To the editor:
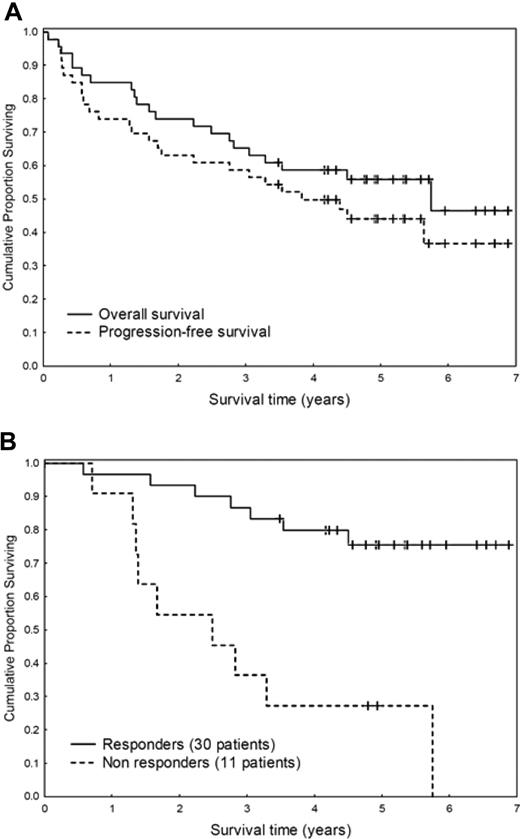
Patients with immunoglobulin light chain (AL) amyloidosis with cardiac or multiorgan involvement are at high risk of death during or immediately after chemotherapy with intravenous melphalan and autologous stem cell transplantation (ASCT), which is considered the most effective treatment for this disease. A risk-adapted reduction of the dose of melphalan renders ASCT feasible in patients with advanced disease at the cost of a reduced response rate and of a still-considerable mortality.1,2 Between 1999 and 2002, we treated with oral melphalan and dexamethasone (M-Dex) 46 consecutive patients ineligible for ASCT, 67% of whom achieved a hematologic response (complete remission [CR] in 33%) in a median of 4.5 months.3 Only 2 patients died while on treatment. A subsequent randomized study showed no difference in response rate and survival between M-Dex and ASCT.4 However, the follow-up of patients treated with M-Dex was limited, and there was concern that response could be short-lived, resulting in reduced survival.5 Thus, we extended the follow-up of the original cohort of patients treated with M-Dex. Median follow-up of living patients is 5.0 years (range, 3.5-6.7 years). A total of 21 patients—13 nonresponders and 8 responders—died after a median of 1.6 years (range, 0.1-5.7 years). Death was not amyloid-related in 4 patients who responded to M-Dex, whereas it was due to progressive cardiac amyloidosis in the remaining cases. Median progression-free and overall survival are 3.8 and 5.1 years, respectively (Figure 1A). Hematologic response significantly prolonged survival (Figure 1B).
Survival of 46 patients with AL amyloidosis ineligible for stem cell transplantation treated with M-Dex. (A) Progression-free (median, 3.8 years) and overall (median, 5.1 years) survival. (B) Effect of hematologic response on survival (6 months landmark). The median is 2.1 years for nonresponders versus not reached for responders (P < .001).
Survival of 46 patients with AL amyloidosis ineligible for stem cell transplantation treated with M-Dex. (A) Progression-free (median, 3.8 years) and overall (median, 5.1 years) survival. (B) Effect of hematologic response on survival (6 months landmark). The median is 2.1 years for nonresponders versus not reached for responders (P < .001).
Two of the 15 patients who achieved CR died due to unrelated causes while still in CR after 2.2 and 3.5 years, respectively. CR was maintained in 9 patients after a median of 4.8 years (range, 3.2-6.1 years). The monoclonal component reappeared in 4 patients after a median of 2.0 years (range, 0.8-2.5 years). In all relapsing patients, CR was restored after 3 cycles (4 cycles in 1 patient) of M-Dex, and is maintained after a median follow-up of 3.0 years (range, 2.1-5.1 years). One patient developed a myelodysplastic syndrome after 2 cycles of M-Dex. This low incidence is probably due to exposure to a modest total dose of melphalan (median, 288 mg; range, 48-912 mg), also considering the additional cycles performed in the 4 relapsing patients. Second-line treatment with thalidomide and dexamethasone (T-Dex)6 was offered to 12 patients. All 5 patients who achieved partial hematologic remission after M-Dex responded to T-Dex, whereas T-Dex induced a response in only 2 of 7 patients who failed to respond to M-Dex, suggesting that T-Dex can improve response to M-Dex, but is less effective in refractory patients. CRs induced by M-Dex are durable, being maintained for at least 3 years in 70% of patients. In relapsing patients, the amyloid clone remains sensitive to M-Dex, and CR can be restored by repeating treatment. The possibility of inducing a high rate of durable responses with M-Dex and its low cost and high practicability should be taken into consideration when planning the treatment strategy of patients with AL amyloidosis.
Authorship
G.P., M.N., and F.L. are partly supported by an investigator fellowship from Collegio Ghislieri, Pavia, Italy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giampaolo Merlini, Amyloid Center, Biotechnology Research Laboratories—Fondazione IRCCS Policlinico San Matteo, Piazzale Golgi, 19, 27100 Pavia, Italy; e-mail: gmerlini@unipv.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal