Abstract
Tissue regeneration involves the formation of new blood vessels regulated by angiogenic factors. We reported recently that the expression of the angiogenic factor CCN1 is up-regulated under various pathophysiologic conditions within the cardiovascular system. Because CD34+ progenitor cells participate in cardiovascular tissue regeneration, we investigated whether CCN1—detected for the first time in human plasma—promotes the recruitment of CD34+ progenitor cells to endothelial cells, thereby enhancing endothelial proliferation and neovascularization. In this study, we demonstrated that CCN1 and supernatants from CCN1-stimulated human CD34+ progenitor cells promoted proliferation of endothelial cells and angiogenesis in vitro and in vivo. In addition, CCN1 induced migration and transendothelial migration of CD34+ cells and the release of multiple growth factors, chemokines, and matrix metalloproteinase-9 (MMP-9) from these cells. Moreover, the CCN1-specific integrins αMβ2 and αVβ3 are expressed on CD34+ cells and CCN1 stimulated integrin-dependent signaling. Furthermore, integrin antagonists (RGD-peptides) suppressed both binding of CCN1 to CD34+ cells and CCN1-induced adhesion of CD34+ cells to endothelial cells. These data suggest that CCN1 promotes integrin-dependent recruitment of CD34+ progenitor cells to endothelial cells, which may contribute to paracrine effects on angiogenesis and tissue regeneration.
Introduction
Bone marrow–derived and circulating hematopoietic progenitor cells have been shown to contribute to cardiovascular tissue regeneration.1,2 CD34+ hematopoietic progenitor cells were widely and successfully used in different transplantation models, including myocardial regeneration.3 A subset of circulating CD34+ cells—expressing markers such as CD133 and vascular endothelial growth factor (VEGF) receptor-2 (flk1)—are endothelial progenitor cells (EPCs) with the ability to differentiate into mature endothelial cells, thus contributing to re-endothelialization and neovascularization.4 In contrast, the ability of hematopoietic cells to transdifferentiate into functional cardiomyocytes is controversial.5-8 Moreover, enhanced angiogenesis in response to progenitor cells may be influenced mainly by the release of growth factors rather than by cellular differentiation.9
CCN1 (formerly known as CYR61) is a cysteine-rich heparin-binding protein that is encoded by an immediate early gene and belongs to the novel CCN gene family (connective tissue growth factor [CTGF], cysteine-rich angiogenic protein 61 [CYR61], and nephroblastoma overexpressed [Nov]).10 The expression of CCN1 is rapidly induced in response to growth factors.11 CCN1 is expressed by all types of vascular cells, is associated with the extracellular matrix (ECM) and mediates cell adhesion, migration, proliferation and neovascularization through cell type-specific binding mainly to α6β1, αMβ2, and αVβ3 integrins.10,12-14 It is noteworthy that CCN1-deficient mice suffer embryonic death as a result of placental vascular insufficiency and compromised vessel integrity because of impaired vessel bifurcations and impaired VEGF-C expression, establishing CCN1 as a novel and essential regulator of vascular development.15 In addition, it has been shown recently that CCN1 is critically involved in cardiac development.16 Besides its ability to promote tumor growth,17 we recently reported that enhanced CCN1 expression is associated with several cardiovascular pathophysiologic settings, such as atherosclerotic plaque formation,18 enhanced vascular mechanical stretch,19 cardiac pressure overload, and ischemic cardiomyopathy.20
Recent evidence suggested that CCN1 expression is induced in various cardiovascular disorders, suggesting a role for CCN1 in cellular and tissue self-renewal, potentially involving circulating progenitor cells. Because the impact of CCN1 on progenitor cells is largely unknown, we used circulating human bone marrow-derived CD34+ cells to study CCN1 effects on migration, release of growth factors, endothelial proliferation and neovascularization, which are important for cardiovascular regeneration.
Materials and methods
Reagents
All chemicals were obtained from Sigma (Taufkirchen, Germany) unless otherwise specified. All cell culture plates were from TTP (Trasadingen, Switzerland). RGD peptides (Arg-Gly-Asp) and GRGDSP peptides (Gly-Arg-Gly-Asp-Ser-Pro) were from Bachem (Weil am Rhein, Germany). Human recombinant fibronectin was obtained from Harbor Bio-Products (Norwood, MA) and 5-carboxytetramethylrhodamine (5-TAMRA) was purchased from Invitrogen (Karlsruhe, Germany). Recombinant CCN1 (Abnova Corporation, Taipei, Taiwan) was obtained as a glutathione transferase (GST) fusion protein. GST protein was removed using PreScission protease (GE Healthcare, Heidelberg Germany) according to the manufacturer's protocol, and cleavage was verified by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and silver staining (Roth, Karlsruhe, Germany). To exclude carryover of remaining CCN1 in the supernatants of CD34+ cells if used in subsequent experiments with human umbilical vein endothelial cells (HUVECs), cell culture medium was exchanged against fresh medium 30 minutes after stimulation with CCN1 and left for additional 24 hours on the CD34+ cells. For control experiments, supernatants or plasma samples were preabsorbed overnight under permanent agitation at 4°C with an antibody against CCN1 (1 μg/100 μL) recently shown to neutralize CCN1-dependent effects.20 CCN1/antibody complexes were removed using protein A agarose (Invitrogen) according to the manufacturer's instructions. Antibodies used in this study included mouse monoclonal anti-CD34-PE (8G12; BD Biosciences, San Jose, CA), mouse monoclonal anti-CD45-APC (HI30; Caltag/Invitrogen, Karlsruhe, Germany), rabbit polyclonal anti-CCN1 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-GAPDH (6C5; Santa Cruz Biotechnology), rabbit polyclonal anti–von Willebrand factor (VWF; Dako Deutschland GmbH, Hamburg, Germany), rabbit polyclonal anti-integrin α6 (CD49f; Santa Cruz Biotechnology), rabbit polyclonal anti-integrin β1 (CD29; Santa Cruz Biotechnology), mouse monoclonal anti-integrin αM (CD11b, ICRF44; BD Biosciences), mouse monoclonal anti-integrin β2 (CD18, YFC118.3; Serotec, Düsseldorf, Germany), mouse monoclonal anti-integrin αV (CD51, P2W7; Abcam, Cambridge, United Kingdom), mouse monoclonal anti-integrin β3 (CD61, SZ21; Beckman Coulter, Krefeld, Germany), rabbit polyclonal anti-focal adhesion kinase (FAK; Santa Cruz, CA), rabbit polyclonal anti-p-FAK (Ser722; Santa Cruz Biotechnology) and peroxidase conjugated secondary polyclonal donkey antigoat (Dianova, Hamburg, Germany).
Mice and cells
C57BL/6 (Charles River Laboratories, Sulzbach, Germany) were maintained at the animal facility of the Medical School Hannover. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996). The use of human CD34+ cells was approved by the local ethics committee of the Medical School Hannover, Germany. HUVECs (PromoCell, Heidelberg, Germany) were cultured in endothelial cell growth medium (EGM; PromoCell) and kept in endothelial cell basal medium (EBM) during experiments. HUVECs between passages 2 and 4 were used for all cell culture experiments. Source of peripheral blood CD34+ cells were leukapheresis products from healthy donors after stem cell mobilization with recombinant granulocyte-colony stimulating factor (G-CSF). For isolation of CD34+ cells, we used the CliniMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). The majority of these isolated cells were positive for the common leukocyte antigen CD45 (< 98%, Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Proliferation assay
HUVECs were cultured in gelatin-coated 96-well plates in EGM to approximately 50% confluence and maintained overnight in EBM. Cell proliferation after 16 hours was measured on the basis of DNA synthesis by 5-bromo-2′-deoxyuridine (BrdU) incorporation in the presence of CCN1 (100 μg/mL), VEGF (50 μg/mL; PeproTech, Rocky Hill, NJ), or supernatants from CD34+ cells with a commercial colorimetric quantification kit (Roche, Mannheim, Germany) according to the manufacturer's protocol. The amount of reaction product was determined by measuring the absorbance at 450 nm using a plate reader (μQuant; Bio-Tek Instruments, Bad Friedrichshall, Germany).
Matrigel angiogenesis assay in vitro (tube formation)
HUVECs were cultured in EGM before being plated in 48-well plates (1 × 104 cells per well) previously coated with 50 μL Matrigel (growth factor-reduced; BD Biosciences), in the presence of CCN1 (100 ng/mL), VEGF (50 ng/mL; PeproTech), or supernatants from CD34+ cells. After 24 hours of culturing, tube-like structures were visualized using an inverted cell culture microscope (Olympus, CKX31, 40× magnification, 0.13 aperture), captured with a digital camera (C-5060; Olympus, Hamburg, Germany), and processed using Adobe Photoshop software (Adobe Systems, San Jose, CA).
Matrigel plug angiogenesis assay in vivo
C57BL/6 mice were anesthetized with ketamine (400 mg/kg) and xylazine (5 mg/kg) and received 0.5-mL injections of sterile chilled Matrigel (growth factor reduced; BD Biosciences) supplemented with CCN1 (20 and 50 ng/mL) and VEGF (50 ng/mL; PeproTech) into the abdominal wall. After 24 hours, 2.5 × 105 CD34+ cells were mixed with Matrigel and injected in the center of the polymerized plug. In addition, supernatants from CCN1-stimulated and unstimulated CD34+ cells were used in a 1:20 dilution in Matrigel. Control mice were injected with Matrigel without added growth factors. Matrigel plugs were removed 6 days after implantation, photographed, and divided into 2 blocks. One Matrigel plug was digested with Dispase (BD Biosciences), and hemoglobin content was determined by the Drabkin method according to the manufacturer's protocol (Sigma, Taufkirchen, Germany). Hemoglobin content is considered as a reflection of the number of blood vessels in the plug.21 Absorbance was measured in a microplate reader (μQuant; Bio-Tek) at 540 nm. Hemoglobin concentration in the Matrigel plugs was calculated with the help of a hemoglobin (Sigma, Taufkirchen, Germany) standard curve and normalized to the plug weight. The other Matrigel block was fixed overnight in 3.7% formalin and embedded in paraffin. Sections (6 μm) were stained with hematoxylin and eosin (H&E) and antibodies against VWF. H and E sections were visualized using a fluorescence microscope (Leica, DM 4000 B, 100× magnification, 0.30 aperture), captured with a CCD camera (Leica, DFC 320, room temperature) and Leica QWin V3.1 software and processed using Adobe Photoshop software. Vascular structures were expressed as percentage vascular area per field.
Cell migration and transendothelial migration assay
CD34+ cells (1 × 105) in 100 μL of EBM containing 0.1% BSA were placed in the upper chamber of transwell cell culture inserts (5-μm pore size; Costar; Corning Life Sciences, Acton, MA). The lower chamber contained 600 μL of EBM plus 0.1% BSA supplemented with CCN1 (100 ng/mL) or stromal cell-derived factor-1 (SDF-1; 100 ng/mL) (Hölzel Diagnostika, Köln, Germany). For transendothelial migration, HUVECs were cultured on the gelatin-coated upper chamber of the transwell cell culture inserts to complete confluence. Migration was carried out for 5 hours at 37°C, 5% CO2. Migrated cells into the lower chamber were quantified by counting in a Neubauer counting chamber using an inverted cell culture microscope (magnification, ×100; CKX31; Olympus, Hamburg, Germany). To test the specificity of cell migration, CD34+ cells were stained with TAMRA for 10 minutes at 37°C in endothelium cell basal medium containing 0.1% BSA. All of the migrated cells were found to be TAMRA-positive. Data obtained by the Neubauer counting chamber were confirmed by quantification of TAMRA-specific fluorescence (549 nm excitation/577 mm emission) using a fluorescence plate reader (Fluorostar Galaxy; BMG Lab Technology, Helsinki, Finland).
Gelatin zymography
Supernatants from CD34+ cells were separated by 10% SDS-PAGE supplemented with 1 mg/mL gelatin. Recombinant mouse matrix metalloproteinases (MMP) 2 and 9 were used as control. Gelatinolytic activity was quantified densitometrically using a gel image analysis system (GeneGenius; Syngene, Cambridge, United Kingdom) and the software Quantity One (Bio-Rad Laboratories, Hercules, CA). Gels were renaturated by 2.5% Triton X-100 for 30 minutes, followed by a substrate buffer incubation (50 mM Tris/HCl, pH 7.5, containing 5 mM CaCl2 and 0.02% Brij-35) for 16 hours. Gels were stained with 0.5% Coomassie blue.
Protein array
Supernatants from CD34+ cells were analyzed with a commercial custom-made protein array for cytokines and growth factors (VEGF, VEGF-D, platelet-derived growth factor [PDGF]-BB, placental growth factor [PlGF], transforming growth factor-1β [TGF-1β], angiogenin, granulocyte-colony-stimulating factor [G-CSF], granulocyte macrophage-colony-stimulating factor [GM-CSF], SDF-1, hepatocyte growth factor [HGF], basic fibroblast growth factor [bFGF], epidermal growth factor [EGF], insulin-like growth factor [IGF-1], macrophage-colony-stimulating factor [M-CSF], stem cell factor [SCF], interleukins 6 and 8, monocyte chemoattractant protein-1 [MCP-1], regulated on activation normal T cell expressed and secreted [RANTES], growth-related oncogene [GRO]-α/β; RayBiotech, Norcross, GA) according to the manufacturer's instructions. In brief, membranes were blocked for 30 minutes with blocking buffer and incubated with 2 mL of supernatants (1:2 diluted with blocking buffer) overnight at 4°C. After washing, biotin-conjugated antibody cocktail was added and again incubated overnight at 4°C, followed by 2-hour incubation with streptavidin-conjugated peroxidase at room temperature. Membranes were incubated with peroxidase substrate and exposed to ECL films (Hyperfilm ECL; GE Healthcare). Films were digitalized and quantified densitometrically using a gel image analysis system (GeneGenius) and the software Quantity One.
Reverse transcription-polymerase chain reaction
Total RNA was isolated using TriFast-Reagent (peqLAB, Erlangen, Germany) and reverse-transcribed with SuperScript reverse transcriptase (RT; Invitrogen), oligo(dT) primers, and deoxynucleoside triphosphates. The RT products were amplified using Taq DNA polymerase (Invitrogen). Polymerase chain reaction (PCR) for CCN1 (forward primer, 5′-CAG CCC TGC GAC CAC AC-3′; reverse primer, 5′-TGC CCT TTT TCA GGC TGC-3′; 648 base pairs) was carried out for 38 cycles and for GADPH (forward primer, 5′-ACC ACC ATG GAG AAG GCT GG-3′; reverse primer, 5′-CTC AGT GTA GCC CAG GAT GC-3′, 527 base pairs) for 20 cycles. Oligonucleotides were obtained from MWG-Biotech (Ebersberg, Germany). PCR products were separated by 1% agarose gel electrophoresis and analyzed using a gel image analysis system (GeneGenius).
Immunoblotting
Proteins from cellular extracts, from cell culture supernatants and from plasma samples were separated by denaturing SDS (10%) PAGE, and transferred to PVDF membrane (GE Healthcare). Transferred proteins were probed with an anti-CCN1 antibody (1:1000) and visualized using a horseradish peroxidase-conjugated secondary anti-goat (1:3000), ECL solution, and ECL film (Hyperfilm ECL; GE Healthcare). Equal protein loading was verified by reprobing the membrane with an anti-GAPDH antibody (1:2000). Gels were analyzed using a gel image analysis system (GeneGenius).
Immunofluorescent staining
HUVECs were cultured to ∼50% confluence in EGM on gelatin-coated cover slips in 24-well plates. CD34+ cells were kept in EBM containing 0.1% BSA and applied to slides at a density of 5 × 104 cells per dot using a cytocentrifuge (Shandon Cytospin 3; Thermo Fisher, Waltham, MA). After fixation with ice-cold acetone cells were blocked with 15% donkey serum containing 2% FCS for 1 hour at room temperature to eliminate unspecific background signals. As indicated, cells were preincubated with RGD-peptides (1 mM RGD and GRGDSP; Bachem, Weil am Rhein, Germany) and CCN1 (100 ng/mL), respectively, each for 30 minutes. Cells were incubated with antibodies against CCN1, FAK, p-FAK, CD34, or von Willebrand factor (each 1:200) overnight at 4°C followed by incubation with appropriate fluorescein isothiocyanate (FITC)- or tetramethylrhodamine B isothiocyanate (TRITC)-conjugated secondary antibodies for 1 hour at room temperature and mounted on slides. Species-specific IgG served as control. Nuclei were counterstained with Hoechst stain. Immunofluorescent staining was visualized using a fluorescence microscope (Leica, DM 4000 B, 1000× magnification, 1.30 oil aperture) for following fluorochromes: CCN1(FITC), FAK(TRITC), p-FAK(FITC), CD34(TRITC), VWF(TRITC). Images were captured with a CCD camera (Leica, DFC 350 FX, room temperature) and Leica QWin V3.1 software and processed using Adobe Photoshop software.
Flow cytometry
Expression of integrins on peripheral blood CD34+ cells was measured by flow cytometry. Cells were stained with allophycocyanin (APC)-conjugated anti-CD34 antibody and primary antibodies against integrins, either directly conjugated with FITC (integrins αM, β2, β3) or followed by FITC-labeled secondary antibodies (integrins αV, α6, β1,). Stained cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Cell adhesion assay
Cell-matrix adhesion was investigated in 96-well plates uncoated or coated overnight (4°C) with CCN1 (1 μg/mL) or fibronectin (1 μg/mL). CD34+ cells in EBM containing 0.1% BSA were seeded at 5 × 104 cells/well in 100 μL. As indicated, cells were pretreated with RGD peptides (1 mM RGD and GRGDSP) for 30 minutes at 37°C. Adhesion was carried out for 4 hours at 37°C, 5% CO2. After removal of nonadherent cells by 2 washing steps with endothelial cell basal medium containing 0.1% BSA at room temperature, adhesion was quantified in duplicates by counting adherent cells using an inverted cell culture microscope (magnification ×100; CKX31, Olympus, Hamburg, Germany).
Cell-cell adhesion was performed by culturing HUVECs in gelatin coated 96-well plates in EGM to complete confluence and maintained overnight in EBM. CD34+ cells in EBM containing 0.1% BSA were stained with TAMRA for 10 minutes at 37°C incubated with CCN1 (1 μg/mL) or fibronectin (1 μg/mL) for 30 minutes at 37°C and seeded at 1 × 105 cells/well in 100 μL. For some experiments, CD34+ cells were preincubated with RGD peptides (1 mM RGD and GRGDSP) for 30 minutes at 37°C. Adhesion was carried out for 4 hours at 37°C, 5% CO2. Nonadherent cells were removed by 2 washing steps with endothelial cell basal medium containing 0.1% BSA. Adherent CD34+ were identified by TAMRA fluorescence and quantified by counting in at least triplicates using an inverted cell culture microscope (magnification ×100; CKX31; Olympus, Hamburg, Germany). Data were confirmed by quantification of TAMRA-specific fluorescence (549 nm excitation/577 nm emission) using a fluorescence plate reader (Fluorostar Galaxy; BMG Lab Technology, Helsinki, Finland).
Statistical analysis
All data are presented as the mean ± SEM of at least 3 separate experiments. Data were compared using 2-tailed Student t test for independent samples. Values of P less than .05 were considered statistically significant.
Results
CCN1 stimulated endothelial proliferation and tube formation
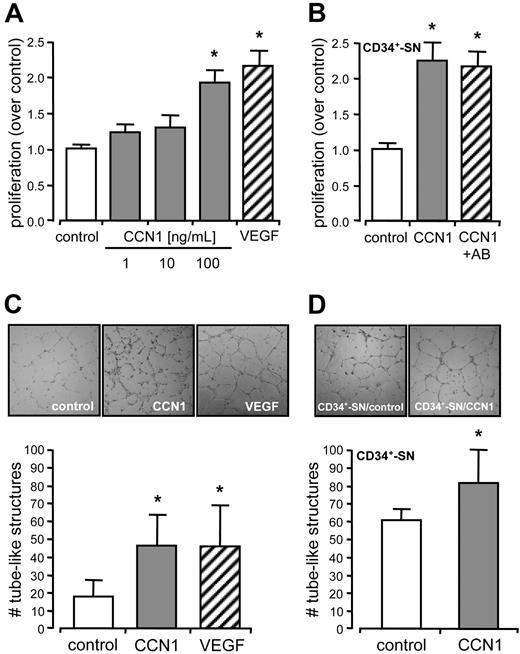
Because proliferation of endothelial cells—among many other factors—contributes to angiogenesis, we determined BrdU incorporation of endothelial cells in response to CCN1. The rate of proliferation in the presence of CCN1 was enhanced, reaching significant levels at 100 ng/mL after 16 hours, and was comparable with that of VEGF (Figure 1A). In addition, supernatants from CCN1 stimulated CD34+ cells significantly accelerated proliferation of endothelial cells after 16 hours compared with unstimulated supernatants. Supernatants preabsorbed with neutralizing antibodies against CCN1 did not block their proliferative effect, excluding carryover of recombinant CCN1 (Figure 1B). To demonstrate the angiogenic potential of CCN1, we performed Matrigel angiogenesis assay in vitro with endothelial cells (HUVECs). The numbers of tube-like structures after 24-hour build up by CCN1 were comparable with those for VEGF, used as positive control (Figure 1C). It is noteworthy that supernatants taken from CCN1-stimulated CD34+ cells significantly increased tube formation by endothelial cells compared with supernatants taken from unstimulated CD34+ cells after 24 hours (Figure 1D). To investigate the angiogenic potential of CCN1 in vivo, we injected Matrigel subcutaneously into the abdominal wall of C57/BL6 mice. Neovascularization was quantified on the basis of the hemoglobin content of the Matrigel plugs21 and by ingrowing vascular structures at day 6. In addition, the endothelial character of these structures was confirmed by immunohistochemistry using antibodies against VWF. CCN1 (50 ng/mL) significantly induced angiogenesis in vivo compared with control levels demonstrating the biological activity of the human protein in mice (Figure 2A). CCN1 at lower concentration of 20 ng/mL and CD34+ cells alone had no significant effect on angiogenesis in vivo. Injection of CD34+ cells into a Matrigel plug already containing 20 ng/mL CCN1 significantly increased neovascularization, indicating an interaction between CCN1 and CD34+ cells in terms of angiogenesis (Figure 2B). Finally, we found that supernatants from CCN1-stimulated CD34+ cells were sufficient to induce angiogenesis in vivo (Figure 2C).
CCN1 stimulated endothelial proliferation and angiogenesis in vitro. (A) Proliferation of HUVECs was determined by BrdU incorporation in the absence (control) and in the presence of 1, 10, and 100 ng/mL CCN1 or 50 ng/mL VEGF for 16 hours. (B) Proliferation of HUVECs was determined by BrdU incorporation after 16 hours of incubation with supernatants (SN) from CD34+ cells unstimulated (control), stimulated with 100 ng/mL CCN1 for 24 hours or preabsorbed with a neutralizing antibody (AB) against CCN1. Unspecific control IgG did not show effects (data not shown). Increase in proliferation was significant different from control levels (*P < .05). (C) HUVECs were cultured in Matrigel for 24 hours in the absence (control) or in the presence of 100 ng/mL CCN1 or 50 ng/mL VEGF. (D) HUVECs were cultured in Matrigel for 24 hours with supernatants (SN) from CD34+ cells unstimulated (control) or stimulated with 100 ng/mL CCN1 for 24 hours. Increase in tube-like structures was significant different from control levels (*P < .05).
CCN1 stimulated endothelial proliferation and angiogenesis in vitro. (A) Proliferation of HUVECs was determined by BrdU incorporation in the absence (control) and in the presence of 1, 10, and 100 ng/mL CCN1 or 50 ng/mL VEGF for 16 hours. (B) Proliferation of HUVECs was determined by BrdU incorporation after 16 hours of incubation with supernatants (SN) from CD34+ cells unstimulated (control), stimulated with 100 ng/mL CCN1 for 24 hours or preabsorbed with a neutralizing antibody (AB) against CCN1. Unspecific control IgG did not show effects (data not shown). Increase in proliferation was significant different from control levels (*P < .05). (C) HUVECs were cultured in Matrigel for 24 hours in the absence (control) or in the presence of 100 ng/mL CCN1 or 50 ng/mL VEGF. (D) HUVECs were cultured in Matrigel for 24 hours with supernatants (SN) from CD34+ cells unstimulated (control) or stimulated with 100 ng/mL CCN1 for 24 hours. Increase in tube-like structures was significant different from control levels (*P < .05).
CCN1 stimulated angiogenesis in vivo. (A-C) Matrigel (0.5 mL) was injected into C57BL/6 mice subcutaneously. Angiogenesis after 6 days was measured by the hemoglobin content of the Matrigel plug and by ingrowing vascular structures confirmed by staining for VWF. Pictures represent H&E sections of Matrigel plugs, upper left insets show the total Matrigel plug and the upper right insets shows sections stained with antibodies against VWF (A) Matrigel in the absence (control) and in the presence of 50 ng/mL CCN1 or 50 ng/mL VEGF. Increase in angiogenesis was significant different from control levels (*P < .05). (B) Matrigel (0.5 mL) in the absence (control) and in the presence of 20 ng/mL CCN1 or 2.5 × 105 CD34+ cells. In addition, 2.5 × 105 CD34+ cells were injected into Matrigel plugs already containing 20 ng/mL CCN1. Increase in angiogenesis was significant different from CCN1 alone and CD34+ cells alone (*P < .05). (C) Matrigel in the absence (control) or in the presence of supernatants (SN) from CD34+ cells unstimulated (control) or stimulated with 100 ng/mL CCN1 for 24 hours. Increase in angiogenesis was significant different from control levels and supernatants from unstimulated CD34+ cells (*P < .05). Hemoglobin content is given in nanograms per milliliter per plug weight in milligrams. Vascular structures are expressed as percentage vascular area per field. Data represent the mean (± SEM) of at least 3 independent experiments.
CCN1 stimulated angiogenesis in vivo. (A-C) Matrigel (0.5 mL) was injected into C57BL/6 mice subcutaneously. Angiogenesis after 6 days was measured by the hemoglobin content of the Matrigel plug and by ingrowing vascular structures confirmed by staining for VWF. Pictures represent H&E sections of Matrigel plugs, upper left insets show the total Matrigel plug and the upper right insets shows sections stained with antibodies against VWF (A) Matrigel in the absence (control) and in the presence of 50 ng/mL CCN1 or 50 ng/mL VEGF. Increase in angiogenesis was significant different from control levels (*P < .05). (B) Matrigel (0.5 mL) in the absence (control) and in the presence of 20 ng/mL CCN1 or 2.5 × 105 CD34+ cells. In addition, 2.5 × 105 CD34+ cells were injected into Matrigel plugs already containing 20 ng/mL CCN1. Increase in angiogenesis was significant different from CCN1 alone and CD34+ cells alone (*P < .05). (C) Matrigel in the absence (control) or in the presence of supernatants (SN) from CD34+ cells unstimulated (control) or stimulated with 100 ng/mL CCN1 for 24 hours. Increase in angiogenesis was significant different from control levels and supernatants from unstimulated CD34+ cells (*P < .05). Hemoglobin content is given in nanograms per milliliter per plug weight in milligrams. Vascular structures are expressed as percentage vascular area per field. Data represent the mean (± SEM) of at least 3 independent experiments.
CCN1 induced cytokine release, migration, and MMP release of CD34+ cells
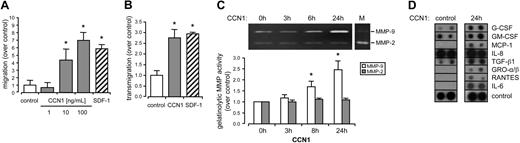
Vascular regeneration and angiogenesis requires migration of various cells. To determine whether CCN1 promoted the migration of CD34+ cells, we performed in vitro migration assays using CCN1 as chemoattractant. CCN1 significantly induced the migration of CD34+ cells already at concentrations of 10 ng/mL; the migration was further enhanced at 100 ng/mL. SDF-1 served as a positive control (Figure 3A). In addition, CCN1 induced the ability of CD34+ cells to migrate across an endothelial monolayer after 5 hours (Figure 3B) comparable with SDF-1. Because transmigration is accompanied by structural changes of the extracellular matrix (ECM) predominantly regulated by MMPs, we investigated the impact of CCN1 on MMP-2/MMP-9 release from CD34+ cells using gelatin zymography. Gelatinolytic activity of MMP-9 in the supernatants of CD34+ cells was significantly induced by CCN1 after 8 hours and further enhanced after 24 hours, whereas MMP-2 remained unchanged (Figure 3C). To investigate which factors derived from CD34+ cells in response to CCN1 might be involved in the observed effects on endothelial proliferation and tube formation, we performed a protein array for selected cytokines and growth factors. 24 hours after CCN1 stimulation we detected an abundant accumulation of several cytokines in the supernatant of CD34+ cells compared with unstimulated controls. Among these were prominent growth factors such as G-CSF and GM-CSF as well as MCP-1, TGF-β1, GRO-α/β, RANTES, and IL-6, pointing to their increased release from CD34+ cells in response to CCN1. In contrast, IL-8 was already heavily secreted in supernatants from unstimulated controls but not induced by CCN1 (Figure 3D).
CCN1 induced migration, MMP, and cytokine release of CD34+cells. (A) Migration of CD34+ cells was carried out in the absence (control) or in the presence of 1, 10 and 100 ng/mL CCN1 or 100 ng/mL SDF-1 for 5 hours. (B) Transendothelial migration of CD34+ cells was carried out in the absence (control) or in the presence of 100 ng/mL CCN1 or 100 ng/mL SDF-1 for 5 hours. Increase in migration was significant different from control levels (*P < .05). (C) Supernatants of CD34+ cells stimulated with 100 ng/mL CCN1 for the indicated time were subjected to gelatin zymography (M = MMP-2/-9 marker). Increase in gelatinolytic MMP activity was significant different from unstimulated control levels (*P < .05). (D) Supernatants of unstimulated (control) or with 100 ng/mL CCN1 for 24 hours stimulated CD34+ cells were subjected to a commercial protein array for selected cytokines and growth factors. Data present the mean (± SEM) of at least 3 independent experiments.
CCN1 induced migration, MMP, and cytokine release of CD34+cells. (A) Migration of CD34+ cells was carried out in the absence (control) or in the presence of 1, 10 and 100 ng/mL CCN1 or 100 ng/mL SDF-1 for 5 hours. (B) Transendothelial migration of CD34+ cells was carried out in the absence (control) or in the presence of 100 ng/mL CCN1 or 100 ng/mL SDF-1 for 5 hours. Increase in migration was significant different from control levels (*P < .05). (C) Supernatants of CD34+ cells stimulated with 100 ng/mL CCN1 for the indicated time were subjected to gelatin zymography (M = MMP-2/-9 marker). Increase in gelatinolytic MMP activity was significant different from unstimulated control levels (*P < .05). (D) Supernatants of unstimulated (control) or with 100 ng/mL CCN1 for 24 hours stimulated CD34+ cells were subjected to a commercial protein array for selected cytokines and growth factors. Data present the mean (± SEM) of at least 3 independent experiments.
CCN1 expression in CD34+ cells and in human plasma
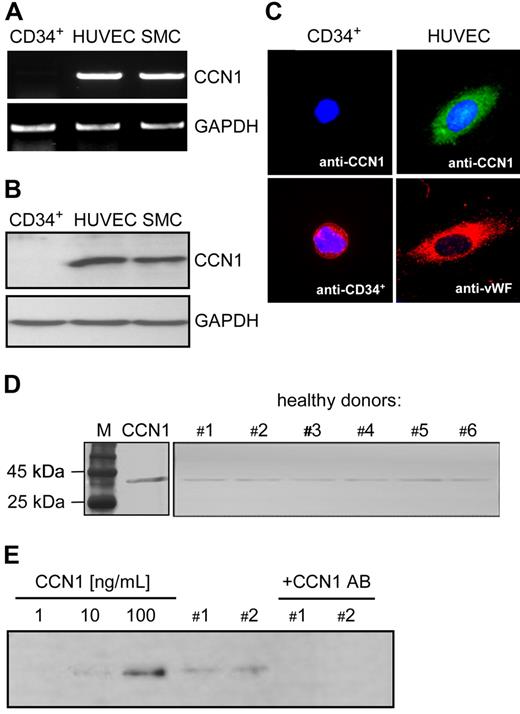
Next, we investigated whether CD34+ cells express CCN1. Here, we showed that CCN1 was not detectable in CD34+ cells (neither the mRNA by PCR [Figure 4A] nor the protein by immunoblotting or immunofluorescent staining [Figure 4B,C]), indicating that circulating CD34+ cells do not express CCN1. In contrast, endothelial cells and smooth muscle cells were tested positive for CCN1 (Figure 4B,C). Immunofluorescence analysis showed an even distribution of the protein over the whole surface area of endothelial cells (Figure 4C). Analysis of plasma samples from healthy donors (male, n = 6; mean age, 33.5 ± 4.6 years; mean body mass index, 22.3 ± 0.9; mean cholesterol, 176.9 ± 5.3) using an antibody against CCN1 revealed detectable amounts of CCN1 protein in the plasma (Figure 4D). Additional Western blot analysis using CCN1 standards and samples from donors pointed to CCN1 plasma levels of approximately 10 to 100 ng/mL in healthy subjects. Plasma samples could be depleted of CCN1 by preabsorption with a neutralizing antibody against CCN1 (Figure 4E).
CCN1 expression in CD34+cells and in human plasma. (A) RNA from CD34+ cells, HUVECs and SMCs were isolated, reverse transcribed and amplified by PCR using specific primers for CCN1 and GAPDH. (B) Cell lysates from CD34+ cells, HUVECs and SMCs were subjected to Western blot analysis using antibodies against CCN1 and GAPDH. (C) Immunofluorescent staining of CD34+ cells and HUVEC with antibodies against CCN1, CD34+ and CCN1, von Willebrand factor (VWF), respectively. Nuclei were counterstained with Hoechst (original magnification ×1000). (D) Plasma samples from healthy donors (#1-#6) were subjected to Western blot analysis using antibodies against CCN1. Recombinant CCN1 was used as positive control (M, protein marker). (E) CCN1 standards containing 1, 10, or 100 ng/mL of recombinant CCN1 protein and plasma from healthy donors (1 and 2), untreated and preabsorbed with a neutralizing antibody against CCN1 were subjected to Western blot analysis using antibodies against CCN1. One representative blot of 3 independent experiments is shown.
CCN1 expression in CD34+cells and in human plasma. (A) RNA from CD34+ cells, HUVECs and SMCs were isolated, reverse transcribed and amplified by PCR using specific primers for CCN1 and GAPDH. (B) Cell lysates from CD34+ cells, HUVECs and SMCs were subjected to Western blot analysis using antibodies against CCN1 and GAPDH. (C) Immunofluorescent staining of CD34+ cells and HUVEC with antibodies against CCN1, CD34+ and CCN1, von Willebrand factor (VWF), respectively. Nuclei were counterstained with Hoechst (original magnification ×1000). (D) Plasma samples from healthy donors (#1-#6) were subjected to Western blot analysis using antibodies against CCN1. Recombinant CCN1 was used as positive control (M, protein marker). (E) CCN1 standards containing 1, 10, or 100 ng/mL of recombinant CCN1 protein and plasma from healthy donors (1 and 2), untreated and preabsorbed with a neutralizing antibody against CCN1 were subjected to Western blot analysis using antibodies against CCN1. One representative blot of 3 independent experiments is shown.
CCN1 binding to CD34+ cells and CCN1-stimulated adhesion of CD34+ cells are integrin-dependent
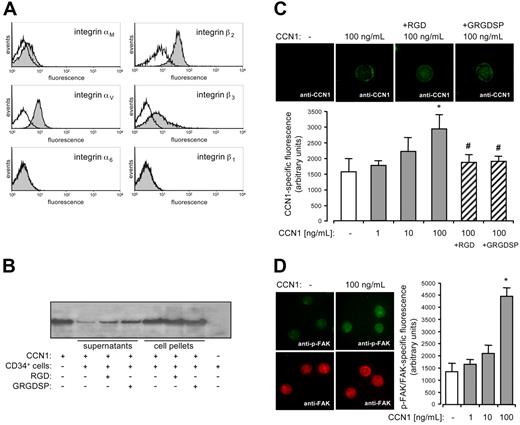
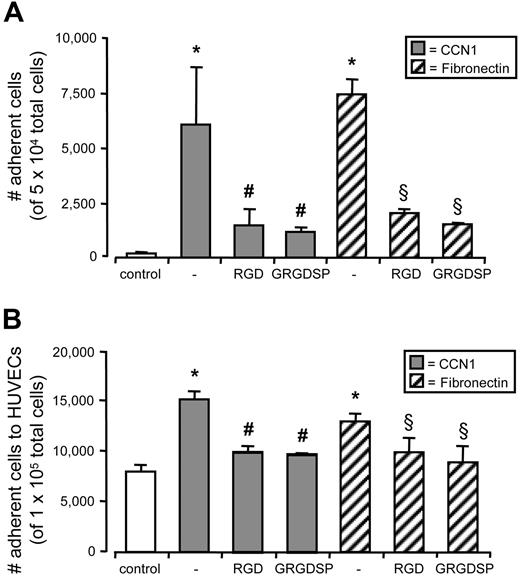
To analyze the underlying mechanism of a potential interaction of CCN1 with CD34+ cells, we determined the expression of integrin receptors on CD34+ cells known to bind specifically to CCN1 on other cell types.9-11 Surface expression of αMβ2 and αVβ3 integrin on CD34+ cells could be demonstrated by flow cytometry, whereas α6β1 integrin was not detectable (Figure 5A). Incubation of CD34+ cells with CCN1 for 4 hours at 37°C and subsequent centrifugation of the cells led to a markedly depletion of CCN1 in the supernatant as assessed by Western blot. Preincubation of the cells with integrin-antagonizing RGD peptides for 30 minutes partly prevented binding of CCN1 to CD34+ cells (Figure 5B). Consequently, dose-dependent incubation of CD34+ cells with CCN1 for 30 minutes followed by several washing steps led to a CCN1-specific immunofluorescent staining, reaching significant levels at CCN1 concentrations of 100 ng/mL. CCN1-specific immunofluorescence was significantly suppressed by RGD peptides (Figure 5C) suggesting an integrin-dependent binding of CCN1 to CD34+ cells. In addition, activation of the intracellular integrin-dependent signaling pathway in CD34+ cells 30 minutes after CCN1 stimulation was demonstrated by enhanced phosphorylation of the focal adhesion kinase at Ser722, which is a downstream target of integrin receptors. CCN1 dose-dependently enhanced FAK phosphorylation reaching significant levels at 100 ng/mL (Figure 5D). Finally, adhesion assays were performed to study the adhesion of CD34+ cells to CCN1. Fibronectin, reported to promote adherence of CD34+ cells served as positive control.4 The cell-matrix adhesion of CD34+ cells to immobilized CCN1 was substantially up-regulated after 4 hours and clearly inhibited by integrin blockade with RGD peptides (Figure 6A). It is noteworthy that cell-cell adhesion of CD34+ cells to an endothelial monolayer was affected in the same way. Treatment of CD34+ cells with CCN1 up-regulated their adhesion to endothelial cells after 4 hours, which was significantly suppressed by integrin inhibition (Figure 6B), indicating a CCN1-driven and integrin-dependent enhancement of circulating CD34+ cell attachment to the endothelium.
Integrin expression on CD34+cells and integrin-dependent binding of CCN1 to CD34+cells. (A) Expression of integrins on CD34+ cells was analyzed by flow cytometry using antibodies against integrins αM, β2, αV, β3, α6, and β1, respectively (filled graphs). Open graphs represent isotype controls. Cells in the histograms are gated for CD34 expression. (B) 1 × 105 CD34+ cells pretreated with 1 mM RGD peptides (RGD and GRGDSP) or left untreated were incubated with 50 ng of CCN1 for 4 hours. After centrifugation of the cells supernatants and cell pellets were subjected to Western blot analysis using antibodies against CCN1. One representative blot of 3 independent experiments is shown. (C) Immunofluorescent staining of CD34+ cells with an antibody against CCN1 in the absence or after preincubation with 1, 10, and 100 ng/mL CCN1 and/or with 1 mM RGD peptides (RGD and GRGDSP) for 30 minutes followed by several washing steps. Immunofluorescence was significantly increased compared with conditions without CCN1 (*P < .01) and significantly reduced by RGD-peptides compared with 100 μg/mL CCN1 (#P < .05). Data represent the mean (± SEM) immunofluorescence of 25 to 50 analyzed cells per condition. (D) Immunofluorescent staining of CD34+ cells in the absence (control) or after 30-minute stimulation with 1, 10, and 100 ng/mL CCN1 using antibodies against phosphorylated focal adhesion kinase (p-FAK, Ser722) or focal adhesion kinase (FAK). p-FAK-specific immunofluorescence was normalized to FAK-specific immunofluorescence. Immunofluorescence was significantly increased compared with conditions without CCN1 (*P < .01). Data represent the mean (± SEM) immunofluorescence of 25 to 50 analyzed cells per condition.
Integrin expression on CD34+cells and integrin-dependent binding of CCN1 to CD34+cells. (A) Expression of integrins on CD34+ cells was analyzed by flow cytometry using antibodies against integrins αM, β2, αV, β3, α6, and β1, respectively (filled graphs). Open graphs represent isotype controls. Cells in the histograms are gated for CD34 expression. (B) 1 × 105 CD34+ cells pretreated with 1 mM RGD peptides (RGD and GRGDSP) or left untreated were incubated with 50 ng of CCN1 for 4 hours. After centrifugation of the cells supernatants and cell pellets were subjected to Western blot analysis using antibodies against CCN1. One representative blot of 3 independent experiments is shown. (C) Immunofluorescent staining of CD34+ cells with an antibody against CCN1 in the absence or after preincubation with 1, 10, and 100 ng/mL CCN1 and/or with 1 mM RGD peptides (RGD and GRGDSP) for 30 minutes followed by several washing steps. Immunofluorescence was significantly increased compared with conditions without CCN1 (*P < .01) and significantly reduced by RGD-peptides compared with 100 μg/mL CCN1 (#P < .05). Data represent the mean (± SEM) immunofluorescence of 25 to 50 analyzed cells per condition. (D) Immunofluorescent staining of CD34+ cells in the absence (control) or after 30-minute stimulation with 1, 10, and 100 ng/mL CCN1 using antibodies against phosphorylated focal adhesion kinase (p-FAK, Ser722) or focal adhesion kinase (FAK). p-FAK-specific immunofluorescence was normalized to FAK-specific immunofluorescence. Immunofluorescence was significantly increased compared with conditions without CCN1 (*P < .01). Data represent the mean (± SEM) immunofluorescence of 25 to 50 analyzed cells per condition.
CCN1 induced adhesion of CD34+cells is integrin-dependent. (A) CD34+ cells were preincubated with 1 mM RGD peptides (RGD and GRGDSP) or left untreated. Cells (5 × 104) were added to uncoated wells (control) and wells coated with 1 μg/mL CCN1 or 1 μg/mL fibronectin and subjected to adhesion for 4 hours. Nonadherent cells were washed, and adherent cells were counted microscopically. (B) CD34+ cells were labeled with TAMRA, left untreated (control) or preincubated with 1 μg/mL CCN1 or 1 μg/mL fibronectin and/or with 1 mM RGD peptides (RGD and GRGDSP). Cells (1 × 105) were added to confluent HUVEC monolayers and subjected to adhesion for 4 hours. Nonadherent cells were washed, and adherent cells were identified by TAMRA fluorescence and counted microscopically. Increase in cell adhesion was significantly different from control (*P < .05) and significantly reduced compared with CCN1 alone (#P < .05) and fibronectin alone (§P < .05). Data represent the mean (± SEM) of at least 3 independent experiments.
CCN1 induced adhesion of CD34+cells is integrin-dependent. (A) CD34+ cells were preincubated with 1 mM RGD peptides (RGD and GRGDSP) or left untreated. Cells (5 × 104) were added to uncoated wells (control) and wells coated with 1 μg/mL CCN1 or 1 μg/mL fibronectin and subjected to adhesion for 4 hours. Nonadherent cells were washed, and adherent cells were counted microscopically. (B) CD34+ cells were labeled with TAMRA, left untreated (control) or preincubated with 1 μg/mL CCN1 or 1 μg/mL fibronectin and/or with 1 mM RGD peptides (RGD and GRGDSP). Cells (1 × 105) were added to confluent HUVEC monolayers and subjected to adhesion for 4 hours. Nonadherent cells were washed, and adherent cells were identified by TAMRA fluorescence and counted microscopically. Increase in cell adhesion was significantly different from control (*P < .05) and significantly reduced compared with CCN1 alone (#P < .05) and fibronectin alone (§P < .05). Data represent the mean (± SEM) of at least 3 independent experiments.
Discussion
Cardiovascular tissue regeneration is mainly mediated by the formation of new blood vessels via induction of angiogenesis. Beyond VEGF, other angiogenic factors, such as FGF, PlGF, PDGF, angiopoietins, and many more, contribute to vessel formation.22 Although the application of a single factor—in the case of VEGF—has entered the clinical area, large scale trials have not yet yielded consistent beneficial results.23,24 This may be related to recent observations that several other potent angiogenic factors, such as CCN1,15,25,26 act in concert with VEGF for vessel formation. However, the underlying mechanism of cardiovascular regeneration through neovascularization and probably the interaction of different proangiogenic factors remain poorly understood. Up-regulated expression of CCN1 accompanies diverse cardiovascular pathophysiologic conditions18-20,27 and percutaneous transluminal coronary angioplasty (PTCA).28 In particular, smooth muscle cells and cardiomyocytes are a major source of CCN1, suggesting that CCN1 may be a part of an important self-renewal program specifically promoting cardiovascular tissue regeneration. Accumulating evidence suggests that circulating progenitors contribute to vascular healing and remodeling under pathologic conditions. In this regard, CD34+ hematopoietic progenitor cells have been shown to promote neovascularization in mice29 and regeneration of the infarcted myocardium in rats.3 In addition, purified human CD34+ progenitor cells are known to improve cardiac function in mice30 and in patients after myocardial infarction.31,32 It is noteworthy that the highly proangiogenic cell population of EPCs coexpressing markers such as CD133 and flk1 can be isolated from CD34+ hematopoietic progenitors.4 The potential of hematopoietic cells to transdifferentiate into myocardial cells is controversial, because there are reports describing their transdifferentiation into cardiomyocytes5,6 and reports that challenge this potential.7,8 Enhanced angiogenesis in response to progenitor cells may be mainly influenced by the release of growth factors rather than by cellular differentiation.9 Accordingly, we observed no enhanced angiogenesis in response to unstimulated CD34+ cells alone.
We report here that endothelial proliferation and neovascularization is directly mediated by CCN1 as well as indirectly by CCN1-stimulated CD34+ cells, suggesting a close relationship of these players in cardiovascular regeneration. We assume that both pathways are coexisting. The indirect effects of CCN1 mediated by CD34+ cells (described herein) may offer an additional regenerative pathway, providing a more flexible response to tissue damage compared with locally restricted direct effects of CCN1. Especially the interaction of CCN1 with CD34+ cells is of high biological relevance because cardiac ischemia is associated with both increased levels of CCN118 and circulating CD34+ cells.33 A mechanism critically involved in tissue regeneration is migration of progenitor cells, especially transendothelial migration.34 Transendothelial migration of CD34+ progenitor cells occurs during mobilization in the bone marrow and during homing of these cells to sites of cardiovascular injury. Paracrine factors may play a role in progenitor cell trafficking, because growth factor-stimulated progenitor cells produce cytokines that act on endothelial cells, modifying their proliferation, motility, permeability, and fenestration, which are critically involved in angiogenesis. We observed that CCN1 induced both migration and transendothelial migration of CD34+ cells comparable with the potent positive control SDF-1. In this regard, we detected a CCN1-induced increase of secreted MMP-9 from CD34+ cells, whereas MMP-2 remained unchanged. It is noteworthy that Mo et al16 recently reported CCN1-induced MMP-2 levels in cardiomyocytes. Enhanced MMP activity has been shown to be associated with angiogenesis.35 First of all, MMP-9 is critically involved in the mobilization of progenitor cells from the bone marrow36 ; nevertheless, our findings are in concert with the notion that cytokines and growth factor induced MMP-9 release from peripheral blood CD34+ cells, promoting their transmigration35,37 with potential implication in angiogenesis. Cardiovascular regeneration by neovascularization and restoration of cardiac function is partly mediated by bone marrow-derived stem cells, and several key signaling factors, including cytokines, chemokines, and growth factors are known to drive the repair process.38 In vivo studies on angiogenesis using supernatants from CCN1-stimulated CD34+ cells revealed that secreted factors rather than the progenitor cells itself promote neovascularization. Several secreted factors with angiogenic and proliferative potential (eg, G-CSF, GM-CSF, and TGF-β1) were found to be induced from circulating CD34+ cells incubated with CCN1 by protein array. It is noteworthy that the common angiogenic factors VEGF and PDGF were not found to be induced, suggesting that CCN1-induced CD34+ cells promote endothelial tube formation and proliferation via other mechanisms. So far, we could not exclude that CCN1 induces factors responsible for the observed effects that are not represented on the array. Taken together, appropriate regulation of signaling between the bone marrow, the peripheral circulation, and damaged tissue is important in orchestrating cardiovascular regeneration, potentially driven—at least in part—by CCN1 and its interaction with circulating CD34+ cells.
Vascular cells such as endothelial cells and smooth muscle cells themselves express CCN110 and react to different cardiovascular pathophysiologic settings with augmented CCN1 expression,18-20,26,27 allowing autocrine cell stimulation in terms of tissue regeneration. In contrast, peripheral blood CD34+ cells do not show endogenous CCN1 expression, excluding a mechanism of auto stimulation. It is noteworthy that we were able to detect CCN1 protein in human plasma for the first time, suggesting paracrine CCN1 effects on circulating CD34+ cells. Comparable with secreted chemokines, CCN1 may act on formation of a gradient attracting circulating CD34+ cells to sites of injured endothelium or damaged tissue. CCN1 plasma levels of approximately 10 to 100 ng/mL were found to be in the same range compared with CCN1 levels causing significant effects in cell culture and in the in vivo studies. In vascular cells, CCN1 mediates its effects in terms of cell adhesion, migration, proliferation, and neovascularization basically through specific binding to integrins such as α6β1, αMβ2, and αVβ3.10,12-14 Furthermore, integrins are known to be expressed on hematopoietic CD34+ progenitor cells involved in their interaction with the bone marrow microenvironment.39 In accordance with other groups, we demonstrated the expression of the CCN1-specific integrins αMβ2 and αVβ3 on peripheral blood CD34+ cells, providing the structural basis for a general responsiveness of these cells to CCN1.40,41 As recently reported by Chavakis et al,42 β2-integrins are involved in the homing of hematopoietic Sca-1+/Lin− progenitor cells to sites of ischemia and are critical for their neovascularization capacity. In fact, binding of CCN1 to CD34+ cells could be blocked using integrin antagonizing RGD-peptides. In addition, CCN1-induced adhesion of CD34+ cells to an endothelial monolayer is mediated by integrins, which is of biological relevance because cell recruitment depends on integrin-mediated firm adhesion and diapedesis.
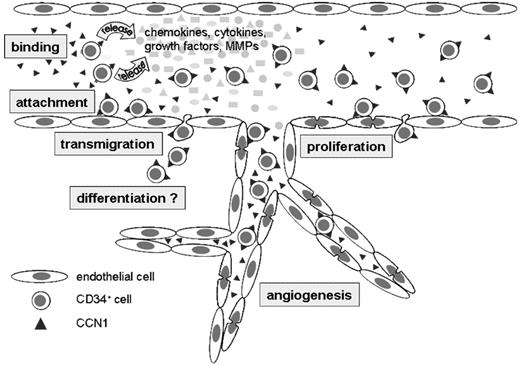
The present study indicates that the angiogenic factor CCN1, which is known to be up-regulated under various pathophysiologic conditions within the cardiovascular system, is released into the peripheral blood. CCN1 could bind as a soluble factor via integrin receptors to circulating CD34+ progenitor cells, inducing the release of chemokines, growth factors, and MMPs from these cells. In addition, CCN1 promotes the attachment of CD34+ cells to the endothelium, their endothelial transmigration and—in combination with the induced factors—subsequent proliferation of endothelial cells and angiogenesis (Figure 7). Thus, CCN1 may be a specific vascular factor enhancing paracrine effects on angiogenesis and tissue regeneration mediated by CD34+ progenitor cells and thereby may be a suitable target for future drug therapy.
CCN1—mode of action. Schematic model of CCN1 effects on circulating CD34+ cells and endothelial cells.
CCN1—mode of action. Schematic model of CCN1 effects on circulating CD34+ cells and endothelial cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Silke Pretzer, Christina Reimer, Maria Carla Casale, and Valentina Boasi for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) grants Schie 386/8-1 (Klinische Forschergruppe 136) and Schie 386/7-2.
Authorship
Contribution: K.G. designed and performed research and wrote the manuscript; G.S. designed and performed research; M.B. performed research; M.D. performed research; H.D. wrote the manuscript; and B.S. designed research and wrote the manuscript. K.G. and G.S. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Karsten Grote, PhD, Abteilung Kardiologie und Angiologie, Medizinische Hochschule Hannover, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany; e-mail: grote.karsten@mh-hannover.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal