phosphorylated in vitro using recombinant ZAP-70. An antiphosphotyrosine blot of the stripped filter is shown as phosphorylation control. A Coomassie staining of the input GST fusion is also shown. (D, top) Immunoblot analysis with anti-Shc antibodies of postnuclear supernatants of Jurkat T-cell lines stably transfected with either empty vector (ctr) or HA-tagged wild-type p52Shc (Shc) or p52Shc mutants lacking either YY239/240 (Shc2F) or Y317 (Shc1F). (D, bottom) Flow cytometric analysis of CXCR4 surface expression on the Jurkat transfectants. (E,F) Immunoblot analysis with anti-Lck (E) or anti–ZAP-70 and anti-Vav (F) antibodies of HA-specific immunoprecipitates from lysates of the Jurkat T-cell transfectants treated for 1 minute with SDF-1α. Control anti-Shc blots of the stripped filters are shown below. The migration of molecular mass markers is indicated.
To further define the interactions involving YY239/240 or Y317 of p52Shc in CXCR4 signal transduction, in vitro binding assays were carried out using the isolated CH1 domain, expressed as recombinant GST fusion protein, and used either as such or after in vitro tyrosine phosphorylation. As shown in Figure 1C, both Vav and ZAP-70 were found to preferentially interact with tyrosine-phosphorylated CH1. Grb2, which is recruited to phosphorylated YY239/240 and Y317, was used as a positive control. Hence, p52Shc interacts with Lck and, following phosphorylation in response to CXCR4 engagement, forms a complex with ZAP-70 and Vav.
To determine the outcome of YY239/240 and Y317 mutation on p52Shc interaction with Lck, ZAP-70, and Vav, we used Jurkat T-cell transfectants stably expressing HA-tagged p52Shc point mutants lacking either tyrosines 239/240 (Shc2F) or tyrosine 317 (Shc1F). A line expressing HA-tagged wild-type Shc at similar levels as the Shc mutants was used as control (Figure 1D). All lines had similar levels of surface CXCR4 (Figure 1D). Recombinant wild-type or mutated p52Shc was immunoprecipitated from lysates of SDF-1α–stimulated cells using anti-HA antibodies. In agreement with the constitutive interaction of Shc with Lck, immunoblot analysis showed that the association of p52Shc with Lck was not affected by the tyrosine mutations (Figure 1E). On the other hand, both Shc1F and Shc2F failed to interact with ZAP-70 and Vav in SDF-1α–treated cells (Figure 1F), indicating that YY239/240 and Y317 are responsible for these interactions.
p52Shc phosphorylation requires Lck and is Gi-dependent
Tyrosine kinases of the Src, Syk, and Tec families have been implicated in signaling by chemokine receptors.8–11 While Lck, ZAP-70, and Itk are all required for CXCR4-dependent signaling, the current evidence points to Lck as the initiator PTK in this pathway.8 The role of Lck in p52Shc phosphorylation was assessed using JCaM1 cells, an Lck-defective Jurkat T-cell variant.21 Notwithstanding the high levels of surface CXCR4 (Figure 2A), p52Shc phosphorylation in response to SDF-1α was completely abrogated in these cells (Figure 2B). Furthermore, Jurkat T-cell treatment with the Src kinase inhibitor, PP2, resulted in impaired SDF-1α–dependent p52Shc phosphorylation (Figure 2C). Of note, no increase in basal p52Shc phosphorylation was observed in Jurkat cells expressing a constitutively active Fyn mutant (96% ± 3.7%), while phosphorylation was significantly enhanced (> 180%) in cells expressing a constitutively active Lck mutant (data not shown). Taken together with the fact that JCaM1 cells express Fyn, this result supports a selective role for Lck in p52Shc phosphorylation. In agreement with the data presented in Figure 1, the failure of p52Shc to become phosphorylated following CXCR4 engagement in JCaM1 cells resulted in the loss of its ability to form a complex with ZAP-70 and Vav (Figure 2B).
p52Shc phosphorylation is Lck- and Gi-dependent. (A) Flow cytometric analysis of CXCR4 surface expression on Jurkat cells and on the Lck-deficient Jurkat T-cell variant (JCaM1). (B, left) Immunoblot analysis with anti–phospho-Shc antibodies of Shc-specific immunoprecipitates from lysates of Jurkat or JCaM1 cells, either untreated or treated for the indicated times with SDF-1α. (B, right) Immunoblot analysis with anti–ZAP-70 (top) and anti-Vav (middle) antibodies of Shc-specific immunoprecipitates from lysates of Jurkat or JCaM1 cells treated as above. Control anti-Shc blots are shown at the bottom. (C) Immunoblot analysis with anti–phospho-Shc antibodies of lysates of Jurkat cells treated as above in the presence or the absence of 20 μM PP2. A control anti-Shc blot is shown at the bottom. (D) Immunoblot analysis with anti–phospho-Shc (left) or anti–phospho-Erk (right) antibodies of lysates of Jurkat cells treated as above in the presence or the absence of 500 ng/mL PTX. (E) Immunoblot analysis with anti–phospho-Shc antibodies of lysates of Jurkat cells treated as above in the presence or the absence of 100 μM Jak2 inhibitor, AG490, or after Jak2 knock down by siRNA (> 85% reduction in Jak2-specific mRNA as assessed by laser densitometric analysis of semiquantitative RT-PCR). A representative experiment is shown in the box. Cells transfected with nonspecific siRNA (ctr siRNA) are used as Jak2 controls (Jak2 siRNA, indicated as kDa in the RT-PCR). Control blots are shown at the bottom. The migration of molecular mass markers is indicated.
p52Shc phosphorylation is Lck- and Gi-dependent. (A) Flow cytometric analysis of CXCR4 surface expression on Jurkat cells and on the Lck-deficient Jurkat T-cell variant (JCaM1). (B, left) Immunoblot analysis with anti–phospho-Shc antibodies of Shc-specific immunoprecipitates from lysates of Jurkat or JCaM1 cells, either untreated or treated for the indicated times with SDF-1α. (B, right) Immunoblot analysis with anti–ZAP-70 (top) and anti-Vav (middle) antibodies of Shc-specific immunoprecipitates from lysates of Jurkat or JCaM1 cells treated as above. Control anti-Shc blots are shown at the bottom. (C) Immunoblot analysis with anti–phospho-Shc antibodies of lysates of Jurkat cells treated as above in the presence or the absence of 20 μM PP2. A control anti-Shc blot is shown at the bottom. (D) Immunoblot analysis with anti–phospho-Shc (left) or anti–phospho-Erk (right) antibodies of lysates of Jurkat cells treated as above in the presence or the absence of 500 ng/mL PTX. (E) Immunoblot analysis with anti–phospho-Shc antibodies of lysates of Jurkat cells treated as above in the presence or the absence of 100 μM Jak2 inhibitor, AG490, or after Jak2 knock down by siRNA (> 85% reduction in Jak2-specific mRNA as assessed by laser densitometric analysis of semiquantitative RT-PCR). A representative experiment is shown in the box. Cells transfected with nonspecific siRNA (ctr siRNA) are used as Jak2 controls (Jak2 siRNA, indicated as kDa in the RT-PCR). Control blots are shown at the bottom. The migration of molecular mass markers is indicated.
As the activator of protein kinase A (PKA), cAMP is a negative regulator of T-cell activation.29 One of its most upstream molecular targets in the TCR signaling pathway is Csk, the PTK that inhibits Src kinases by phosphorylating their C-terminal tyrosine residue.30 Csk activity is enhanced by PKA phosphorylation of Ser364.30 Src family PTK activation in response to chemokines may therefore be causally related to Gi activation. To understand whether p52Shc phosphorylation relies on the capacity of CXCR4 to decrease intracellular cAMP concentrations, cells were treated with PTX, which irreversibly inhibits the Gi subunits of heterotrimeric G-proteins by ADP ribosylation.31 This treatment resulted in inhibition of p52Shc phosphorylation in response to SDF-1α, as well as in inhibition of the Ras/MAPK pathway, as assessed by the state of Erk1/2 phosphorylation (Figure 2D). Hence p52Shc phosphorylation is Gi-dependent.
Although the involvement of the Jak2/STAT3 pathway in CXCR4 signaling is controversial,14,15,32 the potential cross-talk of the Lck-dependent pathway with the Jak2/STAT3 pathway in p52Shc phosphorylation was also addressed. SDF-1α stimulation did not significantly enhance Jak2 phosphorylation in Jurkat cells (data not shown). Accordingly, cell treatment with AG490, a specific pharmacological inhibitor of Jak2, as well as Jak2 knockdown by siRNA, did not affect CXCR4-dependent p52Shc phosphorylation, ruling out the Jak2/STAT3 pathway in this process (Figure 2E).
p52Shc is an early component in CXCR4-dependent signaling
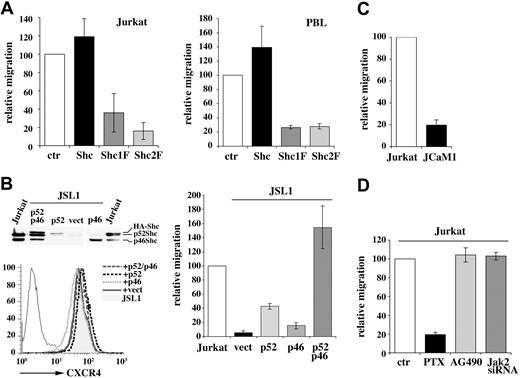
The failure of the tyrosine mutants of p52Shc to associate with ZAP-70 and Vav suggests that they may function as dominant-negative inhibitors of the endogenous protein. To identify which step in the CXCR4 signaling cascade is perturbed in cells expressing Shc1F and Shc2F, the capacity of SDF-1α to promote activation/phosphorylation of the principal components of the Lck-dependent pathway was assessed. Lck was activated in response to SDF-1α to a similar extent in all transfectants, as determined by in vitro kinase assays (Figure 3A). On the other hand, activation of ZAP-70 by CXCR4 was impaired in cells expressing Shc1F or Shc2F, as shown by the significant reduction of ZAP-70 phosphorylation on Y493, which positively controls its activity33 (Figure 3B). A dramatic reduction in inducible tyrosine phosphorylation was also observed for Vav and LAT, both of which are ZAP-70 substrates (Figure 3C,E). The impairment in Vav phosphorylation was paralleled by the failure of cells expressing either Shc1F or Shc2F to reorganize F-actin, as shown by flow cytometric analysis of phalloidin-stained cells (Figure 3D). SDF-1α–dependent phosphorylation of Itk, which is recruited to phospho-LAT through SLP-76 in TCR signaling,34 was also impaired in cells expressing Shc1F or Shc2F (Figure 3F). Furthermore, PLCγ activation was impaired, as shown by the defective [Ca2+]c mobilization from intracellular stores, which is triggered by IP3 (Figure 3H). Of note, this defect was observed only in cells expressing Shc1F, indicating a differential requirement of the 2 phosphorylation sites on p52Shc for CXCR4-dependent PLCγ activation. In agreement with the key role of both Grb2 binding sites of p52Shc in the positive control of the Ras/MAPK pathway,20 Erk1/2 phosphorylation was enhanced by wild-type Shc, and this activity was completely abrogated by YY239/240 or Y317 mutation (Figure 3G). Furthermore, Shc1F or Shc2F expression in transfected human peripheral blood lymphocytes (PBLs) resulted in a reduction of approximately 40% in Erk phosphorylation. A profound deficiency in CXCR4 signaling was also observed in the Jurkat T-cell variant, JSL1, which lacks Shc expression,22 but expresses similar levels of surface CXCR4 (Figure 3I-K). Hence, by promoting intermolecular interactions downstream of Lck, p52Shc acts as an early component in the CXCR4 signaling pathway, contributing both to remodeling of the actin cytoskeleton through Vav and to modifying gene expression through pathways involving MAP kinases and Ca2+.
CXCR4 signaling is inhibited by p52Shc mutants lacking YY239/240 or Y317. (A) Quantification of Lck autophosphorylation, as determined by in vitro kinase assays of Lck-specific immunoprecipitates from lysates of the Jurkat T-cell transfectants expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants, activated for 5 minutes with SDF-1α. The results are presented for each transfectant as fold activation of SDF-1α–treated versus untreated cells (n = 2). Error bars indicate SD. (B) Immunoblot analysis with a ZAP-70 phosphospecific antibody of postnuclear supernatants of the Jurkat T-cell transfectants activated for 1 minute with 10 ng/mL SDF-1α. (C) Immunoblot analysis with antiphosphotyrosine antibodies of Vav-specific immunoprecipitates from lysates of Jurkat cells activated for 1 minute with SDF-1α. (D) Flow cytometric analysis of F-actin polymerization in response to SDF-1α treatment for 30 seconds or 1 minute in Jurkat T cells transfected with empty vector or stably expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants. The data are expressed as fold increase in F-actin (detected as fluorochrome-conjugated phalloidin staining) in stimulated versus unstimulated cells (n = 3). (E-F) Immunoblot analysis with antiphosphotyrosine antibodies of LAT-specific (E) or Itk-specific (F) immunoprecipitates from lysates of the Jurkat transfectants activated for 1 minute (E) or 5 minutes (F) with SDF-1α. Control blots with the indicated antibodies are shown at the bottom. (G). Immunoblot analysis with a Erk1/2 phosphospecific antibody of postnuclear supernatants of the Jurkat T-cell transfectants activated for 5 minutes with SDF-1α. A control anti-Erk immunoblot is shown below. Vertical lines have been inserted to indicate where a gel lane was cut. These gels came from 2 different experiments. (H) Fluorimetric analysis of [Ca2+]c in Jurkat T cells transfected with empty vector or stably expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants. The arrow indicates the time of addition of SDF-1α. Experiment were carried out in Ca2+-free medium to detect Ca2+ release from intracellular stores. The total levels of store-associated Ca2+, as measured after cell solubilization by digitonin treatment in the presence of EGTA, were similar in all cell lines. Representative experiments are shown (n = 3). (I) Flow cytometric analysis of CXCR4 surface expression on Jurkat cells and the Jurkat Shc-deficient JSL1 variant. An anti-Shc immunoblot of the respective cell lysates, together with a control antiactin blot, is shown above. (J) Immunoblot analysis with ZAP-70 (left), Vav (middle), and Erk1/2 (right) phosphospecific antibodies of postnuclear supernatants of Jurkat and JSL1 cells activated for 1 minute (ZAP-70, Vav) or 5 minutes (Erk1/2) with SDF-1α. The immunoblots shown in the figure are representative of at least 3 independent experiments. (K) Fluorimetric analysis of [Ca2+]c in Jurkat cells, JSL1 cells, and JSL1 cells stably transfected with a vector encoding p52/46Shc (for expression, see Figure 4). Experimental setting and data analysis were as in panel H. Representative experiments are shown (n ≥ 2).
CXCR4 signaling is inhibited by p52Shc mutants lacking YY239/240 or Y317. (A) Quantification of Lck autophosphorylation, as determined by in vitro kinase assays of Lck-specific immunoprecipitates from lysates of the Jurkat T-cell transfectants expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants, activated for 5 minutes with SDF-1α. The results are presented for each transfectant as fold activation of SDF-1α–treated versus untreated cells (n = 2). Error bars indicate SD. (B) Immunoblot analysis with a ZAP-70 phosphospecific antibody of postnuclear supernatants of the Jurkat T-cell transfectants activated for 1 minute with 10 ng/mL SDF-1α. (C) Immunoblot analysis with antiphosphotyrosine antibodies of Vav-specific immunoprecipitates from lysates of Jurkat cells activated for 1 minute with SDF-1α. (D) Flow cytometric analysis of F-actin polymerization in response to SDF-1α treatment for 30 seconds or 1 minute in Jurkat T cells transfected with empty vector or stably expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants. The data are expressed as fold increase in F-actin (detected as fluorochrome-conjugated phalloidin staining) in stimulated versus unstimulated cells (n = 3). (E-F) Immunoblot analysis with antiphosphotyrosine antibodies of LAT-specific (E) or Itk-specific (F) immunoprecipitates from lysates of the Jurkat transfectants activated for 1 minute (E) or 5 minutes (F) with SDF-1α. Control blots with the indicated antibodies are shown at the bottom. (G). Immunoblot analysis with a Erk1/2 phosphospecific antibody of postnuclear supernatants of the Jurkat T-cell transfectants activated for 5 minutes with SDF-1α. A control anti-Erk immunoblot is shown below. Vertical lines have been inserted to indicate where a gel lane was cut. These gels came from 2 different experiments. (H) Fluorimetric analysis of [Ca2+]c in Jurkat T cells transfected with empty vector or stably expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants. The arrow indicates the time of addition of SDF-1α. Experiment were carried out in Ca2+-free medium to detect Ca2+ release from intracellular stores. The total levels of store-associated Ca2+, as measured after cell solubilization by digitonin treatment in the presence of EGTA, were similar in all cell lines. Representative experiments are shown (n = 3). (I) Flow cytometric analysis of CXCR4 surface expression on Jurkat cells and the Jurkat Shc-deficient JSL1 variant. An anti-Shc immunoblot of the respective cell lysates, together with a control antiactin blot, is shown above. (J) Immunoblot analysis with ZAP-70 (left), Vav (middle), and Erk1/2 (right) phosphospecific antibodies of postnuclear supernatants of Jurkat and JSL1 cells activated for 1 minute (ZAP-70, Vav) or 5 minutes (Erk1/2) with SDF-1α. The immunoblots shown in the figure are representative of at least 3 independent experiments. (K) Fluorimetric analysis of [Ca2+]c in Jurkat cells, JSL1 cells, and JSL1 cells stably transfected with a vector encoding p52/46Shc (for expression, see Figure 4). Experimental setting and data analysis were as in panel H. Representative experiments are shown (n ≥ 2).
p52Shc is essential for CXCR4-dependent chemotaxis
p52Shc has been implicated in TCR-dependent Rac activation.20 The interaction of tyrosine-phosphorylated p52Shc with Vav, the principal guanine nucleotide exchanger for Rho GTPases, that was observed in SDF-1α–treated cells suggests that p52Shc may contribute to the cytoskeletal reorganization required for T-cell migration toward the chemokine. To address this issue, we used the Jurkat T-cell transfectants stably expressing either HA-tagged p52Shc or the Shc1F/Shc2F mutants. A line stably transfected with empty vector was used as control (Figure 1D). As shown in Figure 4A (left), HA-Shc enhanced to a moderate extent SDF-1α–dependent cell chemotaxis. Expression of either Shc1F or Shc2F resulted in a dramatic reduction in the chemotactic response, to levels comparable with the ones observed in Lck-deficient JCaM1 cells (Figure 4C). Similar results were obtained on freshly isolated peripheral T cells transiently transfected with empty vector or with the same expression vector encoding HA-Shc or the 2 mutated isoforms (Figure 4A right). CXCR4-dependent migration was also profoundly impaired in Shc-deficient JSL1 cells (Figure 4B). Interestingly, migration was fully rescued in these cells by p52/p46Shc, and partially rescued by p52Shc (notwithstanding the low levels of p52Shc expression in the stable transfectant), but not by p46Shc (Figure 1B). A similar differential rescue by p52Shc was observed in these cells when SDF-1α–dependent Ca2+ mobilization was analyzed (data not shown). Hence p52Shc is a key component of the CXCR4 signaling pathway controlling T-cell chemotaxis, and this function is regulated by phosphorylation of both YY239/240 and Y317. Of note, SDF-1α–dependent cell chemotaxis was inhibited by PTX not only in control cells, as expected (Figure 4D), but also in HA-Shc expressing cells, which have approximately twice the level of total p52Shc (Figure 1A; data not shown), further underlining the Gi-dependence of p52Shc in the CXCR4 pathway. In agreement with the lack of inhibition of p52Shc phosphorylation by Jak2 inhibitors (Figure 2E), neither AG490 nor Jak2 knockdown by siRNA affected SDF-1α–dependent chemotaxis (Figure 4D), further ruling out a requirement of the Jak2/STAT3 pathway in CXCR4 signaling in these cells.
CXCR4-dependent chemotaxis is inhibited by p52Shc mutants lacking YY239/240 or Y317. (A) Migration of the Jurkat T-cell transfectants expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants (left), or of PBLs from 3 different donors transiently transfected with empty vector (ctr) or HA-tagged p52Shc or the Shc1F/Shc2F mutants (right), measured after treatment for 2 hours with SDF-1α. The data are presented as relative migration, with the migration index of SDF-1α–treated control cells (transfected with empty vector) taken as 100% (n ≥ 3). PBLs were cotransfected with a GFP reporter, and migration was measured on GFP+ cells. Transfection efficiencies were consistently approximately 50%. The relative amount of HA-Shc (wild-type or mutant) compared with endogenous p52Shc in transiently transfected PBLs was 40% to 50%, as detected by laser densitometric analysis of anti-Shc immunoblots. (B, left) Flow cytometric analysis of CXCR4 surface expression on Shc-deficient JSL1 cells stably transfected with either empty vector (vect) or expression constructs encoding either HA-tagged p52Shc (p52), or p46Shc (p46), or both isoforms (p52/p46). Cells transfected with the latter construct frequently harbor, in addition to p52Shc and p46Shc, an immunoreactive band with a slightly higher electrophoretic mobility than p52Shc, which may result from usage of a 36-bp upstream in-frame ATG in the genomic locus when the p66Shc initiator ATG is mutated. An anti-Shc immunoblot of the respective cell lysates is shown above. Equal amounts of lysates were loaded, as evaluated by reprobing the filter with antiactin mAb (not shown). (B, right) Migration of Jurkat cells and of the JSL1 cell transfectants, measured after 2-hour treatment with SDF-1α (n ≥ 3). (C) Migration of Jurkat cells and JCaM1 cells, measured after 2-hour treatment with SDF-1α. (D) Migration of Jurkat cells, measured after 2-hour treatment with SDF-1α in the presence or absence of PTX (500 ng/mL) or AG490 (100 μM) or after Jak2 knockdown by siRNA (n ≥ 3). Data in panels B-D are presented as relative migration, with the migration index of SDF-1α–treated Jurkat cells taken as 100%. Error bars indicate SD.
CXCR4-dependent chemotaxis is inhibited by p52Shc mutants lacking YY239/240 or Y317. (A) Migration of the Jurkat T-cell transfectants expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants (left), or of PBLs from 3 different donors transiently transfected with empty vector (ctr) or HA-tagged p52Shc or the Shc1F/Shc2F mutants (right), measured after treatment for 2 hours with SDF-1α. The data are presented as relative migration, with the migration index of SDF-1α–treated control cells (transfected with empty vector) taken as 100% (n ≥ 3). PBLs were cotransfected with a GFP reporter, and migration was measured on GFP+ cells. Transfection efficiencies were consistently approximately 50%. The relative amount of HA-Shc (wild-type or mutant) compared with endogenous p52Shc in transiently transfected PBLs was 40% to 50%, as detected by laser densitometric analysis of anti-Shc immunoblots. (B, left) Flow cytometric analysis of CXCR4 surface expression on Shc-deficient JSL1 cells stably transfected with either empty vector (vect) or expression constructs encoding either HA-tagged p52Shc (p52), or p46Shc (p46), or both isoforms (p52/p46). Cells transfected with the latter construct frequently harbor, in addition to p52Shc and p46Shc, an immunoreactive band with a slightly higher electrophoretic mobility than p52Shc, which may result from usage of a 36-bp upstream in-frame ATG in the genomic locus when the p66Shc initiator ATG is mutated. An anti-Shc immunoblot of the respective cell lysates is shown above. Equal amounts of lysates were loaded, as evaluated by reprobing the filter with antiactin mAb (not shown). (B, right) Migration of Jurkat cells and of the JSL1 cell transfectants, measured after 2-hour treatment with SDF-1α (n ≥ 3). (C) Migration of Jurkat cells and JCaM1 cells, measured after 2-hour treatment with SDF-1α. (D) Migration of Jurkat cells, measured after 2-hour treatment with SDF-1α in the presence or absence of PTX (500 ng/mL) or AG490 (100 μM) or after Jak2 knockdown by siRNA (n ≥ 3). Data in panels B-D are presented as relative migration, with the migration index of SDF-1α–treated Jurkat cells taken as 100%. Error bars indicate SD.
SDF-1α–dependent CXCR4 internalization does not require p52Shc phosphorylation
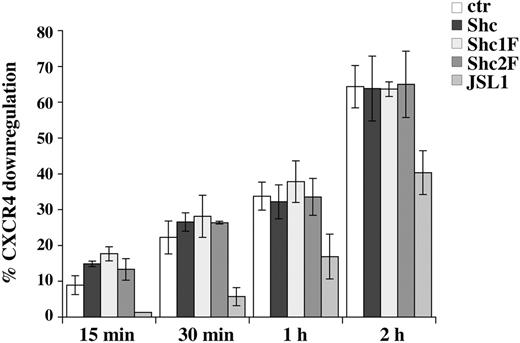
Ligand-dependent internalization of surface receptors is an essential step in signal termination. This process is controlled by the coordinated activity of Rab and Rho GTPases and is initiated by the AP2 adaptor complex that promotes the formation of clathrin-coated pits.35,36 p52Shc has been proposed to participate in receptor-mediated endocytosis. Indeed, an interaction of AP2 with the CH1 domain, which does not involve the phosphorylatable tyrosine residues, has been reported.37 Furthermore, the large GTPase dynamin has been shown to bind to p52Shc through Grb2.38 To address the potential role of YY239/240 and Y317, which mediate Grb2 recruitment, in SDF-1α–dependent receptor endocytosis, a time course analysis of CXCR4 internalization was carried out. As shown in Figure 5, neither Shc1F nor Shc2F affected CXCR4 endocytosis. On the other hand, CXCR4 internalization was impaired in the Shc-deficient JSL1 cells (Figure 5). Hence, while essential for CXCR4 signal transduction, p52Shc phosphorylation does not appear to be required for signal termination through receptor down-regulation, however p52Shc is implicated in this process in a phosphotyrosine-independent manner.
Mutation of YY239/240 or Y317 of p52Shc does not affect ligand-dependent CXCR4 internalization. Flow cytometric analysis of surface CXCR4 on Jurkat T cell transfected with empty vector or stably expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants, or JSL1 cells, after treatment for the indicated times with SDF-1α at 37°C. The results are plotted as percentage CXCR4 internalization (n ≥ 3). Error bars indicate SD.
Mutation of YY239/240 or Y317 of p52Shc does not affect ligand-dependent CXCR4 internalization. Flow cytometric analysis of surface CXCR4 on Jurkat T cell transfected with empty vector or stably expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants, or JSL1 cells, after treatment for the indicated times with SDF-1α at 37°C. The results are plotted as percentage CXCR4 internalization (n ≥ 3). Error bars indicate SD.
CXCR4 promotes p52Shc phosphorylation by transactivating the TCR
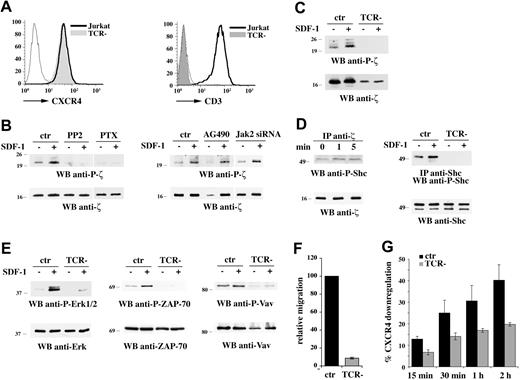
Chemokine receptors have been reported to transactivate receptor PTKs, such as the EGFR.17,39 CXCR4 has been recently shown to physically associate with the TCR in response to ligand binding, and this interaction appears essential for activation of the Ras/MAPK pathway as well as for calcium signaling, suggesting that a similar mechanism may be operational.40 To address the role of the TCR in CXCR4 signaling to p52Shc, we capitalized on a Jurkat T-cell line (E6.157) lacking surface TCR/CD3 complex due to defective expression of the TCRαβ.23 These cells express levels of surface CXCR4 comparable with the parental line (Figure 6A). The outcome of CXCR4 engagement on the TCR activation state was investigated, using as a readout CD3ζ phosphorylation. Immunoblot analysis of cell lysates with an antibody that recognizes phospho-CD3ζ showed that SDF-1α promotes CD3ζ phosphorylation in Jurkat T cells but not in the TCR-deficient variant (Figure 6B,C), indicating that CXCR4 transactivates surface TCR/CD3, resulting in its switch to a signaling-competent state. TCR transactivation was abrogated both by PP2 and by PTX treatment, but not by AG490 or Jak2-specific siRNA (Figure 6B), indicating that this process specifically involves the Lck- and Gi-dependent pathways initiated by CXCR4. Interestingly, phosphorylated p52Shc was found to interact with CD3ζ following CXCR4 engagement (Figure 6D left), while no significant interaction with ZAP-70 was detected in these conditions (data not shown).
Transactivation of the TCR by CXCR4 is required for CXCR4-dependent p52Shc phosphorylation and downstream signaling. (A) Flow cytometric analysis of CXCR4 and CD3 surface expression on Jurkat cells and the E6.157 Jurkat T-cell variant lacking surface TCR expression (TCR−). (B) Immunoblot analysis with an antibody specific for phosphorylated CD3ζ of postnuclear supernatants from Jurkat cells pretreated with either PP2, or PTX, or AG490, or after Jak2 knockdown by siRNA, and stimulated for 5 minutes with SDF-1α. (C) Anti–P-CD3ζ immunoblot of postnuclear supernatants from Jurkat and E6.157 cells treated for 5 minutes with SDF-1α. (D, left) Immunoblot analysis with anti–phospho-Shc antibodies of CD3ζ-specific immunoprecipitates from lysates of Jurkat cells activated for 1 or 5 minutes with SDF-1α. A control anti-CD3ζ blot is shown at the bottom. (D, right) Immunoblot analysis with anti–phospho-Shc antibodies of Shc-specific immunoprecipitates from lysates of Jurkat and E6.157 cells activated for 5 minutes with SDF-1α. (E) Immunoblot analysis with an antibody specific for phosphorylated Erk1/2 (left), ZAP-70 (middle), or Vav (right) of postnuclear supernatants from Jurkat and E6.157 cells treated for 5 minutes (Erk1/2) or 1 minute (ZAP-70, Vav) with SDF-1α. Control blots of the stripped filters are shown (bottom). The migration of molecular mass markers is indicated. (F) Migration of Jurkat and E6.157 cells, measured after 1-hour treatment with SDF-1α. The data are presented as relative migration, with the migration index of SDF-1α–treated Jurkat cells taken as 100% (n ≥ 3). (G) Flow cytometric analysis of surface CXCR4 on Jurkat or E6.157 cells after treatment for the indicated times with SDF-1α at 37°C. The results are plotted as percentage CXCR4 internalization (n ≥ 3). Error bars indicate SD.
Transactivation of the TCR by CXCR4 is required for CXCR4-dependent p52Shc phosphorylation and downstream signaling. (A) Flow cytometric analysis of CXCR4 and CD3 surface expression on Jurkat cells and the E6.157 Jurkat T-cell variant lacking surface TCR expression (TCR−). (B) Immunoblot analysis with an antibody specific for phosphorylated CD3ζ of postnuclear supernatants from Jurkat cells pretreated with either PP2, or PTX, or AG490, or after Jak2 knockdown by siRNA, and stimulated for 5 minutes with SDF-1α. (C) Anti–P-CD3ζ immunoblot of postnuclear supernatants from Jurkat and E6.157 cells treated for 5 minutes with SDF-1α. (D, left) Immunoblot analysis with anti–phospho-Shc antibodies of CD3ζ-specific immunoprecipitates from lysates of Jurkat cells activated for 1 or 5 minutes with SDF-1α. A control anti-CD3ζ blot is shown at the bottom. (D, right) Immunoblot analysis with anti–phospho-Shc antibodies of Shc-specific immunoprecipitates from lysates of Jurkat and E6.157 cells activated for 5 minutes with SDF-1α. (E) Immunoblot analysis with an antibody specific for phosphorylated Erk1/2 (left), ZAP-70 (middle), or Vav (right) of postnuclear supernatants from Jurkat and E6.157 cells treated for 5 minutes (Erk1/2) or 1 minute (ZAP-70, Vav) with SDF-1α. Control blots of the stripped filters are shown (bottom). The migration of molecular mass markers is indicated. (F) Migration of Jurkat and E6.157 cells, measured after 1-hour treatment with SDF-1α. The data are presented as relative migration, with the migration index of SDF-1α–treated Jurkat cells taken as 100% (n ≥ 3). (G) Flow cytometric analysis of surface CXCR4 on Jurkat or E6.157 cells after treatment for the indicated times with SDF-1α at 37°C. The results are plotted as percentage CXCR4 internalization (n ≥ 3). Error bars indicate SD.
The relevance of TCR transactivation to CXCR4 signaling was addressed using the TCR-deficient Jurkat line. Both SDF-1α–dependent p52Shc phosphorylation and Erk1/2 activation were severely impaired in TCR-deficient cells (Figure 6D right; Figure 6E). Consistent with the requirement for p52Shc phosphorylation in CXCR4-dependent ZAP-70 and Vav activation, SDF-1α stimulation did not induce activation of these molecules in the TCR-deficient variant (Figure 6E). CXCR4-dependent chemotaxis was also impaired in these cells (Figure 6F). Furthermore, lack of surface TCR expression resulted in impaired ligand-dependent CXCR4 internalization (Figure 6G). Collectively, these results strongly support a mechanism of signaling by CXCR4 mediated primarily by TCR transactivation and, taken together with the finding that similar defects in CXCR4 signaling can be observed in TCR-deficient and Shc-deficient cells, suggest that p52Shc may be a central early player in CXCR4 coupling to the TCR.
Discussion
Since its initial identification as an adaptor responsible for coupling receptor tyrosine kinases to Ras, Shc has been implicated in a variety of processes controlling cell growth, differentiation, and death.18,19 By providing evidence that p52Shc is an essential component of a signaling pathway that controls T-cell chemotaxis, the data presented above add a novel twist to this multifunctional molecule. The observed inhibition of Vav and Itk phosphorylation by Shc1F and Shc2F is the most likely explanation of the suppressive effect of these mutants on T-cell chemotaxis. Vav is indeed a guanine nucleotide exchanger expressed in the hematopoietic cell lineage and specific for Rho family GTPases that, by stimulating F-actin reorganization, controls remodeling of the cytoskeleton and redistribution of the intracellular components associated with cell motility.41 Itk on the other hand is required for Vav activation and actin polymerization in T cells, an activity that in TCR signal transduction is mediated through a kinase-independent scaffolding function of Itk in the regulation of Vav recruitment to the plasma membrane.42 Although deficiency of the gene encoding either of these molecules or expression of dominant-negative mutants thereof results in suppression of CXCR4-mediated T-cell migration,11,43,44 the mechanistic basis of Vav and Itk participation in chemokine receptor signaling awaits as-yet-complete elucidation. The suppressive effects of the Shc1F/Shc2F mutants on Vav/Itk phosphorylation highlight p52Shc as the link between CXCR4 and the Vav-Itk–dependent pathway of actin reorganization that controls T-cell chemotaxis. While the mechanism of Itk recruitment remains to be clarified, the finding that SDF-1α promotes the assembly of a p52Shc/ZAP-70/Vav complex suggests that p52Shc may control Vav activation by promoting the juxtaposition of Vav close to its activating kinase, ZAP-70, either directly, or indirectly through Grb2.45 It is noteworthy that expression of the p52Shc tyrosine mutants in the human monocytic line, U937, results in inhibition of MIP-1α–dependent cell migration (L.P. and C.T.B., unpublished results, April 2007), suggesting a general role for p52Shc in chemotaxis.
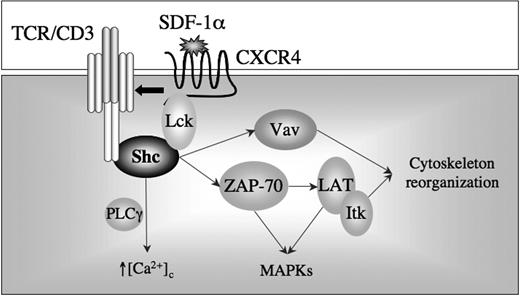
One possible scenario of how p52Shc participates in the CXCR4 signaling cascade is presented in Figure 7. Lck has been shown to associate with CXCR4 following cell stimulation with SDF-1α, which results in its activation.8 The reduction in intracellular cAMP resulting from activation of the heterotrimeric Gi protein coupled to CXCR4 may be causal to the SDF-1α–dependent enhancement in Lck activity, as the negative Src kinase regulator, Csk, is positively controlled by PKA.30 The crucial role of Lck in CXCR4 signaling is supported both by genetic and pharmacological evidence.8,46 As recently reported28 and further confirmed in this study, Lck interacts constitutively with Shc in T cells. Following CXCR4 engagement, p52Shc would be phosphorylated by Lck, as shown for other signaling pathways in T cells.47,48 This event would result in recruitment of ZAP-70 to phosphorylated p52Shc. Since CXCR4 is inducibly recruited to lipid rafts,16 where signaling by chemokine receptors is orchestrated,49 ZAP-70 would be strategically placed to phosphorylate the resident transmembrane adaptor LAT. By analogy with the TCR signaling cascade,50 LAT would become a scaffold for the formation of multimolecular assemblies that would include SLP-76, Itk, Vav, and Grb2. The latter would further enhance Erk1/2 activation. PLCγ, another ZAP-70 substrate also coupled to chemokine receptors,51 may further contribute to Erk1/2 activation through the diacylglycerol-dependent Ras exchange factor RasGRP.52 The early role of p52Shc in CXCR4 signaling, and the resulting impairment of ZAP-70 activation when its recruitment to p52Shc is prevented, could fully account for pleiotropic inhibitory effects of Shc1F and Shc2F on the CXCR4 cascade. Interestingly, while expression of these mutants has no effect on CXCR4 down-regulation, this process is impaired in Shc-deficient cells, suggesting that the tyrosine-independent interaction of p52Shc with the AP2 clathrin adaptor complex, which has been mapped to a short sequence within the CH1 domain,37 may be required for ligand-dependent receptor endocytosis in T cells. Furthermore, this result shows that p52Shc plays a distinct role in CXCR4 signaling to the actin cytoskeleton and to the molecular endocytic machinery.
CXCR4 has been recently reported to physically interact with the TCR in response to SDF-1α. This interaction appears functionally relevant, as CXCR4 would use the low levels of ZAP-70 constitutively bound to the TCR ITAMs to signal.40 Our finding of a requirement of surface TCR both for CXCR4 signaling and for ligand-dependent CXCR4 endocytosis is in full agreement with the concept of a vicarious use of the TCR signaling machinery by CXCR4, from signal initiation to signal termination. Our data are, however, at variance with the data reported by Kumar et al40 on a key issue—CD3ζ phosphorylation and ZAP-70 activation. These authors find, indeed, ITAM-dependent activation of the Ras/MAPK pathway and downstream events by CXCR4 in the absence of ITAM and ZAP-70 phosphorylation, and suggest that “tonic” signaling by the TCR may account for these effects of CXCR4. Conversely, our data show that CXCR4 engagement results in actual transactivation of the TCR beginning from the earliest step, CD3ζ phosphorylation. The time course analysis presented in Figure S1 shows that ZAP-70 activation by CXCR4 is very transient. As Kumar et al40 measured ZAP-70 phosphorylation at a time point when it has already returned to baseline in our experiments, they may have been unable to observe the transactivation reported here. Their finding of an association of CXCR4 with the TCR provides, however, an important contribution to our understanding of how chemokine receptors may transactivate surface receptors, a phenomenon previously described for the EGFR and, with the data presented in this report, for the TCR. The physical proximity of CXCR4-bound and active Lck to the TCR/CD3 ITAMs is likely to promote their phosphorylation, thereby initiating the assembly of a multimolecular signaling complex. It is noteworthy that p52Shc is required for ZAP-70 activation, which places this adaptor upstream of ZAP-70. Based on the observation that CD3ζ associates with p52Shc in response to SDF-1α in the absence of simultaneous enhancement in ZAP-70 recruitment (the latter also reported by Kumar et al40 ), we propose that, at variance with the canonical TCR signaling pathway, it is p52Shc, and not ZAP-70, that initiates signaling by interacting with CD3ζ in the TCR transactivation pathway triggered by CXCR4 (Figure 7).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was generously supported by the Italian Association for Cancer Research (AIRC). The support of the Ministero dell'Università e della Ricerca (MIUR; Fondo per gli Investimenti della Ricerca di Base [FIRB], Progetti di Ricerca di Interesse Nazionale [PRIN]) is also gratefully acknowledged.
The authors wish to thank S. Grassini for technical assistance; A. Di Bello for secretarial assistance; M. Iwashima, B. Rubin, and S. Plyte for the generous gift of JSL1 cells, E6.157 cells, and GST-ZAP-70, respectively; and J. L. Telford for critical reading of the paper.
Authorship
L.P., C.U., O.M.L., S.R.P., and A.G. performed research and analyzed data; L.L. and P.G.P. contributed key reagents and analyzed data; C.T.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cosima T. Baldari, Department of Evolutionary Biology, University of Siena, Via Aldo Moro 2, 53100 Siena, Italy; e-mail baldari@unisi.it.


![Figure 3. CXCR4 signaling is inhibited by p52Shc mutants lacking YY239/240 or Y317. (A) Quantification of Lck autophosphorylation, as determined by in vitro kinase assays of Lck-specific immunoprecipitates from lysates of the Jurkat T-cell transfectants expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants, activated for 5 minutes with SDF-1α. The results are presented for each transfectant as fold activation of SDF-1α–treated versus untreated cells (n = 2). Error bars indicate SD. (B) Immunoblot analysis with a ZAP-70 phosphospecific antibody of postnuclear supernatants of the Jurkat T-cell transfectants activated for 1 minute with 10 ng/mL SDF-1α. (C) Immunoblot analysis with antiphosphotyrosine antibodies of Vav-specific immunoprecipitates from lysates of Jurkat cells activated for 1 minute with SDF-1α. (D) Flow cytometric analysis of F-actin polymerization in response to SDF-1α treatment for 30 seconds or 1 minute in Jurkat T cells transfected with empty vector or stably expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants. The data are expressed as fold increase in F-actin (detected as fluorochrome-conjugated phalloidin staining) in stimulated versus unstimulated cells (n = 3). (E-F) Immunoblot analysis with antiphosphotyrosine antibodies of LAT-specific (E) or Itk-specific (F) immunoprecipitates from lysates of the Jurkat transfectants activated for 1 minute (E) or 5 minutes (F) with SDF-1α. Control blots with the indicated antibodies are shown at the bottom. (G). Immunoblot analysis with a Erk1/2 phosphospecific antibody of postnuclear supernatants of the Jurkat T-cell transfectants activated for 5 minutes with SDF-1α. A control anti-Erk immunoblot is shown below. Vertical lines have been inserted to indicate where a gel lane was cut. These gels came from 2 different experiments. (H) Fluorimetric analysis of [Ca2+]c in Jurkat T cells transfected with empty vector or stably expressing HA-tagged p52Shc or the Shc1F/Shc2F mutants. The arrow indicates the time of addition of SDF-1α. Experiment were carried out in Ca2+-free medium to detect Ca2+ release from intracellular stores. The total levels of store-associated Ca2+, as measured after cell solubilization by digitonin treatment in the presence of EGTA, were similar in all cell lines. Representative experiments are shown (n = 3). (I) Flow cytometric analysis of CXCR4 surface expression on Jurkat cells and the Jurkat Shc-deficient JSL1 variant. An anti-Shc immunoblot of the respective cell lysates, together with a control antiactin blot, is shown above. (J) Immunoblot analysis with ZAP-70 (left), Vav (middle), and Erk1/2 (right) phosphospecific antibodies of postnuclear supernatants of Jurkat and JSL1 cells activated for 1 minute (ZAP-70, Vav) or 5 minutes (Erk1/2) with SDF-1α. The immunoblots shown in the figure are representative of at least 3 independent experiments. (K) Fluorimetric analysis of [Ca2+]c in Jurkat cells, JSL1 cells, and JSL1 cells stably transfected with a vector encoding p52/46Shc (for expression, see Figure 4). Experimental setting and data analysis were as in panel H. Representative experiments are shown (n ≥ 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2007-01-068411/7/m_zh80180706830003.jpeg?Expires=1770236765&Signature=3eAu-affR2Ag45DtXvuHOc~Q~Utk-RYUzI9Myz9dvbv4DrfsGLylh-ZRGvjPQfKlvTkeaUXweDFjtRDIN8jqCjqMvY2v62K-oMOypB7WYfkkK3CymRYiB89BdEN2Zm8wKea8~x5Fmt~LcfZyG2vx~7G9Bu0HAmJMGDIWqU1dTWcu6-FwWZ1Y~a5BaXJtsOKTX6h9VWk8gCL8-Wp1QGNBT6MV6gbFepc5eYsdkAg~B5tHLuxrdpHip1WLO1Vz6sA8hXQ3Y06sPbFi9D~CthPmXMfL1D4lBVEgBgCMeE~DZMFMF4i0PFJgYd6~HTJAexlMOuERExizQKz0Ugh-k~3tnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal