Abstract
The ISCL/EORTC recommends revisions to the Mycosis Fungoides Cooperative Group classification and staging system for cutaneous T-cell lymphoma (CTCL). These revisions are made to incorporate advances related to tumor cell biology and diagnostic techniques as pertains to mycosis fungoides (MF) and Sézary syndrome (SS) since the 1979 publication of the original guidelines, to clarify certain variables that currently impede effective interinstitution and interinvestigator communication and/or the development of standardized clinical trials in MF and SS, and to provide a platform for tracking other variables of potential prognostic significance. Moreover, given the difference in prognosis and clinical characteristics of the non-MF/non-SS subtypes of cutaneous lymphoma, this revision pertains specifically to MF and SS. The evidence supporting the revisions is discussed as well as recommendations for evaluation and staging procedures based on these revisions.
Purpose of revision
When it became recognized that mycosis fungoides (MF), Sézary syndrome (SS), and other cutaneous T-cell lymphomas arising in the skin were part of a broader spectrum of cutaneous T-cell lymphoma (CTCL),1 the Mycosis Fungoides Cooperative Group (MFCG) developed a staging system for CTCL2 aimed at the specific findings in the MF/SS subtypes and based on the TNM (tumor-node-metastasis) classification advocated by the International Union Against Cancer (UICC)3 and American Joint Committee on Cancer.4 This classification and staging system was modified in conjunction with the National Cancer Institute (NCI) and the Veteran's Administration (VA) Hospital and published in 1979 (Tables 1,2).5 The MFCG system has proved to be an extremely useful tool in the management of patients with MF/SS and is the standard for the staging and classification of MF/SS patients today.
Original Mycosis Fungoides Cooperative Group TNM classification of cutaneous T-cell lymphoma (CTCL)
| Classification . | Description . |
|---|---|
| T: Skin* | |
| T0 | Clinically and/or histopathologically suspicious lesions |
| T1 | Limited plaques, papules, or eczematous patches covering < 10% of the skin surface |
| T2 | Generalized plaques, papules, or erythematous patches covering ≥ 10% or more of the skin surface |
| T3 | Tumors, one or more |
| T4 | Generalized erythroderma |
| N: Lymph nodes† | |
| N0 | No clinically abnormal peripheral lymph nodes; pathology negative for CTCL |
| N1 | Clinically abnormal peripheral lymph nodes; pathology negative for CTCL |
| N2 | No clinically abnormal peripheral lymph nodes; pathology positive for CTCL |
| N3 | Clinically abnormal peripheral lymph nodes; pathology positive for CTCL |
| B: Peripheral blood | |
| B0 | Atypical circulating cells not present (< 5%) |
| B1 | Atypical circulating cells present (> 5%); record total white blood count and total lymphocyte counts, and number of atypical cells/100 lymphocytes |
| M: Visceral organs | |
| M0 | No visceral organ involvement |
| M1 | Visceral involvement (must have pathology confirmation and organ involved should be specified) |
| Classification . | Description . |
|---|---|
| T: Skin* | |
| T0 | Clinically and/or histopathologically suspicious lesions |
| T1 | Limited plaques, papules, or eczematous patches covering < 10% of the skin surface |
| T2 | Generalized plaques, papules, or erythematous patches covering ≥ 10% or more of the skin surface |
| T3 | Tumors, one or more |
| T4 | Generalized erythroderma |
| N: Lymph nodes† | |
| N0 | No clinically abnormal peripheral lymph nodes; pathology negative for CTCL |
| N1 | Clinically abnormal peripheral lymph nodes; pathology negative for CTCL |
| N2 | No clinically abnormal peripheral lymph nodes; pathology positive for CTCL |
| N3 | Clinically abnormal peripheral lymph nodes; pathology positive for CTCL |
| B: Peripheral blood | |
| B0 | Atypical circulating cells not present (< 5%) |
| B1 | Atypical circulating cells present (> 5%); record total white blood count and total lymphocyte counts, and number of atypical cells/100 lymphocytes |
| M: Visceral organs | |
| M0 | No visceral organ involvement |
| M1 | Visceral involvement (must have pathology confirmation and organ involved should be specified) |
Pathology of T1-4 is diagnostic of a CTCL. When more than 1 T exists, both are recorded and the highest is used for staging (eg, T4(3)).
Record number of sites of abnormal nodes (eg, cervical; left + right), axillary (left + right), inguinal (left + right), epitrochlear, and submandibular/submaxillary.
Original Mycosis Fungoides Cooperative Group staging system for cutaneous T-cell lymphoma (CTCL)
| Clinical stage . | T . | N . | M . |
|---|---|---|---|
| IA | 1 | 0 | 0 |
| IB | 2 | 0 | 0 |
| IIA | 1,2 | 1 | 0 |
| IIB | 3 | 0,1 | 0 |
| III | 4 | 0,1 | 0 |
| IVA | 1-4 | 2,3 | 0 |
| IVB | 1-4 | 0-3 | 1 |
| Clinical stage . | T . | N . | M . |
|---|---|---|---|
| IA | 1 | 0 | 0 |
| IB | 2 | 0 | 0 |
| IIA | 1,2 | 1 | 0 |
| IIB | 3 | 0,1 | 0 |
| III | 4 | 0,1 | 0 |
| IVA | 1-4 | 2,3 | 0 |
| IVB | 1-4 | 0-3 | 1 |
The “B” classification does not alter clinical stage.
Since the publication of the MFCG classification and staging system, there have been steady advances in the areas of molecular biology, immunohistochemistry, and imaging as well as new data on prognostic variables in MF and SS that affect staging. In addition, it has become clear that the non-MF/non-SS subtypes of CTCL neither share the same T or N stages nor have the same prognosis as MF and SS.6,7 The purpose of revising the MFCG staging and classification system now is to incorporate these advances and new data; to exclude the non-MF/non-SS variants; to provide for identification, subsequent tracking, and validation of certain variables that appear to have prognostic importance; to provide clear definitions of certain variables necessary to carry out the staging and classification that in the current system have been open to variations in interpretation; and to incorporate blood (B) involvement, a major prognostic factor for patients with MF and SS.
To address these issues, the International Society for Cutaneous Lymphomas (ISCL), which includes key leaders of the EORTC, sponsored a series of workshops in 2002 to 2006 on the classification and staging of MF and SS, and the resulting revision to the MFCG classification and staging represents a consensus of both groups. The updated ISCL/EORTC staging and classification applies specifically and solely to MF and SS and has maintained the primary components of the MFCG system to allow for continued comparison of patient outcomes within both systems.
Establishment of the diagnosis of MF/SS
MF and SS represent approximately 65% of the cases of CTCL.6 Both are characterized by a monoclonal proliferation of predominantly CD4+/CD45R0+ helper T cells and the loss of mature T-cell antigens in the skin and other involved organs.6,8,9 SS is currently defined by the ISCL as a distinctive erythrodermic CTCL (albeit potentially lacking the diagnostic histologic features in the skin10 ) with hematologic evidence of leukemic involvement.11 The WHO/EORTC considers SS to be a separate entity from cases that otherwise meet the criteria for SS but have been preceded by clinically typical MF.6,9 Such latter cases have been designated as “SS preceded by MF” and also as “secondary” SS.12
In some instances, the diagnosis of MF can be rendered with confidence on a skin biopsy specimen based on typical light microscopic changes, that is, marked epidermotropism of cytologically atypical T lymphocytes, clusters of these cells in the epidermis (Pautrier microabscesses), or a bandlike infiltrate containing abnormal lymphocytes in the upper dermis.13–16 However, a definitive histopathologic diagnosis by light microscopy alone may be difficult to make in early MF17,18 or in erythroderma in which inflammatory cells often predominate.19 The ISCL recently proposed a diagnostic algorithm for early MF (Table 3).15 No patient with clinically suspect patch- or plaque-stage disease who does not at least fulfill this algorithm nor any patient with tumor-stage disease with histologic findings only “suggestive of MF” should be entered into MF/SS databases or into therapeutic trials for MF/SS. In the case of erythrodermic CTCL, multiple skin biopsies may be necessary to establish a firm diagnosis or a definitive diagnosis may be made by blood studies and/or by biopsy of an enlarged lymph node or other tissue.19,20
Algorithm for the diagnosis of early MF15
| Criteria . | Major (2 points) . | Minor (1 point) . |
|---|---|---|
| Clinical | ||
| Persistent and/or progressive patches and plaques plus | Any 2 | Any 1 |
| (1) Non–sun-exposed location | ||
| (2) Size/shape variation | ||
| (3) Poikiloderma | ||
| Histopathologic | ||
| Superficial lymphoid infiltrate plus | Both | Either |
| (1) Epidermotropism without spongiosis | ||
| (2) Lymphoid atypia* | ||
| Molecular/biologic: clonal TCR gene rearrangement | NA† | Present |
| Immunopathologic | ||
| (1) CD2,3,5 less than 50% of T cells | NA† | Any 1 |
| (2) CD7 less than 10% of T cells | ||
| (3) Epidermal discordance from expression of CD2,3,5 or CD7 on dermal T cells |
| Criteria . | Major (2 points) . | Minor (1 point) . |
|---|---|---|
| Clinical | ||
| Persistent and/or progressive patches and plaques plus | Any 2 | Any 1 |
| (1) Non–sun-exposed location | ||
| (2) Size/shape variation | ||
| (3) Poikiloderma | ||
| Histopathologic | ||
| Superficial lymphoid infiltrate plus | Both | Either |
| (1) Epidermotropism without spongiosis | ||
| (2) Lymphoid atypia* | ||
| Molecular/biologic: clonal TCR gene rearrangement | NA† | Present |
| Immunopathologic | ||
| (1) CD2,3,5 less than 50% of T cells | NA† | Any 1 |
| (2) CD7 less than 10% of T cells | ||
| (3) Epidermal discordance from expression of CD2,3,5 or CD7 on dermal T cells |
— indicates not applicable.
Lymphoid atypia is defined as cells with enlarged hyperchromatic nuclei and irregular or cerebriform nuclear contours.
Not applicable since it cannot fulfill any major criteria.
For patients presenting with tumors, it is important to differentiate tumor-stage MF from non-MF subtypes of CTCL. In classic MF, the tumor lesions generally develop in the presence of patch or plaque disease and not de novo. In the past, this latter presentation was classified as MF tumor d'emblee, but many of these cases would now, with immunophenotypic markers, likely be classified as various types of non-MF T-cell lymphoma or even B-cell lymphoma of the skin.6,7 Given the large number of neoplastic lymphocytes in tumoral lesions of CTCL, molecular analysis of tumor lesions will usually, but not invariably,21 demonstrate evidence of a T-cell clone; however, if this is lacking, chromosomal analysis22 and/or additional molecular studies to rule out a T-cell–rich B-cell lymphoma are in order. Histologic evaluation of an enlarged node can also be used to confirm the type of non-MF CTCL and/or to confirm (and stage) MF.
Proposed revisions to the T (skin) classification
The original MFCG skin scoring system for CTCL (Table 1) included a T0 category for “clinically and/or histopathologically suspicious lesions.” While T0 may be a useful category for tracking disorders with malignant potential, current practice dictates that clinical staging be applied only to cases in which a diagnosis of cancer has been established. Therefore, the T0 category has been eliminated in the ISCL/EORTC updated staging and classification scheme (Table 4) that requires all staged patients have a definitive diagnosis of MF/SS and/or algorithmic diagnosis of early MF.
ISCL/EORTC revision to the classification of mycosis fungoides and Sézary syndrome
| TNMB stages . | . |
|---|---|
| Skin | |
| T1 | Limited patches,* papules, and/or plaques† covering < 10% of the skin surface. May further stratify into T1a (patch only) vs T1b (plaque ± patch). |
| T2 | Patches, papules or plaques covering ≥ 10% of the skin surface. May further stratify into T2a (patch only) vs T2b (plaque ± patch). |
| T3 | One or more tumors‡ (≥ 1-cm diameter) |
| T4 | Confluence of erythema covering ≥ 80% body surface area |
| Node | |
| N0 | No clinically abnormal peripheral lymph nodes§; biopsy not required |
| N1 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 1 or NCI LN0-2 |
| N1a | Clone negative# |
| N1b | Clone positive# |
| N2 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 2 or NCI LN3 |
| N2a | Clone negative# |
| N2b | Clone positive# |
| N3 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grades 3-4 or NCI LN4; clone positive or negative |
| Nx | Clinically abnormal peripheral lymph nodes; no histologic confirmation |
| Visceral | |
| M0 | No visceral organ involvement |
| M1 | Visceral involvement (must have pathology confirmation¶ and organ involved should be specified) |
| Blood | |
| B0 | Absence of significant blood involvement: ≤ 5% of peripheral blood lymphocytes are atypical (Sézary) cells‖ |
| B0a | Clone negative# |
| B0b | Clone positive# |
| B1 | Low blood tumor burden: > 5% of peripheral blood lymphocytes are atypical (Sézary) cells but does not meet the criteria of B2 |
| B1a | Clone negative# |
| B1b | Clone positive# |
| B2 | High blood tumor burden: ≥ 1000/μL Sézary cells‖ with positive clone# |
| TNMB stages . | . |
|---|---|
| Skin | |
| T1 | Limited patches,* papules, and/or plaques† covering < 10% of the skin surface. May further stratify into T1a (patch only) vs T1b (plaque ± patch). |
| T2 | Patches, papules or plaques covering ≥ 10% of the skin surface. May further stratify into T2a (patch only) vs T2b (plaque ± patch). |
| T3 | One or more tumors‡ (≥ 1-cm diameter) |
| T4 | Confluence of erythema covering ≥ 80% body surface area |
| Node | |
| N0 | No clinically abnormal peripheral lymph nodes§; biopsy not required |
| N1 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 1 or NCI LN0-2 |
| N1a | Clone negative# |
| N1b | Clone positive# |
| N2 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 2 or NCI LN3 |
| N2a | Clone negative# |
| N2b | Clone positive# |
| N3 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grades 3-4 or NCI LN4; clone positive or negative |
| Nx | Clinically abnormal peripheral lymph nodes; no histologic confirmation |
| Visceral | |
| M0 | No visceral organ involvement |
| M1 | Visceral involvement (must have pathology confirmation¶ and organ involved should be specified) |
| Blood | |
| B0 | Absence of significant blood involvement: ≤ 5% of peripheral blood lymphocytes are atypical (Sézary) cells‖ |
| B0a | Clone negative# |
| B0b | Clone positive# |
| B1 | Low blood tumor burden: > 5% of peripheral blood lymphocytes are atypical (Sézary) cells but does not meet the criteria of B2 |
| B1a | Clone negative# |
| B1b | Clone positive# |
| B2 | High blood tumor burden: ≥ 1000/μL Sézary cells‖ with positive clone# |
For skin, patch indicates any size skin lesion without significant elevation or induration. Presence/absence of hypo- or hyperpigmentation, scale, crusting, and/or poikiloderma should be noted.
For skin, plaque indicates any size skin lesion that is elevated or indurated. Presence or absence of scale, crusting, and/or poikiloderma should be noted. Histologic features such as folliculotropism or large-cell transformation (> 25% large cells), CD30+ or CD30−, and clinical features such as ulceration are important to document.
For skin, tumor indicates at least one 1-cm diameter solid or nodular lesion with evidence of depth and/or vertical growth. Note total number of lesions, total volume of lesions, largest size lesion, and region of body involved. Also note if histologic evidence of large-cell transformation has occurred. Phenotyping for CD30 is encouraged.
For node, abnormal peripheral lymph node(s) indicates any palpable peripheral node that on physical examination is firm, irregular, clustered, fixed or 1.5 cm or larger in diameter. Node groups examined on physical examination include cervical, supraclavicular, epitrochlear, axillary, and inguinal. Central nodes, which are not generally amenable to pathologic assessment, are not currently considered in the nodal classification unless used to establish N3 histopathologically.
For viscera, spleen and liver may be diagnosed by imaging criteria.
For blood, Sézary cells are defined as lymphocytes with hyperconvoluted cerebriform nuclei. If Sézary cells are not able to be used to determine tumor burden for B2, then one of the following modified ISCL criteria along with a positive clonal rearrangement of the TCR may be used instead: (1) expanded CD4+ or CD3+ cells with CD4/CD8 ratio of 10 or more, (2) expanded CD4+ cells with abnormal immunophenotype including loss of CD7 or CD26.
A T-cell clone is defined by PCR or Southern blot analysis of the T-cell receptor gene.
The definition and differentiation of patch versus plaque23 versus tumor lesions24,25 is more than of just semantic importance because both prognosis and choice of and response to treatment are linked to these different designations. Currently, the distinction between patch and thin plaque lesions and between thick plaque and tumor lesions in MF is quite subjective4,26 (personal communication, E. Vonderheid, ISCL Workshop San Francisco, 1999). The ISCL/EORTC have attempted to add some clarity to the situation by suggesting definitions for the skin lesions (Table 4). Because in patch/plaque disease, histology has been shown to offer an objective means of defining each subtype,27,28 be a validated surrogate for the clinical classification of MF lesions,29 and have prognostic implications,30 there is a provision in the classification system for characterizing exclusively patch-stage disease with the subscript of “a” (T1a and T2a) versus combined patch/plaque disease with the subscript of “b” (T1b and T2b) in order to gather additional longitudinal data on this distinction (Table 4). However, the derivation of all T stages remains a clinical determination.
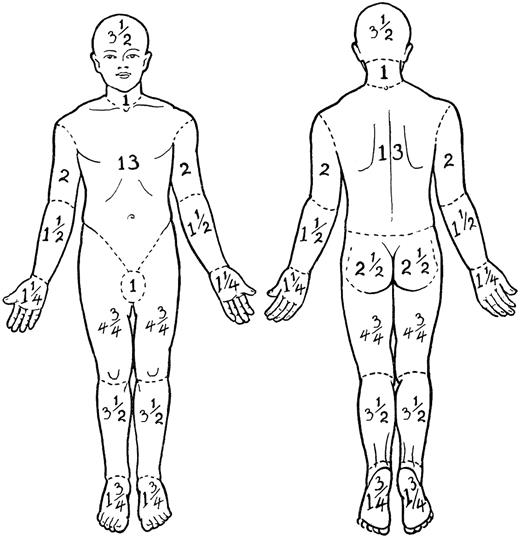
In both the original MFCG5 and the revised staging system, the T1 skin rating is defined as papules, patches, and/or plaques covering less than 10% body surface area (BSA) and T2 skin rating is defined as patches and/or plaques covering 10% or more BSA. In the classification published by the MFCG in 1979, 1% BSA was defined as equal to the “palmar surface of the hand.”5 However, the area of the palm and digits together is actually slightly less than 1% BSA (∼ 0.8%),31–34 and mathematically and reliably, the palm at 0.5% BSA31,34 may be the easiest and most reliable measure to use in assigning BSA of lesions of MF or SS. Another method of determining BSA is to estimate the percentage of skin involvement in each of 12 regions of the body (each with a relative assigned percent BSA35 [Figure 1]), multiplying this number by the percentage of the BSA for that particular region and adding up the regional percentages to obtain the total BSA involved with MF/SS.
Regional percent body surface area (BSA) in the adult. Adapted from Lund and Browder35 with permission.
Regional percent body surface area (BSA) in the adult. Adapted from Lund and Browder35 with permission.
Although ulceration, which may occur in plaques as well as tumors, is generally a poor prognostic sign,4,36,37 ulceration may be caused by infection as well as tumor necrosis and, by multivariable analysis, does not alter the prognosis once the extent of the T rating is known.38 Therefore, the ISCL/EORTC does not recommend using ulceration as the sole criterion to move a patient from plaque (T1 or T2)- to tumor (T3)-stage MF.
Whether any given number of lesions, aggregate volume, size of largest lesion, number of lesions, or specific body regions involved has any predictive prognostic value in tumor-stage MF is unknown at this time. The MFCG originally required that a diagnosis of tumor-stage disease include at least 3 tumors,26 but this was changed to one or more tumors in the final MFCG staging system.5 The proposed ISCL/EORTC classification revision retains the requirement of at least one tumor (≥ 1.5 cm in diameter) for the definition of T3. Whether there should be a minimum histologic depth of infiltrate to distinguish plaque from tumor in order to corroborate this important assignment of T stage based on a single lesion has not been yet been determined.
The skin of erythrodermic CTCL may show some degree of clinically apparent infiltration that may be caused by either dermal infiltration with tumor cells or an inflammatory reaction with or without edema. There is currently no distinction in the updated staging system for subclassifying T4 based on varying degrees of induration, erythema, or scale. Specific grading systems for erythroderma that do include these variables have been published elsewhere39,40 and may be of value for use in clinical trials.
When more than one T rating might apply, the highest is used for staging purposes. In the situations where both tumors and erythroderma exist simultaneously, both T ratings should be recorded (eg, T4(3)).5 This latter nuance of the T staging suggested by the MFCG continues to offer a way of tracking a variable that may impact on the poor prognosis of T4 disease, but would otherwise be buried in the classification hierarchy.
There are at least 2 histologic findings on skin biopsies in MF that appear to have prognostic importance but require further data before modifications to the staging system are justified. Folliculotropic MF is characterized histologically by atypical CD4+ T lymphocytes that surround and infiltrate the hair follicles (folliculotropism), usually without evidence of epidermotropism and with frequent concomitant follicular mucinosis.41–43 Clinically, folliculotropic MF is typically classified under the T1 or T2 skin rating even though the infiltrate extends histologically along the hair follicles deeper than is typical for plaque-stage disease.41,44 Folliculotropic MF has been shown to be associated with a worse prognosis than expected for clinical stage41–43,45 : the 5-year survival is similar and the 10-year survival is worse than in patients with tumor-stage MF.45 Large-cell transformation, defined as a biopsy specimen showing large cells (≥ 4 times the size of a small lymphocyte) in 25% or more of the dermal infiltrate,46–49 is a poor prognostic sign, seen most commonly in tumor-stage MF and less commonly in plaque-stage and erythrodermic MF. Based on molecular analysis, large-cell transformation in MF or SS represents evolution of the original malignant clone.50,51 These large cells may or may not be CD30+.48 The possibility that a patient with CD30+ nodules might have primary cutaneous CD30+ anaplastic large-cell lymphoma coexisting with MF must also be considered, although the coexistence of typical patches and plaques of MF would normally indicate that such lesions represent large-cell transformation (CD30+) of MF rather than a separate primary cutaneous lymphoma. The ISCL/EORTC recommends tracking folliculotropic MF and large-cell transformation to determine if either warrants an advance in stage.
Revisions to the N (node) classification
Defining peripheral adenopathy
The negative impact on survival of “palpable adenopathy” in MF has long been appreciated.14,24,26,36,37,52,53 In the MFCG classification, “N” represented only peripheral lymph nodes. Although there was no size designation for “abnormal” nodes, this was not problematic as all patients, even those without palpable nodes, were to have node biopsies as part of the staging evaluation since each N rating for staging was based on both clinical and histopathologic findings. Although Sausville et al reported that 9 (32%) of 28 patients with MF or SS without adenopathy had either frank lymphoma or advanced dermatopathic findings on histopathologic review of “blind” nodal biopsies,13 biopsies of nonpalpable nodal groups are rarely done in clinical practice today.
The updated ISCL/EORTC classification eliminates biopsies of lymph nodes for staging purposes that are not enlarged on physical examination or imaging studies. In doing so, however, the size of a peripheral lymph node designated to be “clinically abnormal” takes on more importance because a lymph node biopsy is recommended only of such nodes. The ISCL/EORTC revision defines clinically abnormal peripheral nodes as those 1.5 cm or larger in the longest transverse diameter or any palpable peripheral node, regardless of size, that on physical examination is firm, irregular, clustered, or fixed. The 1.5-cm size is different from the 1-cm diameter node designated as abnormal both by the International Workshop on Response Criteria for Non-Hodgkin Lymphoma54 and the ISCL/EORTC staging for non-MF/SS primary cutaneous lymphomas55 since reactive or dermatopathic peripheral lymph nodes commonly occur in MF, SS, and benign inflammatory skin disorders56 but are uncommon in these other lymphomas involving the skin. These clinically enlarged or abnormal nodes should be corroborated by an imaging study (computed tomography [CT] ± 18F-fluorodeoxyglucose positron emission tomography [FDG-PET] or by magnetic resonance imaging (MRI) (in cases where patients may be allergic to contrast dye) or ultrasound prior to biopsy.
Pathology of lymph nodes
The original N rating did not include guidelines for the distinction between nodes judged pathologically “negative” or “positive” for CTCL nor did it give any weight to increasing magnitude of tumor involvement based on light microscopy, flow cytometry, or molecular genetic results. The 2 main histopathologic grading systems for lymph nodes in MF/SS in use today are the NCI/VA classification system,13 first proposed by Matthews and Gazdar,57 and the Dutch System58 (Table 5). The major difference between these classification systems resides in the criteria used to define “abnormal” lymphocytes.58,60 Specifically, the NCI/VA system,13 although it defines abnormal (neoplastic) cells as small (6-10 μm) or large (> 11.5 μm) cells with cerebriform, irregularly folded, hyperconvoluted nuclei (ie, Sézary cells), does not use the size of cells but instead uses the relative numbers of such cells in the paracortex of the lymph node for the LN0-2 definition (Table 5). Conversely, the Dutch system uses the diameter of the cerebriform cells (> 7.5 μm) to define abnormal (neoplastic) cells, and if present, this constitutes early involvement (grade 2).58
Histopathologic staging of lymph nodes in mycosis fungoides and Sézary syndrome
| Updated ISCL/EORTC classification . | Dutch system58 . | NCI-VA classification13,57,59 . |
|---|---|---|
| N1 | Grade 1: dermatopathic lymphadenopathy (DL) | LN0: no atypical lymphocytes |
| LN1: occasional and isolated atypical lymphocytes (not arranged in clusters) | ||
| LN2: many atypical lymphocytes or in 3-6 cell clusters | ||
| N2 | Grade 2: DL; early involvement by MF (presence of cerebriform nuclei > 7.5 μm) | LN3: aggregates of atypical lymphocytes; nodal architecture preserved |
| N3 | Grade 3: partial effacement of LN architecture; many atypical cerebriform mononuclear cells (CMCs) Grade 4: complete effacement | LN4: partial/complete effacement of nodal architecture by atypical lymphocytes or frankly neoplastic cells |
| Updated ISCL/EORTC classification . | Dutch system58 . | NCI-VA classification13,57,59 . |
|---|---|---|
| N1 | Grade 1: dermatopathic lymphadenopathy (DL) | LN0: no atypical lymphocytes |
| LN1: occasional and isolated atypical lymphocytes (not arranged in clusters) | ||
| LN2: many atypical lymphocytes or in 3-6 cell clusters | ||
| N2 | Grade 2: DL; early involvement by MF (presence of cerebriform nuclei > 7.5 μm) | LN3: aggregates of atypical lymphocytes; nodal architecture preserved |
| N3 | Grade 3: partial effacement of LN architecture; many atypical cerebriform mononuclear cells (CMCs) Grade 4: complete effacement | LN4: partial/complete effacement of nodal architecture by atypical lymphocytes or frankly neoplastic cells |
Prognosis in MF/SS is clearly related to partially or completely effaced nodal architecture as defined by either the NCI-VA (LN4) or Dutch (grade 3/4) grading system,60 and continues to define the N3 node rating in the updated ISCL/EORTC staging system. However, the prognostic importance of lesser degrees of involvement as defined by light microscopy alone is less clear.24,59,61,62 Some investigators advocate that the LN3 rating should be considered equivalent to LN4 for staging purposes.13 Although in the context of MF and SS, the survival of patients with an NCI/VA LN3 node rating is worse than patients with LN0-2,24,62 it must be remembered that findings comparable with LN3 have been found in nodes from patients with benign disorders56,60,63 and that the survival of patients with LN4 is worse than for those with LN3.24,60
Prognostic significance of T-cell clonality in lymph nodes
As early as 1988, Sausville et al recognized the potential prognostic significance of a clonal rearrangement of the TCR in nodal staging.24 In 3 studies that have reported on the use of Southern blot technique, which has a 5% detection threshold to show clonal T cells in lymph nodes,62,64,65 none of the lymph nodes classified histologically as uninvolved (NCI/VA LN0-1 or Dutch grade 1) had evidence of a clone. However, 13% of LN2 nodes, 83% of LN3 nodes, and 100% of patients with LN4 nodes had clonal rearrangements in one of the studies.62 These studies indicate that Southern blot analysis is useful as an adjunct study for lymph nodes that show higher histologic grades of involvement, especially given that the prognosis of such patients with evidence of a clone was worse than patients without a clone.62
However, the Southern blot technique has largely been replaced by the technically easier polymerase chain reaction (PCR)–based methods, with much more sensitive detection thresholds.66 Although not significant in a multivariate analysis, Fraser-Andrews et al67 have reported detection of T-ell clones by PCR in 6 of 19 N0-2 lymph nodes and Assaf et al68 in 7 of 14 dermatopathic lymph nodes in patients with MF, and in none of the lymph nodes obtained from patients with benign conditions. In a comparison of Southern blot and PCR determination of clonality of TCR rearrangement in both palpable and nonpalpable lymph nodes in patients with MF/SS, Juarez et al concurred that in a univariate analysis, both were predictive of a poor outcome but that only Southern blot analysis was predictive of a poor prognosis in a multivariate analysis that included skin stage, presence or absence of lymphadenopathy, and histologic lymph node score.69
For staging purposes, the ISCL recommends that nodal rating still be based on histopathology until new molecular markers have been validated, and that uneffaced nodes exhibiting an NCI/VA grade LN3 or Dutch grade 2 be classified as an N2 rating with further division into 2 subgroups: N2a (clone negative) and N2b (clone positive). It is hoped that by capturing this information longitudinally, it can be determined if there is a similar prognosis of patients with N2b and N3 node ratings.
Clinical-only staging of nodes
Because a biopsy of a clinically abnormal node is not always done at initial staging, the revised ISCL/EORTC classification has added a new category, the Nx node rating, to facilitate capture of at least this clinical information.
Lymph node biopsy
The ISCL/EORTC recommends excisional biopsy as the preferred method to evaluate abnormal lymph nodes in MF/SS. In addition to routine histologic examination, a portion of the node can be processed for immunohistochemistry, flow cytometry, and/or molecular genetic or cytogenetic analysis. Since excisional lymph node biopsies put the patient at risk for sepsis, especially in erythrodermic patients whose skin is often colonized with Staphylococcus, alternative methods to obtain nodal tissue (core biopsy or fine needle aspiration [FNA]) have been suggested as potential substitutes particularly if combined with flow cytometry.70 However, these alternate methods may have inadequate or poorly representative sampling71 or incomplete concordance with excisional biopsy results,72,73 and they do not provide the histopathologic assessment of nodal architecture necessary for N staging.
In general, the largest peripheral lymph node draining an area of involved skin and/or one that shows intense uptake on an FDG-PET scan should be selected for biopsy. If there are multiple enlarged nodes, the order of preference for biopsy by location remains cervical, axillary, then inguinal5 since cervical nodes have a higher chance of showing lymphomatous involvement than other sites.74 A biopsy of multiple nodes of a single nodal group may show different LN histopathologic ratings, raising the issue of “sampling error” and whether more than one node should be sampled at the time of excision.75 However, the need to study multiple lymph nodes from one region may be obviated if ancillary studies are performed on the specimen.76
Potential involvement of central nodes in MF or SS was not addressed in the original MFCG classification.5 Central adenopathy may be secondary to a second malignancy (especially a second lymphoma), infection, a reactive process, or MF. The ISCL/EORTC recommends that central enlarged nodes be excluded from the determination of “N” status except in cases where an excisional biopsy of a central node has proven lymphomatous (N3) involvement with MF.
Revision to M classification
Visceral involvement with MF/SS is a well-documented, independently significant prognostic factor in MF/SS.24,77 But what constitutes “visceral disease” has not been well defined. It is generally asymptomatic and therefore prone to be underdiagnosed, especially in those with more advanced skin involvement. Visceral involvement should be questioned in the absence of node or blood involvement. To be considered as having visceral disease (stage IVb), documentation of involvement by only one organ outside the skin, nodes, or blood is needed.
Splenomegaly in MF patients is generally proven to be either diffuse or nodular involvement with MF14 and is uncommon in healthy persons or in those with benign skin disease. The ISCL/EORTC considers splenomegaly as visceral disease, even without biopsy confirmation, when it is (a) unequivocally present on physical exam and (b) documented radiographically by either enlargement or multiple focal defects that are neither cystic nor vascular (a more rigorous modification of the Cotswolds meeting recommendations on definition of lymphomatous involvement of the spleen in Hodgkin lymphoma).78
Liver disease may be suspected by physical examination, abnormal liver function tests, or radiologic tests (CT, FDG-PET, liver/spleen scan) but should be confirmed by liver biopsy.79 In agreement with the Cotswolds meeting on Hodgkin lymphoma, multiple focal hepatic defects, which are neither cystic nor vascular, on at least 2 imaging techniques may be considered indicative of tumor involvement.78
Bone marrow biopsy confirmation of frank lymphoma is a low-yield procedure in MF unless there is evidence of blood or nodal disease24,38,47,52,61,80 Although it has been suggested that the finding of cytologic atypical lymphoid aggregates in the bone marrow of patients with MF correlates with shorter survival,24,81–83 multivariate analysis has not demonstrated the independent prognostic value of bone marrow involvement.23,80,84,85 The ISCL/EORTC recommends performance of a bone marrow biopsy in patients with MF and SS who have B2 blood involvement (as described in paragraph 4 of “Revisions to the B (blood) rating”) or unexplained hematologic abnormalities. Tracking and recording the specific cytologic findings and whether clonality of the TCR gene rearrangement is present will provide further data on whether bone marrow involvement has independent prognostic significance and should be considered as evidence of visceral disease.
If lung abnormalities or other suggestions of extracutaneous lymphomatous involvement besides splenomegaly are found on radiographic examination, pathological assessment is recommended before ascribing this to visceral involvement with MF/SS. Visceral abnormalities seen radiologically in MF could be secondary to either another unrelated cancer (second malignancies are not uncommon in MF/SS86–88 ) or to an infectious disorder and not to MF/SS.
Revisions to the B (blood) rating
Following the lead of Clendenning et al,89 the original MFCG classification of blood involvement in MF (B1 rating) was defined as more than 5% of the total lymphocytes that exhibited an atypical convoluted appearance by light microscopy.5 Low numbers of atypical cerebriform mononuclear cells have been reported in both benign skin conditions90–94 and in healthy donors,92,95 and can be generated in vivo by incubating lymphocytes with cellular mitogens96 or stimulating peripheral T cells via CD3 or CD2 in the presence of phorbol esters.97 Because the prognostic importance of this criterion was unclear, the B rating was not used for staging purposes.5 Later studies at the NCI and elsewhere indicated that more than 20% atypical (Sézary) lymphocytes was associated with an adverse prognosis in MF,24,98 although no formal revision to the staging classification was done because this more rigorous B1 rating was not found to be an independent prognostic variable.13,24 However, Kim et al have subsequently shown that “significant” blood involvement (> 1000 Sézary cells/mm3 and/or > 20% Sézary cells) has prognostic significance regardless of T or N rating,25 and others have corroborated that blood involvement has independent prognostic significance.61,99,100
The assessment of blood tumor burden in CTCL based on morphologic features of the neoplastic cells alone (eg, Sézary cell counts) is subjective and prone to interobserver variability, although absolute counts of Sézary cells continue to be used in staging at centers where such counts are routinely performed.100,101 Blood flow cytometry offers an alternate objective means of identifying and quantifying these neoplastic lymphocytes in the blood. There has been an increasing awareness that neoplastic T cells in CTCL often have altered surface expression of normal markers such as CD3, CD4, CD7, and CD26.102–108 Deletion of one or more of these markers on the surface of CD4+ cells is typical of Sézary cells, but the blood of many patients with benign inflammatory dermatoses may also show CD7 deletion.107,109,110 Loss of CD26 may be a more specific phenotype for the neoplastic lymphocytes.104,108 Identification of neoplastic cells by flow cytometry is complicated, however, by the fact that all neoplastic lymphocytes may not have the same phenotypic features and several clones may be present in a given patient with SS.111 The correlation of the percentage of abnormal cells determined by flow cytometry and that by Sézary cell preparation is inexact and may offer differing results in some cases.
Clonal expansion of TCR gene rearrangement in the blood is extremely common in early-stage disease even without a significant population of morphologically or immunophenotypically abnormal cells.99,112 It is not synonymous with blood involvement by MF/SS since benign lymphoproliferative disorders113,114 and some healthy elderly volunteers115,116 may have clonal TCR gene rearrangements of the blood T cells. Using spectratyping, MF patients even at early stages demonstrate loss of their T-cell repertoire with emergence of one of more clones.117 The presence of a peripheral blood clone in MF patients, if the same as that in the skin, has been found to have prognostic significance independent of skin stage.99
Previously, the ISCL has categorized blood involvement into prognostically significant B ratings based on the degree of involvement, that is, B0 = absence of significant blood involvement; B1 = aleukemic, low blood tumor burden; and B2 = leukemic, high blood tumor burden.11 The ISCL/EORTC has simplified and clarified the definitions of B0 to B2. B0 remains 5% or less Sézary cells. B2 is now defined as a clonal rearrangement of the TCR in the blood and either 1.0 K/μL or more Sézary cells or one of the 2 criteria outlined by the ISCL,11 that is, (1) increased CD4+ or CD3+ cells with CD4/CD8 of 10 or more or (2) increase in CD4+ cells with an abnormal phenotype (≥ 40% CD4+/CD7− or ≥ 30% CD4+/CD26− has been suggested118 ). B1 is defined as more than 5% Sézary cells but either less than 1.0 K/μL absolute Sézary cells or absence of a clonal rearrangement of the TCR or both.
Evaluation and staging of the patient with MF/SS
The staging of MF/SS according to the TNMB system implies that an appropriate evaluation of the 4 TNMB systems has been performed. The recommended workup is detailed in Table 6.
Recommended evaluation/initial staging of the patient with mycosis fungoides/Sézary syndrome
| Complete physical examination including |
| Determination of type(s) of skin lesions |
| If only patch/plaque disease or erythroderma, then estimate percentage of body surface area involved and note any ulceration of lesions |
| If tumors are present, determine total number of lesions, aggregate volume, largest size lesion, and regions of the body involved |
| Identification of any palpable lymph node, especially those ≥ 1.5 cm in largest diameter or firm, irregular, clustered, or fixed |
| Identification of any organomegaly |
| Skin biopsy |
| Most indurated area if only one biopsy |
| Immunophenotyping to include at least the following markers: CD2, CD3, CD4, CD5, CD7, CD8, and a B-cell marker such as CD20. CD30 may also be indicated in cases where lymphomatoid papulosis, anaplastic lymphoma, or large-cell transformation is considered. |
| Evaluation for clonality of TCR gene rearrangement |
| Blood tests |
| CBC with manual differential, liver function tests, LDH, comprehensive chemistries |
| TCR gene rearrangement and relatedness to any clone in skin |
| Analysis for abnormal lymphocytes by either Sézary cell count with determination absolute number of Sézary cells and/or flow cytometry (including CD4+/CD7− or CD4+/CD26−) |
| Radiologic tests |
| In patients with T1N0B0 stage disease who are otherwise healthy and without complaints directed to a specific organ system, and in selected patients with T2N0B0 disease with limited skin involvement, radiologic studies may be limited to a chest X-ray or ultrasound of the peripheral nodal groups to corroborate absence of adenopathy |
| In all patients with other than presumed stage IA disease, or selected patients with limited T2 disease and the absence of adenopathy or blood involvement, CT scans of chest, abdomen, and pelvis alone ± FDG-PET scan are recommended to further evaluate any potential lymphadenopathy, visceral involvement, or abnormal laboratory tests. In patients unable to safely undergo CT scans, MRI may be substituted. |
| Lymph node biopsy |
| Excisional biopsy is indicated in those patients with a node that is either ≥ 1.5 cm in diameter and/or is firm, irregular, clustered, or fixed |
| Site of biopsy |
| Preference is given to the largest lymph node draining an involved area of the skin or if FDG-PET scan data are available, the node with highest standardized uptake value (SUV). |
| If there is no additional imaging information and multiple nodes are enlarged and otherwise equal in size or consistency, the order of preference is cervical, axillary, and inguinal areas. |
| Analysis: pathologic assessment by light microscopy, flow cytometry, and TCR gene rearrangement. |
| Complete physical examination including |
| Determination of type(s) of skin lesions |
| If only patch/plaque disease or erythroderma, then estimate percentage of body surface area involved and note any ulceration of lesions |
| If tumors are present, determine total number of lesions, aggregate volume, largest size lesion, and regions of the body involved |
| Identification of any palpable lymph node, especially those ≥ 1.5 cm in largest diameter or firm, irregular, clustered, or fixed |
| Identification of any organomegaly |
| Skin biopsy |
| Most indurated area if only one biopsy |
| Immunophenotyping to include at least the following markers: CD2, CD3, CD4, CD5, CD7, CD8, and a B-cell marker such as CD20. CD30 may also be indicated in cases where lymphomatoid papulosis, anaplastic lymphoma, or large-cell transformation is considered. |
| Evaluation for clonality of TCR gene rearrangement |
| Blood tests |
| CBC with manual differential, liver function tests, LDH, comprehensive chemistries |
| TCR gene rearrangement and relatedness to any clone in skin |
| Analysis for abnormal lymphocytes by either Sézary cell count with determination absolute number of Sézary cells and/or flow cytometry (including CD4+/CD7− or CD4+/CD26−) |
| Radiologic tests |
| In patients with T1N0B0 stage disease who are otherwise healthy and without complaints directed to a specific organ system, and in selected patients with T2N0B0 disease with limited skin involvement, radiologic studies may be limited to a chest X-ray or ultrasound of the peripheral nodal groups to corroborate absence of adenopathy |
| In all patients with other than presumed stage IA disease, or selected patients with limited T2 disease and the absence of adenopathy or blood involvement, CT scans of chest, abdomen, and pelvis alone ± FDG-PET scan are recommended to further evaluate any potential lymphadenopathy, visceral involvement, or abnormal laboratory tests. In patients unable to safely undergo CT scans, MRI may be substituted. |
| Lymph node biopsy |
| Excisional biopsy is indicated in those patients with a node that is either ≥ 1.5 cm in diameter and/or is firm, irregular, clustered, or fixed |
| Site of biopsy |
| Preference is given to the largest lymph node draining an involved area of the skin or if FDG-PET scan data are available, the node with highest standardized uptake value (SUV). |
| If there is no additional imaging information and multiple nodes are enlarged and otherwise equal in size or consistency, the order of preference is cervical, axillary, and inguinal areas. |
| Analysis: pathologic assessment by light microscopy, flow cytometry, and TCR gene rearrangement. |
The updated ISCL staging classification (Table 7) takes into account the B stage and also differentiates levels of blood involvement (B1 and B2). B1 is used to separate erythrodermic patients without overt lymph node involvement (T4N0-2M0) into 2 subgroups, IIIA (T4N0-2M0B0) and IIIB (T4N0-2M0B1), which will allow determination of the prognostic significance of low blood tumor burden in the setting of erythrodermic CTCL. The ISCL blood rating of B2 is considered comparable with nodal involvement (N3 nodal rating). Stage IVA is now defined as any skin stage and either blood involvement (B2 [IVA1]) or nodal lymphoma (N3 [IVA2]) that allows for independent tracking of these 2 important prognostic indicators.
ISCL/EORTC revision to the staging of mycosis fungoides and Sézary syndrome
| . | T . | N . | M . | B . |
|---|---|---|---|---|
| IA | 1 | 0 | 0 | 0,1 |
| IB | 2 | 0 | 0 | 0,1 |
| II | 1,2 | 1,2 | 0 | 0,1 |
| IIB | 3 | 0-2 | 0 | 0,1 |
| III | 4 | 0-2 | 0 | 0,1 |
| IIIA | 4 | 0-2 | 0 | 0 |
| IIIB | 4 | 0-2 | 0 | 1 |
| IVA1 | 1-4 | 0-2 | 0 | 2 |
| IVA2 | 1-4 | 3 | 0 | 0-2 |
| IVB | 1-4 | 0-3 | 1 | 0-2 |
| . | T . | N . | M . | B . |
|---|---|---|---|---|
| IA | 1 | 0 | 0 | 0,1 |
| IB | 2 | 0 | 0 | 0,1 |
| II | 1,2 | 1,2 | 0 | 0,1 |
| IIB | 3 | 0-2 | 0 | 0,1 |
| III | 4 | 0-2 | 0 | 0,1 |
| IIIA | 4 | 0-2 | 0 | 0 |
| IIIB | 4 | 0-2 | 0 | 1 |
| IVA1 | 1-4 | 0-2 | 0 | 2 |
| IVA2 | 1-4 | 3 | 0 | 0-2 |
| IVB | 1-4 | 0-3 | 1 | 0-2 |
What has not been dealt with adequately in this updated staging is the continued classification of tumor-stage MF at a stage below that of erythroderma with the data from several centers now demonstrating that the survival curves for T3 patients are similar24,25,29 or even worse than T4 patients53,52,61,84,119,120 However, it remains unclear to what degree other important prognostic factors such as lymph node or blood involvement or large-cell transformation are influencing these observations. Ideally, comparison of the survival curves of patients with T3 and T4 skin ratings who have comparable N and now B ratings should be undertaken before concluding that the hierarchy of T3 and T4 should be modified. It is also possible that erythrodermic patients with coexisting tumors may have this important prognostic variable lost in the final staging because only the highest skin T rating is used for staging purposes. For these reasons, the ISCL/EORTC have elected to retain the existing staging parameters until additional information is available.
Use of the term “stage”
In oncology, the stage assigned to a patient with malignancy at the initial diagnosis and workup is the primary prognostic indicator, and although the condition may go into complete or partial remission, relapse, or progress, the “clinical stage” does not change thereafter.121 In keeping with this tradition, the formal stage of a patient with MF/SS refers to the overall tumor status at initial diagnosis. However, as with other malignancies, changes in the tumor burden in patients with MF/SS often occur during the course of disease, which affects treatment choices and response to treatment. Moreover, it is important to have a means to indicate both the maximum and the current disease status at the time of enrollment into clinical trials. Therefore, the ISCL/EORTC recommends that, in addition to “clinical stage” at diagnosis of MF/SS patients, that TNMB ratings without the corresponding stage be used to indicate the maximum tumor burden and the current tumor burden. These distinctions will provide a means of communicating the initial, maximum, and current level and type of tumor burden for an individual patient.
Conclusions
The ISCL/EORTC recommended revisions to the MFCG classification and staging system for CTCL are made both to incorporate advances since 1979 related to tumor-cell biology and diagnostic techniques as pertains to MF and SS and to clarify certain variables that currently impede effective interinstitution and interinvestigator communication and/or the development of standardized clinical trials in MF and SS. The ISCL/EORTC recognizes that although this revision to the staging and classification of MF and SS further narrows and defines the variables involved, it does not provide a finite staging system that inherently incorporates all potential prognostic factors. There are several current factors, primarily related either to histopathology of lesions (folliculotropic MF, large-cell transformation, patch vs plaque disease) or TCR gene rearrangement in tissue and/or blood, that require further data on their prognostic importance before making formal revisions to the staging system of MF and SS related to them. The revisions to the classification outlined here provide a framework on which to gather data and facilitate validation efforts regarding these variables, all of which can be addressed in an optional fashion for any given MF or SS patient without affecting his/her overall staging. As additional clinical, genetic, or molecular information becomes available, it is anticipated that there will be further revisions to the classification and staging guidelines for MF and SS.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.O. wrote the draft of the paper, participated in paper preparation, and was an active participant in the meetings where revisions to the staging and classification were generated; E.V., N.P., R.W., Y.K., R.K., H.Z., M.D., T.E., S.L., G.W., R.D., A.R., P.H., M.P., M.-G.B., and S.W. participated in paper preparation and were active participants in the meetings where revisions to the staging and classification were generated; G.B. and W.S. participated in paper preparation; L.L. and F.T. were active participants in the meetings where revisions to the staging and classification were generated.
Complete lists of the active members of the International Society for Cutaneous Lymphomas (Document S1) and the European Organization of Research and Treatment of Cancer (Document S2) are available on the Blood website; see the Supplemental Appendices link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elise A. Olsen, Box 3294 DUMC, Durham, NC 27516; e-mail: olsen001@mc.duke.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal