Abstract
To define prognostic impact of Epstein-Barr virus (EBV) infection in diffuse large B-cell lymphoma (DLBCL), we investigated EBV status in patients with DLBCL. In all, 380 slides from paraffin-embedded tissue were available for analysis by EBV-encoded RNA-1 (EBER) in situ hybridization, and 34 cases (9.0%) were identified as EBER-positive. EBER positivity was significantly associated with age greater than 60 years (P = .005), more advanced stage (P < .001), more than one extranodal involvement (P = .009), higher International Prognostic Index (IPI) risk group (P = .015), presence of B symptom (P = .004), and poorer outcome to initial treatment (P = .006). The EBER+ patients with DLBCL demonstrated substantially poorer overall survival (EBER+ vs EBER− 35.8 months [95% confidence interval (CI), 0-114.1 months] vs not reached, P = .026) and progression-free survival (EBER+ vs EBER− 12.8 months [95% CI, 0-31.8 months] vs 35.8 months [95% CI, 0-114.1 months], respectively (P = .018). In nongerminal center B-cell–like subtype, EBER in situ hybridization positivity retained its statistical significance at the multivariate level (P = .045). Nongerminal center B-cell–like patients with DLBCL with EBER positivity showed substantially poorer overall survival with 2.9-fold (95% CI, 1.1-8.1) risk for death. Taken together, DLBCL patients with EBER in situ hybridization+ pursued more rapidly deteriorating clinical course with poorer treatment response, survival, and progression-free survival.
Introduction
Epstein-Barr virus (EBV) preferentially infects B lymphocytes by binding the major viral envelope glycoprotein gp350 to the CD21 receptor on the surface of B cells1 and a second glycoprotein, gp42, to human leukocyte antigen class II molecules.2 Moreover, EBV has the unique ability to transform resting B cells into permanent, latently infected lymphoblastoid cell lines.3,4 In immunocompetent hosts, EBV infection has been implicated in several lymphoid malignancies, including Burkitt lymphoma,5 extranodal natural killer (NK)–T-cell lymphomas,6 aggressive NK leukemia/lymphoma,7 lymphomatoid granulomatosis,7 angioimmunoblastic T-cell lymphoma,8 and a proportion of Hodgkin lymphoma.9 The prognostic significance of EBV infection in lymphoid malignancies has not been established, however. A recent population-based study on 437 classic Hodgkin lymphoma cases showed that EBV positivity was an independent adverse factor for survival.10 Yet another study reported that EBV positivity was associated with better survival in young patients and poorer survival in older patients with nodular sclerosis type, but not in other subtypes.11 In support of these findings, our recent study also demonstrated a varying impact of EBV infection on survival by different age groups.12
Diffuse large B-cell lymphoma (DLBCL) is the most common type of nonHodgkin lymphoma accounting for 30% to 40% of new lymphoma cases,13 and only 40% to 50% of patients achieve durable remissions. Hence, an acquisition of prognostic parameters at initial diagnosis may contribute to implementation of risk-based stratification of therapy in these patients and may facilitate identification of those who may benefit from early intensive therapy. It was not until recently that DLBCL was recognized as a clinically and morphologically heterogeneous disease entity with the advent of cDNA microarray.14 Using a cDNA microarray and immunohistochemical studies of CD10, bcl-6, or MUM-1, DLBCL can be reclassified into prognostically distinct subgroups with germinal center B-cell–like (GCB), and non-GCB phenotypes.15,16
The negative impact of EBV positivity on treatment outcome has been previously observed in peripheral T-cell lymphoma, unspecified,17,18 senile B cell lymphomas,19,20 and DLBCL,21 although the small number of cases makes conclusive attribution difficult. Interestingly, one group observed that 50% of the gastric patients with DLBCL, who did not initially respond or relapsed after chemoradiotherapy, were EBV-positive, suggesting that EBV status might have a role as a predictive factor for resistance to treatment.22 Nevertheless, an independent and systematic approach on the incidence of EBV infection and the impact of EBV status on treatment outcome has not been undertaken in DLBCL. To define the prognostic impact of EBV infection in DLBCL, we retrospectively investigated 380 patients with DLBCL in whom biopsy specimens were available for further pathologic examination.
Patients, materials, and methods
Patients
The criteria for case inclusion were as follows: (1) pathologically confirmed diagnosis of DLBCL, according to the World Health Organization classification23 ; (2) complete set of clinical data; (3) adequate paraffin-embedded biopsy specimen or unstained slides for EBV-encoded RNA-1 (EBER-1) in situ hybridization; and (4) age ≥ 18 years. A complete set of clinical information in this analysis included the following: patient demographics, type of treatment, treatment outcome, and vital status. This study was approved by the Institutional Review Board at Samsung Medical Center, Seoul, Korea, in accordance with the Declaration of Helsinki.
Histology
All pathologic specimens were reviewed by one pathologist with expertise and reclassified in accordance with the World Health Organization criteria for pathologic diagnosis. Immunohistochemical analysis was performed on paraffin sections using monoclonal and polyclonal antibodies for the detection of lineage-specific or lineage-characteristic antigens. Cases of any confirmed follicular architecture or transformed lymphoma were excluded from this study. Immunophenotyping was performed using a panel of monoclonal antibodies, including antibodies against CD10 (Dakopatts, Copenhagen, Denmark), bcl-6 (Dakopatts), and MUM-1 (Dakopatts). Using these markers, DLBCL was categorized into 2 subgroups, GCB and non-GCB type, using the algorithm proposed by Hans et al (n = 296).15
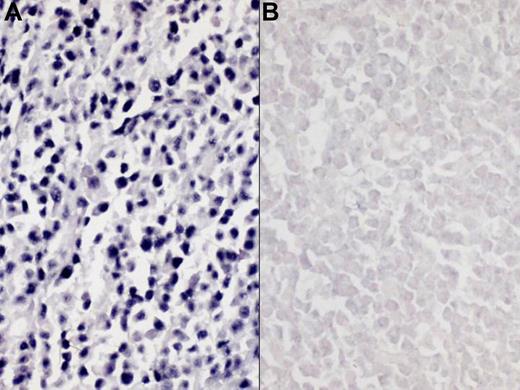
EBV RNA was detected by an in situ hybridization (ISH) technique. The paraffin-embedded sections (5 μm) were dewaxed with xylene followed by treatment with proteinases K and hybridized with fluorescein isothiocyanate conjugated EBER-1 and -2 oligonucleotide probes (Novocastra, Newcastle upon Tyne, United Kingdom). After incubation with antifluorescein isothiocyanate–conjugated antibody tagged with alkaline phosphatase, slides were covered with nitrobluetetrazolium, 5-bromo-4-chloro-3-indolyl phosphates, and 1 M levamisole. We used EBV-negative lymphoid tissues and the hybridization mixture without EBV oligonucleotides as negative controls. To identify cases with strong pathogenic association with EBV, a positive reaction was defined as more than 20% nuclear positivity of examined cells (Figure 1).
Representative results of Epstein-Barr virus–encoded RNA-1 in situ hybridization (EBER ISH). (A) positive EBER ISH; (B) negative EBER ISH. Images were captured using a Polaroid DMC2 digital microscope camera (Polaroid, Tokyo, Japan) and processed using Adobe Photoshop 7.0 (Adobe Systems, Seattle, WA). Original magnification, ×200.
Representative results of Epstein-Barr virus–encoded RNA-1 in situ hybridization (EBER ISH). (A) positive EBER ISH; (B) negative EBER ISH. Images were captured using a Polaroid DMC2 digital microscope camera (Polaroid, Tokyo, Japan) and processed using Adobe Photoshop 7.0 (Adobe Systems, Seattle, WA). Original magnification, ×200.
Treatment
Patients received one of the following initial treatment modalities: (1) an anthracycline-containing chemotherapeutic regimen; (2) a nonanthracycline-containing chemotherapeutic regimen; and (3) supportive care only. The anthracycline-based regimens used were as follows: cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP; n = 216); rituximab-CHOP (n = 63); doxorubicin, bleomycin, vinblastine, dacarbazine (n = 1); ProMACE-CytaBOM (prednisolone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, methotrexate, and leucovorin, n = 1); and others (n = 6). The nonanthracycline-containing regimens used were methotrexate/cytarabine (n = 37); ifosfamide, methotrexate, etoposide (n = 3); cyclophosphamide, vincristine, prednisone (n = 3); and others (n = 2). Forty-eight patients received best supportive care only. The treatment response was assessed according to standard response criteria.24
Statistical analysis
Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier product-limit method. Overall survival was calculated from the date of diagnosis to the date of death from any cause or the last follow up. PFS was measured from the date of diagnosis to the date of the first documented progression, death, or the last follow-up visit. Survival rates were compared for statistical differences by using log-rank analysis. Continuous biologic variables were dichotomized. A backward stepwise Cox regression analysis was performed to delineate prognostic factors at multivariate level and all hazard ratios (HRs) were adjusted for age. P values less than .05 were considered statistically significant and all P values correspond to 2-sided significance tests.
Results
Patient characteristics and Epstein-Barr virus–encoded RNA-1 in situ hybridization results
A total of 380 cases, which were pathologically diagnosed with DLBCL between September 1994 and December 2005, were included in this analysis. All patients were Korean, immunocompetent, and negative for antihuman immunodeficiency virus antibody. Baseline characteristics are provided in Table 1. In all, 380 slides from paraffin-embedded tissue were available for EBV analysis by EBER ISH, and 34 (9.0%) cases were identified as EBER-positive. EBER positivity was significantly associated with age (> 60 years, P = .005), more advanced stage (P < .001), more than one extranodal involvement (P = .009), higher International Prognostic Index (IPI) risk group (P = .015), the presence of B symptom (P = .004), and poorer response to initial treatment (P = .006). There were no significant differences in distribution of primary treatment modalities between the EBER+ and EBER− groups (P = .825). Moreover, proportions of GCB were similar between the 2 groups (EBER+ vs EBER−; 28.6% vs 34.4%, P = .253). Of the 232 (95.4%) patients in whom the treatment responses were available (EBER−, n = 207; EBER+, n = 25), 209 responded to the initial therapy (overall response rate, 90.1%). The overall response rate to initial treatment was significantly lower in the EBER+ patients with DLBCL (19 of 25 [72.0%]) compared with that in the EBER− patients with DLBCL (191 of 207 [92.3%], P = .006). The distributions of primary lesions were similar between the 2 groups (data not shown) except for a low incidence of gastrointestinal involvement in the EBER+ group (8.8% vs 25.2%, P = .026).
Baseline characteristics of patients with diffuse large B-cell lymphoma according to EBV-encoded RNA-1 (EBER) status
| Characteristics and parameters . | All patients . | EBER+ . | EBER− . | P . |
|---|---|---|---|---|
| Total cases, no. (%) | 380 (100) | 34 (9.0) | 346 (91.0) | |
| Median age, y (range) | 56 (18–95) | 65 (20–95) | 56 (18–89) | .009 |
| Age, no. (%) | ||||
| 60 y or less | 241 (63.4) | 14 (41.2) | 227 (65.6) | .005 |
| Older than 60 y | 139 (36.6) | 20 (58.8) | 119 (34.4) | |
| Sex, no. (%) | ||||
| Male | 221 (58.2) | 18 (52.9) | 203 (58.7) | .518 |
| Female | 159 (41.8) | 16 (47.1) | 143 (41.3) | |
| Performance status, no. (%) | ||||
| ECOG 0–1* | 293 (80.1) | 24 (70.6) | 269 (81.0) | .147 |
| ECOG 2–4 | 73 (19.9) | 10 (29.4) | 63 (19.0) | |
| Initial presentation, no. (%) | ||||
| Nodal | 96 (25.3) | 9 (26.5) | 87 (26.1) | .339 |
| Extranodal | 100 (26.3) | 6 (17.6) | 94 (28.2) | |
| Nodal and extranodal | 171 (45.0) | 19 (55.9) | 152 (45.6) | |
| Ann Arbor stage, no. (%) | ||||
| Limited, I-II | 254 (66.8) | 14 (42.4) | 240 (72.1) | <.001 |
| Advanced, III-IV | 112 (29.5) | 19 (57.6) | 93 (27.9) | |
| No. of extranodal involvement, no. (%) | ||||
| 0 or 1 | 312 (82.1) | 23 (67.6) | 289 (83.5) | .009 |
| 2 or more | 58 (15.3) | 11 (32.4) | 47 (13.6) | |
| Lactic dehydrogenase, no. (%) | ||||
| Upper limit of normal or below | 203 (56.2) | 20 (60.6) | 183 (55.8) | .071 |
| Over the upper limit of normal | 158 (43.8) | 13 (39.4) | 145 (44.2) | |
| International Prognostic Index risk groups, no. (%) | ||||
| Low/low intermediate | 279 (73.4) | 20 (62.5) | 259 (79.9) | .015 |
| High intermediate/high | 77 (20.3) | 12 (37.5) | 65 (20.1) | |
| B symptom, no. (%) | ||||
| Positive | 74 (20.4) | 13 (39.4) | 61 (18.5) | .004 |
| Negative | 289 (79.6) | 20 (60.6) | 269 (81.5) | |
| Bone marrow involvement, no. (%) | ||||
| Positive | 74 (20.4) | 13 (39.4) | 61 (18.5) | .104 |
| Negative | 289 (79.6) | 20 (60.6) | 269 (81.5) | |
| Primary treatment, no. (%) | ||||
| Anthracycline-based chemotherapy with or without radiotherapy | 224 (58.9) | 25 (73.5) | 199 (57.5) | |
| Rituximab with cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy with or without radiotherapy | 63 (16.6) | 2 (5.9) | 61 (17.6) | .825* |
| Nonanthracycline-based chemotherapy | 45 (11.8) | 3 (8.8) | 42 (12.1) | |
| Best supportive care only | 48 (12.6) | 4 (11.8) | 44 (12.7) | |
| Response to front-line, no. (%) | ||||
| Chemotherapy, n = 232† | ||||
| Complete remission or partial remission | 209 (90.1) | 18 (72.0) | 191 (92.3) | .006 |
| Stable disease or progressive disease | 23 (9.9) | 7 (28.0) | 16 (7.7) | |
| Histologic subtype, no. (%); n = 296 | ||||
| Germinal center B cell–like | 125 (42.2) | 6 (28.6) | 119 (34.4) | .253 |
| Nongerminal center B cell–like | 171 (57.8) | 15 (71.4) | 156 (45.1) |
| Characteristics and parameters . | All patients . | EBER+ . | EBER− . | P . |
|---|---|---|---|---|
| Total cases, no. (%) | 380 (100) | 34 (9.0) | 346 (91.0) | |
| Median age, y (range) | 56 (18–95) | 65 (20–95) | 56 (18–89) | .009 |
| Age, no. (%) | ||||
| 60 y or less | 241 (63.4) | 14 (41.2) | 227 (65.6) | .005 |
| Older than 60 y | 139 (36.6) | 20 (58.8) | 119 (34.4) | |
| Sex, no. (%) | ||||
| Male | 221 (58.2) | 18 (52.9) | 203 (58.7) | .518 |
| Female | 159 (41.8) | 16 (47.1) | 143 (41.3) | |
| Performance status, no. (%) | ||||
| ECOG 0–1* | 293 (80.1) | 24 (70.6) | 269 (81.0) | .147 |
| ECOG 2–4 | 73 (19.9) | 10 (29.4) | 63 (19.0) | |
| Initial presentation, no. (%) | ||||
| Nodal | 96 (25.3) | 9 (26.5) | 87 (26.1) | .339 |
| Extranodal | 100 (26.3) | 6 (17.6) | 94 (28.2) | |
| Nodal and extranodal | 171 (45.0) | 19 (55.9) | 152 (45.6) | |
| Ann Arbor stage, no. (%) | ||||
| Limited, I-II | 254 (66.8) | 14 (42.4) | 240 (72.1) | <.001 |
| Advanced, III-IV | 112 (29.5) | 19 (57.6) | 93 (27.9) | |
| No. of extranodal involvement, no. (%) | ||||
| 0 or 1 | 312 (82.1) | 23 (67.6) | 289 (83.5) | .009 |
| 2 or more | 58 (15.3) | 11 (32.4) | 47 (13.6) | |
| Lactic dehydrogenase, no. (%) | ||||
| Upper limit of normal or below | 203 (56.2) | 20 (60.6) | 183 (55.8) | .071 |
| Over the upper limit of normal | 158 (43.8) | 13 (39.4) | 145 (44.2) | |
| International Prognostic Index risk groups, no. (%) | ||||
| Low/low intermediate | 279 (73.4) | 20 (62.5) | 259 (79.9) | .015 |
| High intermediate/high | 77 (20.3) | 12 (37.5) | 65 (20.1) | |
| B symptom, no. (%) | ||||
| Positive | 74 (20.4) | 13 (39.4) | 61 (18.5) | .004 |
| Negative | 289 (79.6) | 20 (60.6) | 269 (81.5) | |
| Bone marrow involvement, no. (%) | ||||
| Positive | 74 (20.4) | 13 (39.4) | 61 (18.5) | .104 |
| Negative | 289 (79.6) | 20 (60.6) | 269 (81.5) | |
| Primary treatment, no. (%) | ||||
| Anthracycline-based chemotherapy with or without radiotherapy | 224 (58.9) | 25 (73.5) | 199 (57.5) | |
| Rituximab with cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy with or without radiotherapy | 63 (16.6) | 2 (5.9) | 61 (17.6) | .825* |
| Nonanthracycline-based chemotherapy | 45 (11.8) | 3 (8.8) | 42 (12.1) | |
| Best supportive care only | 48 (12.6) | 4 (11.8) | 44 (12.7) | |
| Response to front-line, no. (%) | ||||
| Chemotherapy, n = 232† | ||||
| Complete remission or partial remission | 209 (90.1) | 18 (72.0) | 191 (92.3) | .006 |
| Stable disease or progressive disease | 23 (9.9) | 7 (28.0) | 16 (7.7) | |
| Histologic subtype, no. (%); n = 296 | ||||
| Germinal center B cell–like | 125 (42.2) | 6 (28.6) | 119 (34.4) | .253 |
| Nongerminal center B cell–like | 171 (57.8) | 15 (71.4) | 156 (45.1) |
Data were missing as follows: performance status (n = 14), initial disease presentation (n = 13), Ann Arbor stage (n = 14), lactic dehydrogenase level (n = 19), no. of extranodal involvement (n = 10), International Prognostic Index score (n = 24), B symptoms (n = 17), bone marrow involvement (n = 15), and histologic subtype (n = 84).
ECOG indicates Eastern Cooperative Oncology Group.
Two hundred forty-three patients received chemotherapy as a front-line treatment and 232 patients were assessable for response.
Impact of Epstein-Barr virus–encoded RNA-1 in situ hybridization positivity on survival
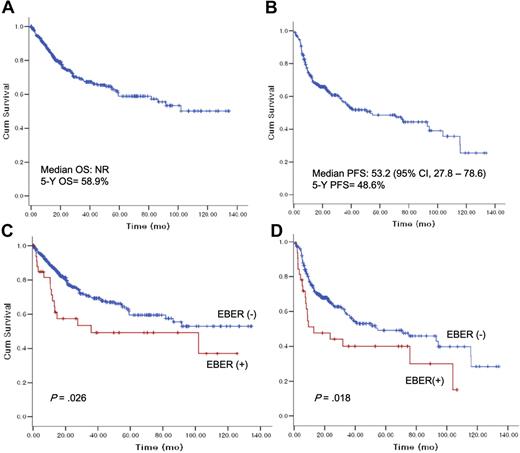
After a median follow-up duration of 40.5 months (range, 1-165.7 months), the 5-year OS and 5-year PFS rates were 58.9% and 48.6%, respectively (Figure 2). The EBER+ patients with DLBCL demonstrated substantially poorer OS (EBER+ vs EBER−; 35.8 months [95% confidence interval {CI}, 0-114.1 months] vs median OS not reached, respectively, P = .026) and PFS (EBER+ vs EBER−; 12.8 [95% CI, 0-31.8 months] vs 35.8 months [95% CI, 0-114.1 months], P = .018).
Overall survival and progression-free survival according to Epstein-Barr virus–encoded RNA-1 status.
Overall survival and progression-free survival according to Epstein-Barr virus–encoded RNA-1 status.
Prognostic significance of Epstein-Barr virus–encoded RNA-1 in situ hybridization positivity in subgroup analyses
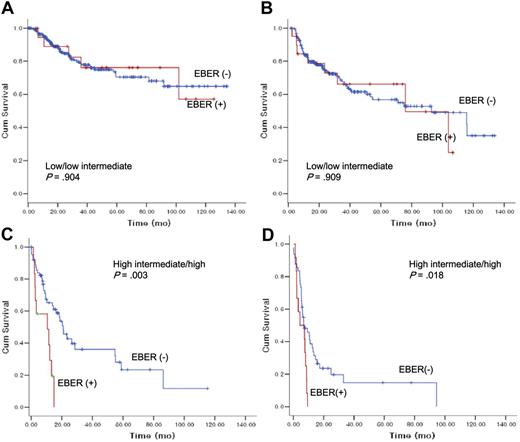
We further performed subgroup analyses according to IPI risk groups (low/low intermediate risk vs high intermediate/high risk), age groups (18-50 years vs 51+), and GCB vs non-GCB (Figures 3,4). In low- to low-intermediate risk groups, there was no significant difference in survival (Figure 3A) or progression-free survival according to EBER status (Figure 3B). In high-intermediate to high-risk groups, however, EBER+ patients with DLBCL pursued a more rapidly deteriorating clinical course (median OS, 10.5 months [95% CI, 0.0–22.7]) compared with EBER− patients with DLBCL (median OS, 20.8 months [95% CI, 15.6-26.2]) in terms of survival with statistical significance (P = .003) (Figure 3C).
The impact of Epstein-Barr virus–encoded RNA-1 status on overall survival and progression-free survival according to International Prognostic Index risk groups.
The impact of Epstein-Barr virus–encoded RNA-1 status on overall survival and progression-free survival according to International Prognostic Index risk groups.
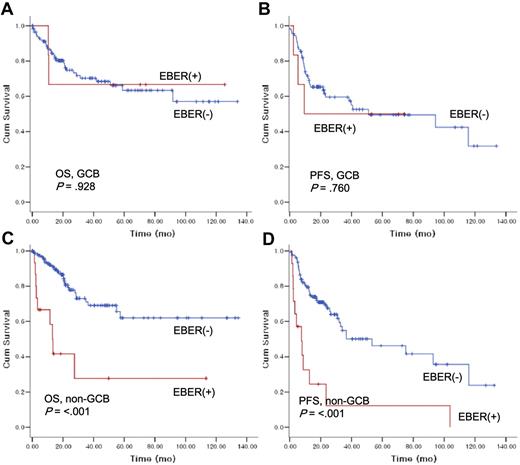
The impact of Epstein-Barr virus–encoded RNA-1 status on overall survival and progression-free survival according to histologic subtypes.
The impact of Epstein-Barr virus–encoded RNA-1 status on overall survival and progression-free survival according to histologic subtypes.
Next, the prognostic value of EBER ISH positivity was evaluated in subgroup analysis according to age groups (data not shown). EBER positivity did not markedly influence OS or PFS in patients younger than 50 years of age; however, there was a trend toward poorer OS and PFS for EBER+ in patients older than 50 years. In non-GCB patients with DLBCL, EBER positivity had an adverse impact on OS (EBER+ vs EBER−, 13.3 months [95% CI, 10.0-16.5] vs median OS not reached; P > .001) and PFS (7.6 months [95% CI, 1.6-13.7] vs 53.2 months [95% CI, 16.1-90.3]; P > .001) with statistical significance (Figure 4C,D). However, the EBER positivity did not influence survival or PFS in GCB DLBCL (Figure 4A,B).
Prognostic factor analyses
The clinical factors predicting poor survival at univariate analysis were as follows: EBER ISH positivity (P = .026), age greater than 60 years (P = .001), poor performance status (Eastern Cooperative Oncology Group 2-4, P<.001), advanced Ann Arbor stage (stage III/IV, P < .001), elevated lactic dehydrogenase level (P < .001), more than one extranodal involvement (P < .001), the presence of B symptom (P > .001), bone marrow involvement (P > .001), and no use of rituximab as part of primary treatment (P = .022) (Table 2). Clinical parameters that were included in the multivariate analysis were EBER ISH status, bone marrow involvement, B symptom, performance status (0-1 vs ≥ 2), age (≤60 vs <60), lactic dehydrogenase level (normal vs elevated), Ann Arbor stage, extranodal involvement (0-1 vs ≥2), histologic subtype (GCB vs non-GCB), and the use of rituximab. The backward conditional Cox regression model was used. Prognostic factors for survival in all patients were performance status (P > .001; HR, 3.4; 95% CI, 2.0-5.9), lactic dehydrogenase (P > .001; HR, 3.2; 95% CI, 1.8-5.5), number of extranodal sites (P > .001; HR, 2.9; 95% CI, 1.7-5.0), and age (P > .001; HR, 2.7; 95% CI, 1.6-5.1) (Table 3). In the non-GCB subtype, however, EBER ISH positivity retained its statistical significance at the multivariate level (P = .045). Non-GCB patients with DLBCL with EBER positivity showed substantially poorer OS with 2.9-fold (95% CI, 1.1-8.1) risk for death. In contrast, the EBER status did not affect survival in the GCB DLBCL with statistical significance (P = .091).
Univariate analysis of prognostic factors for survival in patients with diffuse large B-cell lymphoma
| Parameters . | Overall survival . | |
|---|---|---|
| Median, mo (95% CI) . | P . | |
| Epstein-Barr virus status | .026 | |
| EBER-positive | 35.8 (0-114.1) | |
| EBER-negative | NR | |
| Age, y | <.001 | |
| 60 y old or younger | NR | |
| Older than 60 y | 44.7 (28.5-60.9) | |
| Performance status | <.001 | |
| ECOG, 0 to 1 | NR | |
| ECOG, 2 to 4 | 20.9 (11.1-30.7) | |
| Ann Arbor stage | <.001 | |
| Limited (I/II) | NR | |
| Advanced (III/IV) | 28.5 (1.8-55.1) | |
| Lactic dehydrogenase | <.001 | |
| ULN or below | NR | |
| Over ULN | 58.8 (20.7-96.9) | |
| No. of extranodal involvement | <.001 | |
| 0 or 1 | NR | |
| 2 or more | 21.1 (12.0-29.1) | |
| B symptom | <.001 | |
| Positive | 54.7 (11.0-98.4) | |
| Negative | NR | |
| Bone marrow involvement | <.001 | |
| Positive | 20.4 (8.2-32.5) | |
| Negative | NR | |
| Histologic subtype | .990 | |
| Nongerminal center B cell–like | NR | |
| Germinal center B cell–like | NR | |
| Primary treatment | <.001 | |
| Anthracycline-based | NR | |
| Non-anthracycline-based | 44.7 (22.9-66.4) | |
| The use of rituximab | .022 | |
| Rituximab | NR | |
| No rituximab | 101.9 | |
| Parameters . | Overall survival . | |
|---|---|---|
| Median, mo (95% CI) . | P . | |
| Epstein-Barr virus status | .026 | |
| EBER-positive | 35.8 (0-114.1) | |
| EBER-negative | NR | |
| Age, y | <.001 | |
| 60 y old or younger | NR | |
| Older than 60 y | 44.7 (28.5-60.9) | |
| Performance status | <.001 | |
| ECOG, 0 to 1 | NR | |
| ECOG, 2 to 4 | 20.9 (11.1-30.7) | |
| Ann Arbor stage | <.001 | |
| Limited (I/II) | NR | |
| Advanced (III/IV) | 28.5 (1.8-55.1) | |
| Lactic dehydrogenase | <.001 | |
| ULN or below | NR | |
| Over ULN | 58.8 (20.7-96.9) | |
| No. of extranodal involvement | <.001 | |
| 0 or 1 | NR | |
| 2 or more | 21.1 (12.0-29.1) | |
| B symptom | <.001 | |
| Positive | 54.7 (11.0-98.4) | |
| Negative | NR | |
| Bone marrow involvement | <.001 | |
| Positive | 20.4 (8.2-32.5) | |
| Negative | NR | |
| Histologic subtype | .990 | |
| Nongerminal center B cell–like | NR | |
| Germinal center B cell–like | NR | |
| Primary treatment | <.001 | |
| Anthracycline-based | NR | |
| Non-anthracycline-based | 44.7 (22.9-66.4) | |
| The use of rituximab | .022 | |
| Rituximab | NR | |
| No rituximab | 101.9 | |
ULN indicates upper limit normal; NR, not reached.
Cox proportional hazards model hazard ratios for death from all causes in patients with diffuse large B-cell lymphoma
| Parameters . | All patients . | Non-GCB subtype . | GCB subtype . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| EBV status | .544 | .045 | .091 | |||
| EBER ISH − | — | 1.0 | — | |||
| EBER ISH + | — | 2.9 (1.1-8.1) | — | |||
| Performance status | <.001 | .003 | <.001 | |||
| ECOG, 0 to 1 | 1.0 | 1.0 | 18.1 (7.0-47.0) | |||
| ECOG, 2 to 4 | 3.4 (2.0-5.9) | 3.3 (1.5-7.0) | ||||
| Lactic dehydrogenase | <.001 | .006 | .154 | |||
| ULN or below | 1.00 | 1.0 | — | |||
| Over ULN | 3.2 (1.8-5.5) | 3.1 (1.3-7.3) | — | |||
| No. of extranodal sites | <.001 | .083 | .060 | |||
| 0 or 1 | 1.0 | — | — | |||
| 1 or more | 2.9 (1.7-5.0) | — | — | |||
| Age, years | <.001 | .059 | .198 | |||
| 60 years old or less | 1.0 | — | — | |||
| Over 60 years old | 2.7 (1.6-5.1) | — | — | |||
| B symptom | .246 | .006 | .474 | |||
| Absent | — | 1.0 | — | |||
| Present | — | 3.3 (1.5-7.0) | — | |||
| Ann Arbor stage | .161 | .085 | .020 | |||
| Stage I/II | — | — | 1.0 | |||
| Stage III/IV | — | — | 3.0 (1.2-7.5) | |||
| Parameters . | All patients . | Non-GCB subtype . | GCB subtype . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| EBV status | .544 | .045 | .091 | |||
| EBER ISH − | — | 1.0 | — | |||
| EBER ISH + | — | 2.9 (1.1-8.1) | — | |||
| Performance status | <.001 | .003 | <.001 | |||
| ECOG, 0 to 1 | 1.0 | 1.0 | 18.1 (7.0-47.0) | |||
| ECOG, 2 to 4 | 3.4 (2.0-5.9) | 3.3 (1.5-7.0) | ||||
| Lactic dehydrogenase | <.001 | .006 | .154 | |||
| ULN or below | 1.00 | 1.0 | — | |||
| Over ULN | 3.2 (1.8-5.5) | 3.1 (1.3-7.3) | — | |||
| No. of extranodal sites | <.001 | .083 | .060 | |||
| 0 or 1 | 1.0 | — | — | |||
| 1 or more | 2.9 (1.7-5.0) | — | — | |||
| Age, years | <.001 | .059 | .198 | |||
| 60 years old or less | 1.0 | — | — | |||
| Over 60 years old | 2.7 (1.6-5.1) | — | — | |||
| B symptom | .246 | .006 | .474 | |||
| Absent | — | 1.0 | — | |||
| Present | — | 3.3 (1.5-7.0) | — | |||
| Ann Arbor stage | .161 | .085 | .020 | |||
| Stage I/II | — | — | 1.0 | |||
| Stage III/IV | — | — | 3.0 (1.2-7.5) | |||
— indicates not applicable; HR, hazard ratio.
Discussion
To the best of our knowledge, this study represents the largest one to evaluate the significance of EBV positivity on treatment outcome and survival of patients with DLBCL. Only a few other studies have speculated a negative correlation between EBV status and prognosis in DLBCL based on a limited number of cases.19-22 To elucidate the clinical implication of detecting EBV status by EBER-1 ISH in patients with DLBCL, we systematically performed EBER-1 ISH in 380 tumor specimens of DLBCL. The incidence of EBER+ DLBCL was 9% (34 of 380) in this series, which is comparable with those reported in other studies (8% to 11%). In agreement with previous studies, the EBER+ patients with DLBCL were more likely to be diagnosed older than 60 years, at Ann Arbor stage III/IV, and to present with more than one extranodal involvement and high-intermediate/high risk according to IPI.
Despite a similar distribution of treatment modalities between EBER+ and EBER− groups (anthracycline-based chemotherapy; 76% vs 79%, respectively), EBER+ DLBCL showed substantially poorer response to front-line chemotherapy compared with EBER− DLBCL (72.0% vs 92.3%, P = .006). It is generally known that most EBV-associated tumors respond poorly to intensive chemotherapy regimens or have a significant relapse rate. Moreover, a small Japanese study investigated the role of EBV infection in primary refractory gastric DLBCL to CHOP chemotherapy and observed that 50% (4 of 8) of refractory patients were EBV-positive.22 Attributable to the retrospective nature of this study, the stratification of therapy according to EBV status in DLBCL may not be firmly established.
Univariate analysis of survival indicated that EBER+ patients had a significantly worse OS and PFS compared with EBER− patients. In subgroup analyses, the EBV status adversely influenced only patients with DLBCL with high-intermediate/high IPI risk groups or non-GCB subtype, but not those with low/low-intermediate risk groups or GCB subtype. Furthermore, only in the cohort of patients with non-GCB subtype was this impact still retained in multivariate analysis of survival (HR, 2.9; 95% CI, 1.1-8.9 P = .045). These findings may have clinical implications that are noteworthy. The IPI score is a well-known prognostic index, which effectively separates DLBCL into 4 risk groups.25,26 Nevertheless, patients in each risk group may not pursue a consistent clinical course with a uniform response to treatment. In addition, a recently developed subclassification of DLBCL into GCB and non-GCB phenotypes has shown distinct prognostication.14-16 Yet, the EBV status further categorized non-GCB subtypes into 2 groups with considerably different prognosis (Table 3). A small study reported a substantial correlation between EBV positivity and non-GCB phenotype in HIV-positive patients with DLBCL.27 Taken together, EBER ISH status may be a useful tool in further identifying high-risk patients who may benefit from early intensive treatment such as hematopoietic stem cell transplantation in addition to IPI and immunohistochemical studies. An issue of whether the EBV-associated DLBCL represents an independent disease entity remains unanswered and needs to be clarified in future studies. In addition, because the number of EBER ISH+ patients was only 29 in our series, the impact of the EBER status in DLBCL should be validated in a larger number of patients.
Recently, Kwong et al demonstrated that the level of circulating EBV DNA is correlated with stage and survival, reflecting the tumor load, leading to a conclusion that plasma EBV DNA can be used as a tumor biomarker to monitor treatment response in EBV-positive NK/T-cell lymphoma.28 They hypothesized that the increase of EBV DNA was attributable to tumor release of EBV fragments rather than reactivation of latent EBV infection because patients with NK/T-cell lymphoma are immunocompetent. Thus, the relationship between the quantification of EBV viral load in blood and EBER ISH in tumor specimens should be confirmed in DLBCL.
The role of EBV infection in pathogenesis of DLBCL is not known. EBV can infect resting B lymphocytes efficiently and drive it out of the resting state to become an activated lymphoblastoid cell lines.3,4,29 These transforming effects are associated with the restricted expression of the EBV-encoded latent genes such as latent infection membrane protein 1.30 Latent infection membrane protein 1 is an integral membrane protein that up-regulates antiapoptotic proteins Bcl-231 and functions as a constitutively activated member of the tumor necrosis factor receptor superfamily activating several signaling pathways,32 including nuclear factor kappa-B transcription factor pathway,33 MAP kinase cascade,34 and the phosphatidylinositol 3-kinase/Akt pathway.35 Constitutively activated proteins in these pathways may contribute to the clinical characteristics of EBV-positive tumors. Thus, correlative analyses with EBER ISH and key proteins of these cascades may be interesting to investigate to further clarify the pathogenic role of EBV in DLBCL.
Based on our data, patients with DLBCL who are EBER ISH+ pursue a more rapidly deteriorating clinical course with poorer treatment response, survival, and PFS. Thus, more effective treatment should be adopted in this particular subset of patients with possible addition of EBV-targeted therapy to conventional chemotherapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.P. collected and analyzed data; J.L. analyzed data, wrote and revised the manuscript; Y.H.K. designed the research and performed pathologic examinations; A.H., H.J.J., S.C.L., and I.G.H. performed data collection; J.S.A., C.W.J., K.K., Y.C.A., W.K.K., and K.P. performed patient provision; and W.S.K. designed the research, analyzed the data, and approved the final manuscript.
S.P. and J.L. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Won Seog Kim, Division of Hematology–Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Ilwon-dong Kangnam-Gu, Seoul, Korea, 135-710; e-mail: wskimsmc@smc.samsung.co.kr; or Young Hyeh Ko, Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Ilwon-dong Kangnam-Gu, Seoul, Korea, 135–710; e-mail: yhko@smc.samsung.co.kr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal