Abstract
It is well documented that dendritic cells (DCs), representative antigen-presenting cells, are important sources of Th1-promoting cytokines and are actively involved in the regulation of T-helper–cell differentiation. However, the intracellular event that regulates this process is still largely unknown. In this study, we examined the role of Tyk2, a JAK kinase that is involved in the signaling pathway under IL-12 and IL-23, in DC functions. While the differentiation and maturation of DCs was normal in Tyk2-deficient (Tyk2−/−) mice, IL-12–induced Stat4 phosphorylation was diminished in Tyk2−/− DCs. IL-12–induced IFN-γ production was also significantly diminished in Tyk2−/− DCs to levels similar to those in Stat4−/− DCs. Interestingly, Tyk2−/− DCs were defective in IL-12 and IL-23 production upon stimulation with CpG ODN. Furthermore, Tyk2−/− DCs were impaired in their ability to induce Th1-cell differentiation but not Th2-cell differentiation. Taken together, these results indicate that the expression of Tyk2 in DCs is crucial for the production of Th1-promoting cytokines such as IL-12 and IFN-γ from DCs and thereby for the induction of antigen-specific Th1-cell differentiation.
Introduction
The differentiation and commitment of T helper (Th) cells to Th1 cells or Th2 cells depends not only on the strength of T-cell receptor and coreceptor signals provided by the direct interaction between T cells and antigen-presenting cells (APCs) but also on cytokine environment produced by the crosstalk between T cells and APCs.1-3 Dendritic cells (DCs) are representative APCs4,5 and one of the most important DC-derived cytokines for the induction of Th1 cell differentiation is IL-12.6-9 The essential roles of IL-12 in both innate and acquired immunity are evidenced by the analysis of IL-12–deficient mice, in which NK-cell responses and Th1-cell differentiation are impaired.10 In addition to T cells and NK cells, it has been shown that DCs themselves express a high-affinity receptor for IL-12 and can produce considerable amounts of IFN-γ in response to IL-12.11 These observations suggest that DCs are important sources of Th1-promoting cytokines and are thereby involved in the regulation of Th-cell differentiation
The receptor binding of cytokines results in the activation of the Janus family of protein tyrosine kinases (JAKs) and subsequently the activated JAKs phosphorylate signal transducers and activators of transcriptions (STATs).12-14 There are 4 mammalian JAKs: Jak1, Jak2, Jak3, and Tyk2 and they are differentially activated in response to various cytokines.12-14 From the analysis of T cells and NK cells, it has been demonstrated that IL-12 activates Jak2 and Tyk2 and then activates Stat4 that plays pivotal roles in IL-12–induced gene expression.12-14 Analyses of Stat4-deficient (Stat4−/−) mice have shown that Stat4 is required for IL-12–dependent IFN-γ production in T cells and NK cells.15,16
Tyk2 has originally been identified as an essential molecule for mediating IFN-α/β signaling17 and subsequently has been shown to be activated in response to IL-6,18 IL-10,19 IL-12,20 IL-13,21 and IL-23.22 To address the specific and nonredundant role of Tyk2, Tyk2-deficient (Tyk2−/−) mice were generated, and using Tyk2−/− mice, it has been demonstrated that Tyk2 is not essential for many of the biologic responses upon IFN-α/β, IL-6, and IL-10 stimulation.23,24 In contrast, Tyk2 is required for IL-12–induced IFN-γ production in activated T cells.23,24 However, the role of Tyk2 in DC functions including the cytokine production and the induction of T helper cell differentiation is still largely unknown.
In this study, we show that Tyk2 expression is required for IL-12, IL-23, and IFN-γ production from DCs upon activation. We also show that Tyk2 expression in DCs is vital for the induction of antigen-specific Th1 cell differentiation. Our results indicate that Tyk2 expression in DCs is involved in the production of Th1-promoting cytokines upon activation and thereby in the induction of Th1-cell differentiation.
Materials and methods
All experiments were performed according to the guidelines of Chiba University, Chiba, Japan.
Mice
Tyk2-deficient (Tyk2−/−) mice23 were backcrossed for more than 8 generations onto BALB/c mice (Charles River Laboratories, Atsugi, Japan). Stat4-deficient (Stat4−/−) mice15 on a BALB/c background were purchased from The Jackson Laboratory (Bar Harbor, ME). Ovalbumin (OVA)–specific DO11.10 T-cell receptor (TCR) transgenic (DO11.10+) mice were backcrossed more than 10 generations onto BALB/c mice. All mice were housed in microisolator cages under specific pathogen-free conditions and all experiments were performed according to the guidelines of Chiba University.
Reagents
Murine IL-2 and IL-4 were purchased from PeproTech (Rocky Hill, NJ). Murine IL-12 and IL-23 were purchased from R&D Systems (Minneapolis, MN). Phosphorothioate-stabilized CpG oligodeoxynucleotide (ODN) 1668 and lipopolysaccharide (LPS: Escherichia coli 0111-B4) were purchased from Hokkaido System Science (Hokkaido, Japan) and Sigma (St Louis, MO), respectively.
Flow cytometric analysis
Cells were stained and analyzed on a FACSCalibur (BD Biosciences, San Jose, CA) using CellQuest software (BD Biosciences). The following antibodies were purchased from BD PharMingen (San Diego, CA): anti-CD4 FITC, phycoerythrin (PE), allophycocyanin (H129.19), anti-CD8 FITC, PE, allophycocyanin (53-6.7), anti-B220 FITC, PE, PerCP (RA3-6B2), anti-CD3 PE, PerCP (145-2C11), anti-CD19 PE (ID3), anti-CD11b (Mac-1) PE (M1/70), anti-CD11c FITC (HL3), antierythroid PE (TER-119), anti-pan NK PE (DX5), anti-CD80 PE (16-10A1), anti–I-Ad PE (AMS-32.1), anti–IL-12Rβ1 biotin (114), and anti–IL-12Rβ2 (HAM10B9). Before staining, Fc receptors were blocked with anti-CD16/32 antibody (2.4G2; BD PharMingen). Negative controls consisted of isotype-matched, directly conjugated, nonspecific antibodies (BD PharMingen).
Isolation of dendritic cells (DCs)
Splenic DCs were prepared as described previously.25 In brief, spleens were cut into small fragments and then digested with collagenase A (0.5 mg/mL; Roche, Indianapolis, IN) for 10 minutes at 37°C with continuous agitation. After digested fragments were filtered through a stainless-steel sieve, low-density cells were isolated by using OptiPrep reagent (Axis-Shield, Oslo, Norway). Low-density cells were stained with anti-CD11c FITC and after washing with PBS, FITC-stained cells were positively collected using anti-FITC microbeads (Miltenyi Biotec, Sunnyvale, CA) according to the manufacturer's instructions. The resultant cells were routinely more than 95% pure CD11c+ cells by fluorescence-activated cell sorting (FACS) analysis.
Cell culture
Isolated CD11c+ DCs were stimulated with CpG ODN (10 μg/mL), LPS (10 μg/mL), IL-12 (20 ng/mL), or IL-23 (20 ng/mL) in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 50 μM β-mercaptoethanol, 2 mM l-glutamine, and antibiotics (complete RPMI 1640 medium) at 37°C for the indicated time period. In some experiments, CD11c+ DCs were stimulated with CpG ODN (10 μg/mL) in the presence of anti–IL-12 (p40/p70) antibody (10 μg/mL, clone C15.6; BioSource International, Camarillo, CA), anti–IL-12/23 (p40) antibody (10 μg/mL, clone C17.8; BD PharMingen), or anti–type I IFN receptor antisera (50 μg/mL; R&D Systems).
ELISA
The amounts of IFN-γ, IL-12, IL-4, and IL-5 in the culture supernatant were measured by the enzyme immunoassay using murine IFN-γ, IL-12 (p70), IL-4, and IL-5 enzyme-linked immunosorbent assay (ELISA) kits from BD PharMingen. The amounts of IL-23 in the culture supernatant were measured by an IL-23 ELISA kit from eBioscience (San Diego, CA). The amounts of IL-13 and IL-17 in the culture supernatant were measured by ELISA kits from R&D Systems. The assays were performed in duplicate according to the manufacturers' instructions. The minimum significant values of these assays were 15.6 pg/mL IL-4, IL-5, IL-13, and IL-17, 31.3 pg/mL IFN-γ and IL-23, and 62.5 pg/mL IL-12.
Intracellular staining for IFN-γ
Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were stimulated with IL-12 (20 ng/mL) in the complete RPMI 1640 medium for 72 hours. Monensin (2 μM; Sigma) was added for final 4 hours to prevent cytokine release. After surface staining, cells were fixed with IC FIX (BioSource International), permeabilized with IC PERM (BioSource International), and stained with anti–IFN-γ allophycocyanin (XMG1.2; BD PharMingen) as described previously.25
Intracellular staining for TLR9
Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were washed with PBS, fixed with IC FIX, and permeabilized with IC PERM. Cells were then incubated with biotin-conjugated anti-TLR9 antibody (5G5; HyCult Biotechnology, Uden, the Netherlands) or biotin-conjugated control antibody for 30 minutes at room temperature. After washing, cells were incubated with streptavidin allophycocyanin (BD PharMingen) and analyzed on a FACSCalibur.
Intracellular staining for Stat4 and phospho-Stat4
Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were cultured in the presence of CpG ODN (10 μg/mL) for 72 hours. For the evaluation of the phosphorylated form of Stat4, cells were rested for 12 hours in fresh medium and then stimulated with or without IL-12 (10 ng/mL) for 20 minutes. Cells were harvested, washed with PBS, fixed with IC FIX, and permeabilized with 90% methanol and subsequently with IC PERM. Cells were then incubated with anti-Stat4 antibody (Zymed, San Francisco, CA), anti-phospho Stat4 antibody (Zymed), or control rabbit IgG (Serotec, Raleigh, NC) for 30 minutes at room temperature. After washing, cells were incubated with Alexa Fluor 647–conjugated anti–rabbit IgG antibody (Molecular Probes, Eugene, OR) and analyzed on a FACSCalibur.
Role of Tyk2 expression in DCs in Th1- and Th2-cell differentiation
Splenic CD4+ T cells were purified from DO11.10+ mice using T-cell enrichment columns (R&D Systems).26 CD4+ T cells (> 90% pure by flow cytometry) (1 × 106 cells/well) were stimulated with OVA323-339 peptide (0.2 μg/mL) in the presence of isolated CD11c+ DCs (1 × 105 cells/well) from WT or Tyk2−/− splenocytes as antigen-presenting cells for 72 hours. Where indicated, IL-12 (10 ng/mL) or anti–IFN-γ antibody (15 μg/mL, XMG1.2; BD PharMingen) was added to the culture. Five days later, the culture supernatants were harvested for the cytokine assay and cells were restimulated with plate-bound anti-CD3 antibody (5 μg/mL, 145-2C11; BD PharMingen) in the complete RPMI 1640 medium at 37°C for 6 hours with monensin (2 μM) added for the final 4 hours. Cells were stained with anti-CD4 PerCP and anti-CD11c FITC, fixed with IC FIX, permeabilized with IC PERM, and stained with anti–IL-4 PE (BVD4-1D11; BD PharMingen) and anti–IFN-γ allophycocyanin. Cytokine profile (IL-4 vs IFN-γ) in CD4+ CD11c− cells was analyzed on a FACSCalibur.
In vivo function of Tyk2−/− DCs
In vivo function of Tyk2−/− DCs was assessed by an adoptive transfer assay described by Lugo-Villarino et al.27 Briefly, isolated DO11.10+ CD4+ T cells (1 × 106 cells/mouse) were injected intraperitoneally into recipient BALB/c mice. Two days later, isolated WT or Tyk2−/− CD11c+ DCs (3.5 × 105 cells/mouse) were pulsed with OVA323-339 peptide and then injected into footpad of the recipient BALB/c mice. Five days later, popliteal lymph nodes (LNs) were harvested from the recipient mice and LN cells (5 × 105 cells) were stimulated with OVA323-339–pulsed WT CD11c+ DCs for 96 hours at the indicated ratios. The amounts of IL-4 and IFN-γ in the supernatant were measured by ELISA.
Data analysis
Data are summarized as mean plus or minus the standard deviation (SD). The statistical analysis of the results was performed by the unpaired t test. P values less than .05 were considered significant.
Results
The differentiation and maturation of DCs is normal in Tyk2−/− mice
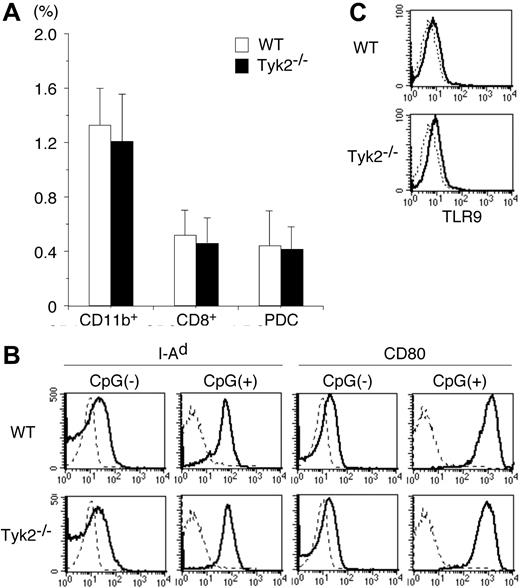
To investigate the role of Tyk2 in DC functions, we first examined whether the differentiation of DCs was normal in Tyk2−/− mice. Although the number of whole splenocytes in Tyk2−/− mice was modestly decreased compared with that in wild-type (WT) mice (Tyk2−/− mice 62.3 ± 8.3 vs WT mice 78.1 ± 11.3, × 106 cells/mouse, mean ± SD, n = 8 mice in each group, P < .01), the frequency of whole DCs (CD11c+ cells), as well as the frequency of each DC subtype, namely CD11b+ DCs, CD8+ DCs, and plasmacytoid DCs (PDCs), was indistinguishable between Tyk2−/− mice and WT mice (Figure 1A).
Differentiation and maturation of DCs are normal in Tyk2−/− mice. (A) Splenocytes from wild-type (WT) mice and Tyk2−/− mice were stained with anti-CD11c FITC, anti-CD11b PE, anti-B220 PerCP, anti-CD3 PerCP, and anti-CD8 allophycocyanin or with anti-CD11c FITC, DX-5 PE, anti-CD11b PE, TER-119 PE, CD19 PE, anti-CD3 PerCP, and anti-B220 allophycocyanin, and the stained cells were analyzed by FACS. The frequencies of CD11b+ DCs (CD11c+ CD11b+ B220− CD3− CD8− cells), CD8+ DCs (CD11c+ CD8+ CD11b− B220− CD3− cells), and plasmacytoid DCs (PDCs) (CD11c+ B220+ CD3− CD11b− CD19− cells) are shown. Data are means (± SD) for 5 mice in each group. (B) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were cultured with CpG ODN (10 μg/mL) for 3 days and analyzed for the expression of I-Ad and CD80 by FACS. As controls, freshly isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were analyzed for the expression of I-Ad and CD80. Shown are representative histograms from 4 independent experiments. Dashed lines indicate the staining with isotype-matched control antibodies. (C) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were subjected to intracellular staining with anti-TLR9 antibody (bold lines) or control antibody (dashed lines). Shown are representative histograms from 3 independent experiments.
Differentiation and maturation of DCs are normal in Tyk2−/− mice. (A) Splenocytes from wild-type (WT) mice and Tyk2−/− mice were stained with anti-CD11c FITC, anti-CD11b PE, anti-B220 PerCP, anti-CD3 PerCP, and anti-CD8 allophycocyanin or with anti-CD11c FITC, DX-5 PE, anti-CD11b PE, TER-119 PE, CD19 PE, anti-CD3 PerCP, and anti-B220 allophycocyanin, and the stained cells were analyzed by FACS. The frequencies of CD11b+ DCs (CD11c+ CD11b+ B220− CD3− CD8− cells), CD8+ DCs (CD11c+ CD8+ CD11b− B220− CD3− cells), and plasmacytoid DCs (PDCs) (CD11c+ B220+ CD3− CD11b− CD19− cells) are shown. Data are means (± SD) for 5 mice in each group. (B) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were cultured with CpG ODN (10 μg/mL) for 3 days and analyzed for the expression of I-Ad and CD80 by FACS. As controls, freshly isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were analyzed for the expression of I-Ad and CD80. Shown are representative histograms from 4 independent experiments. Dashed lines indicate the staining with isotype-matched control antibodies. (C) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were subjected to intracellular staining with anti-TLR9 antibody (bold lines) or control antibody (dashed lines). Shown are representative histograms from 3 independent experiments.
We next examined whether Tyk2 was required for the maturation of DCs. Isolated CD11c+ DCs from spleen of Tyk2−/− mice or littermate WT mice were stimulated with CpG ODN, a ligand for TLR9,28 for 3 days and then analyzed for the expression of I-Ad and CD80 by FACS. The expression levels of I-Ad and CD80 were similarly up-regulated in Tyk2−/− CD11c+ DCs and WT CD11c+ DCs by CpG ODN stimulation (Figure 1B). The expression levels of CD86 were also similarly up-regulated in CpG ODN-stimulated Tyk2−/− CD11c+ DCs and WT CD11c+ DCs (data not shown). Consistent with the similar responses to CpG ODN, the expression levels of TLR9 in the cytoplasm (Figure 1C) as well as on the surface (data not shown) were similar between Tyk2−/− CD11c+ DCs and WT CD11c+ DCs. These results suggest that Tyk2 is not essential for the differentiation and maturation of DCs.
IL-12–induced Stat4 phosphorylation is defective in Tyk2−/− DCs
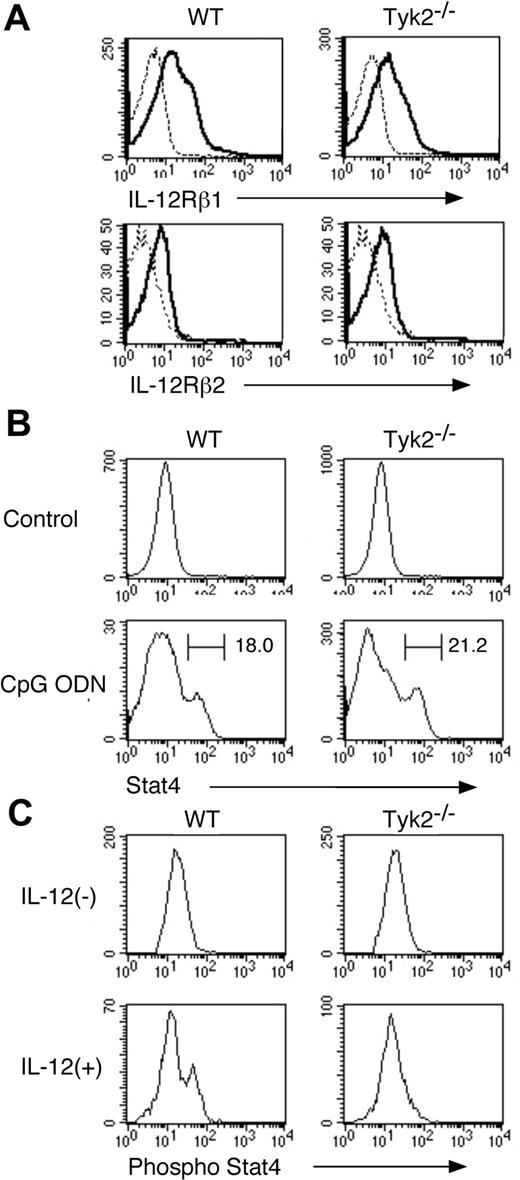
Given that Tyk2 is associated with IL-12 receptor subunit IL-12Rβ1 and Stat4 is an important signaling molecule under IL-12 signaling,12-16 we next examined the expression of IL-12 receptor and Stat4 as well as IL-12–induced phosphorylation of Stat4 in Tyk2−/− CD11c+ DCs. As shown in Figure 2A, the expression levels of IL-12Rβ1 and IL-12Rβ2 in freshly isolated CD11c+ DCs were similar between Tyk2−/− DCs and WT DCs. The expression levels of Stat4 in freshly isolated CD11c+ DCs were very low in both WT DCs and Tyk2−/− DCs (Figure 2B) and upon CpG ODN stimulation, Stat4 expression was similarly up-regulated in Tyk2−/− CD11c+ DCs and WT CD11c+ DCs (Figure 2B). However, when CpG ODN-matured CD11c+ DCs were stimulated with IL-12, IL-12 induced the phosphorylation of Stat4 in WT CD11c+ DCs but not in Tyk2−/− CD11c+ DCs (Figure 2C), indicating that Tyk2 is essential for transducing IL-12 signaling to Stat4 activation in CD11c+ DCs.
IL-12–induced phosphorylation of Stat4 is impaired in Tyk2−/− DCs. (A) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were analyzed for the expression of IL-12Rβ1 and IL-12Rβ2 by FACS. Representative histograms are shown (n = 3). Dashed lines indicate the staining with isotype-matched control antibodies. (B) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were cultured with CpG ODN (10 μg/mL) for 3 days. Intracellular staining for Stat4 was performed and analyzed by FACS. As controls, freshly isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were stained for Stat4. Representative histograms of anti-Stat4 staining gated on CD11c+ cells are shown (n = 4). (C) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were cultured with CpG ODN for 3 days. After cells were washed and rested in fresh medium for 12 hours, cells were stimulated with IL-12 (20 ng/mL) for 20 minutes and intracellular staining for the phosphorylated form of Stat4 was performed and analyzed by FACS. Representative histograms of anti–phospho-Stat4 staining gated on CD11c+ cells are shown (n = 4).
IL-12–induced phosphorylation of Stat4 is impaired in Tyk2−/− DCs. (A) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were analyzed for the expression of IL-12Rβ1 and IL-12Rβ2 by FACS. Representative histograms are shown (n = 3). Dashed lines indicate the staining with isotype-matched control antibodies. (B) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were cultured with CpG ODN (10 μg/mL) for 3 days. Intracellular staining for Stat4 was performed and analyzed by FACS. As controls, freshly isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were stained for Stat4. Representative histograms of anti-Stat4 staining gated on CD11c+ cells are shown (n = 4). (C) Isolated CD11c+ DCs from WT splenocytes or Tyk2−/− splenocytes were cultured with CpG ODN for 3 days. After cells were washed and rested in fresh medium for 12 hours, cells were stimulated with IL-12 (20 ng/mL) for 20 minutes and intracellular staining for the phosphorylated form of Stat4 was performed and analyzed by FACS. Representative histograms of anti–phospho-Stat4 staining gated on CD11c+ cells are shown (n = 4).
IL-12– and IL-23–induced IFN-γ production is diminished in Tyk2−/− DCs
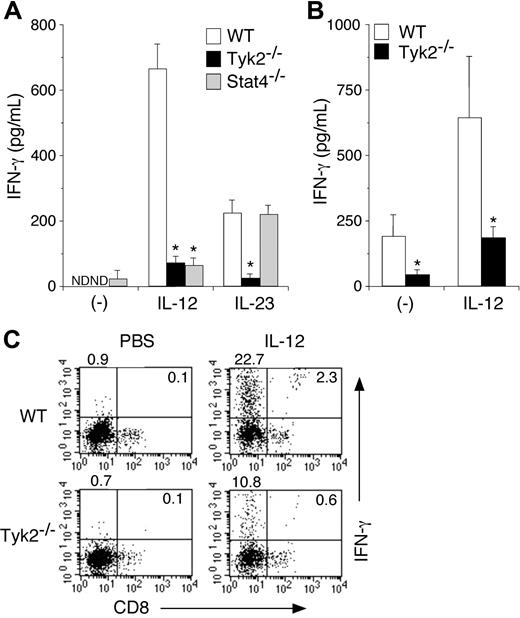
It has been shown that IL-12 induces IFN-γ production from DCs.11,29 It has recently been shown that IL-23, an IL-12–related cytokine that activates Tyk2,22,30 also induces IFN-γ production from DCs.31 We therefore examined whether Tyk2 was required for IL-12– and IL-23–induced IFN-γ production in DCs. Isolated CD11c+ DCs from WT, Tyk2−/−, or Stat4−/− splenocytes were cultured with IL-12 or IL-23 for 3 days, and the amounts of IFN-γ in the supernatants were measured by ELISA. As shown in Figure 3A, IL-12–induced IFN-γ production was significantly decreased in Tyk2−/− CD11c+ DCs and Stat4−/− CD11c+ DCs compared with that in WT CD11c+ DCs (WT 668.3 ± 77.8 vs Tyk2−/− 77.4 ± 22.0 vs Stat4−/− 70.8 ± 24.2, pg/mL, n = 5, P < .01) (Figure 3A). IL-23 also induced IFN-γ production in WT CD11c+ DCs, although the levels were lower than those by IL-12 stimulation (Figure 3A) even when the amount of IL-23 was increased (data not shown). Interestingly, IL-23–induced IFN-γ production was impaired in Tyk2−/− CD11c+ DCs but not in Stat4−/− CD11c+ DCs. These results indicate that both IL-12 and IL-23 induce IFN-γ production through Tyk2 activation in DCs, the former of which is Stat4 dependent but the latter is Stat4 independent.
IL-12– and IL-23–induced IFN-γ production is impaired in Tyk2−/− DCs. (A) Isolated CD11c+ DCs from WT, Tyk2−/−, or Stat4−/− splenocytes were cultured with IL-12 (20 ng/mL) or IL-23 (20 ng/mL) for 3 days. The amounts of IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 5 independent experiments. ND indicates not detectable. *Significantly different from the corresponding mean values of WT DCs, *P < .01. (B) Isolated CD11c+ DCs from WT or Tyk2−/− splenocytes were cultured with CpG ODN for 3 days. After cells were washed and rested in fresh medium for 12 hours, cells were stimulated with IL-12 (20 ng/mL) for 3 days. The amounts of IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 4 independent experiments. *Significantly different from the corresponding mean values of WT DCs, *P < .05. (C) Isolated CD11c+ DCs from WT or Tyk2−/− splenocytes were cultured with IL-12 (20 ng/mL) for 72 hours with monensin added for the final 4 hours. Cells were stained with anti-CD11c FITC and anti-CD8 PE and then the production of IFN-γ was evaluated by intracellular staining by FACS. Representative FACS profiles of CD8 versus IFN-γ gated on CD11c+ cells are shown (n = 4, each).
IL-12– and IL-23–induced IFN-γ production is impaired in Tyk2−/− DCs. (A) Isolated CD11c+ DCs from WT, Tyk2−/−, or Stat4−/− splenocytes were cultured with IL-12 (20 ng/mL) or IL-23 (20 ng/mL) for 3 days. The amounts of IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 5 independent experiments. ND indicates not detectable. *Significantly different from the corresponding mean values of WT DCs, *P < .01. (B) Isolated CD11c+ DCs from WT or Tyk2−/− splenocytes were cultured with CpG ODN for 3 days. After cells were washed and rested in fresh medium for 12 hours, cells were stimulated with IL-12 (20 ng/mL) for 3 days. The amounts of IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 4 independent experiments. *Significantly different from the corresponding mean values of WT DCs, *P < .05. (C) Isolated CD11c+ DCs from WT or Tyk2−/− splenocytes were cultured with IL-12 (20 ng/mL) for 72 hours with monensin added for the final 4 hours. Cells were stained with anti-CD11c FITC and anti-CD8 PE and then the production of IFN-γ was evaluated by intracellular staining by FACS. Representative FACS profiles of CD8 versus IFN-γ gated on CD11c+ cells are shown (n = 4, each).
We next examined IL-12–induced IFN-γ production in CpG ODN–matured Tyk2−/− CD11c+ DCs and WT CD11c+ DCs, both of which express a considerable amount of Stat4 (Figure 2B). IL-12–induced IFN-γ production was also significantly decreased in Tyk2−/− CD11c+ DCs compared with that in WT CD11c+ DCs (Figure 3B).
To determine which DC subtypes produce mainly IFN-γ upon IL-12 stimulation, we performed intracellular IFN-γ staining for IL-12–stimulated CD11c+ DCs. As shown in Figure 3C, among WT CD11c+ DCs, CD8− populations produced mainly IFN-γ upon IL-12 stimulation. Consistent with the data shown in Figure 3A, the number of IFN-γ–producing cells was significantly decreased in Tyk2−/− CD11c+ DCs compared with that in WT CD11c+ DCs (Figure 3C).
CpG ODN–induced IFN-γ production is diminished in Tyk2−/− DCs
Since TLR signaling is crucial for the activation of DCs and their cytokine production,28 we then examined IFN-γ production in Tyk2−/− DCs upon stimulation with CpG ODN. When WT CD11c+ DCs were stimulated with CpG ODN, they produced a considerable amount of IFN-γ (Figure 4). Interestingly, CpG ODN–induced IFN-γ production was significantly decreased in Tyk2−/− CD11c+ DCs and Stat4−/− CD11c+ DCs (n = 5, P < .01) (Figure 4), although Tyk2 and Stat4 have not been shown to be involved in signaling via TLR.28 Indeed, we could not detect the phosphorylation of Tyk2 in WT CD11c+ DCs upon CpG ODN stimulation (data not shown). Taken together, these results suggest that CpG ODN–induced IFN-γ production in DCs is mediated by a cytokine(s) that uses Tyk2 and Stat4 as signaling molecules.
CpG ODN–induced IFN-γ production is diminished in Tyk2−/− DCs. Isolated CD11c+ DCs from WT, Tyk2−/−, or Stat4−/− splenocytes were cultured with CpG ODN for 3 days. The amounts of IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 5 independent experiments. ND indicates not detectable. *Significantly different from the mean value of WT DCs, *P < .01.
CpG ODN–induced IFN-γ production is diminished in Tyk2−/− DCs. Isolated CD11c+ DCs from WT, Tyk2−/−, or Stat4−/− splenocytes were cultured with CpG ODN for 3 days. The amounts of IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 5 independent experiments. ND indicates not detectable. *Significantly different from the mean value of WT DCs, *P < .01.
CpG ODN–induced IL-12 production is diminished in Tyk2−/− DCs and is required for IFN-γ production in DCs
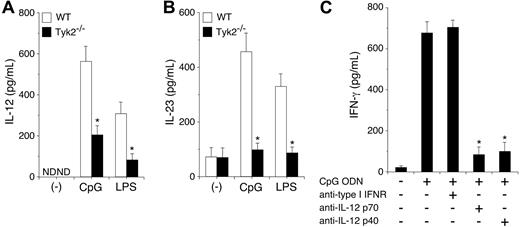
To determine the mechanism by which Tyk2 is involved in CpG ODN–induced IFN-γ production from DCs, we first examined IL-12 and IL-23 production in CpG ODN–stimulated CD11c+ DCs. As shown in Figure 5A-B, CpG ODN induced IL-12 and IL-23 production in WT CD11c+ DCs. Unexpectedly, CpG ODN–induced IL-12 and IL-23 production was significantly decreased in Tyk2−/− CD11c+ DCs compared with WT CD11c+ DCs (n = 5, P < .01) (Figure 5A-B). LPS-induced IL-12 and IL-23 production was also decreased in Tyk2−/− CD11c+ DCs compared with WT CD11c+ DCs (n = 5, P < .01) (Figure 5A-B). These results suggest that the production of IL-12 and IL-23 from TLR-stimulated DCs also depends in part on cytokines that use Tyk2 as a signaling molecule.
CpG ODN–induced IL-12 production depends on Tyk2 and is required for IFN-γ production in DCs. (A-B) Isolated CD11c+ DCs from WT or Tyk2−/− splenocytes were cultured with CpG ODN or LPS for 24 hours. The amounts of (A) IL-12 and (B) IL-23 in the supernatants were measured by ELISA. Data are means (± SD) from 5 independent experiments. ND indicates not detectable. *Significantly different from the corresponding mean values of WT DCs, *P < .01. (C) Isolated CD11c+ DCs from WT splenocytes were cultured with CpG ODN in the presence of anti–type I IFN receptor antibody, anti–IL-12 p70 antibody, or anti–IL-12 p40 antibody for 3 days. The amounts of IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 5 independent experiments. *Significantly different from the mean value of the control response (CpG ODN alone), P < .01.
CpG ODN–induced IL-12 production depends on Tyk2 and is required for IFN-γ production in DCs. (A-B) Isolated CD11c+ DCs from WT or Tyk2−/− splenocytes were cultured with CpG ODN or LPS for 24 hours. The amounts of (A) IL-12 and (B) IL-23 in the supernatants were measured by ELISA. Data are means (± SD) from 5 independent experiments. ND indicates not detectable. *Significantly different from the corresponding mean values of WT DCs, *P < .01. (C) Isolated CD11c+ DCs from WT splenocytes were cultured with CpG ODN in the presence of anti–type I IFN receptor antibody, anti–IL-12 p70 antibody, or anti–IL-12 p40 antibody for 3 days. The amounts of IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 5 independent experiments. *Significantly different from the mean value of the control response (CpG ODN alone), P < .01.
To determine whether the endogenously produced IL-12 and IL-23 from CpG ODN–stimulated DCs are involved in IFN-γ production, we examined the effect of anti-p70 antibody, which specifically inhibits IL-12, and anti-p40 antibody, which inhibits both IL-12 and IL-23, on CpG ODN-induced IFN-γ production in CD11c+ DCs. As shown in Figure 5C, anti-p40 antibody significantly inhibited CpG ODN–induced IFN-γ production in CD11c+ DCs. Anti–IL-12 p70 antibody also significantly inhibited CpG ODN–induced IFN-γ production in CD11c+ DCs to a similar extent as did anti-p40 antibody (n = 5, P < .01). In contrast, anti–type I IFN-receptor antibody did not inhibit CpG ODN–induced IFN-γ production in CD11c+ DCs (Figure 5C). Together with the finding of Tyk2 and Stat4 dependency of CpG ODN–induced IFN-γ production in DCs (Figure 4), these results indicate that IL-12 mediates mainly CpG ODN–induced IFN-γ production through Tyk2 and Stat4 activation in DCs.
The expression of Tyk2 in DCs is required for optimal Th1 cell differentiation
We next examined the possible involvement of Tyk2 expression in DCs in Th1- and Th2-cell differentiation. CD4+ T cells from DO11.10+ mice were stimulated with OVA323-339 peptide in the presence of WT CD11c+ DCs or Tyk2−/− CD11c+ DCs as APCs, and the differentiation of Th1 cells and Th2 cells was evaluated by intracellular staining for IL-4 and IFN-γ. As shown in Figure 6A, without exogenous cytokines, CD4+ T cells that produce IFN-γ but not IL-4 (Th1 cells) were significantly decreased when CD4+ T cells were stimulated with Tyk2−/− DCs (Tyk2−/− DCs 14.4% ± 3.6% vs WT DCs 20.8% ± 5.2%, n = 5, P < .05). In contrast, when CD4+ T cells were stimulated in the presence of exogenous IL-12, Th1-cell differentiation was similarly induced by Tyk2−/− DCs and WT DCs (Figure 6A). When endogenously produced IFN-γ was neutralized with anti–IFN-γ antibody, Th1-cell differentiation was similarly reduced regardless of whether CD4+ T cells were stimulated with Tyk2−/− DCs or WT DCs (Figure 6A). Accordingly, the levels of IFN-γ but not of IL-4, IL-5, or IL-13 in the culture supernatants were significantly decreased when CD4+ T cells were stimulated by Tyk2−/− DCs in the absence of exogenous IL-12 (Figure 6B and data not shown). On the other hand, proliferation of CD4+ T cells was similarly induced by Tyk2−/− DCs and WT DCs (Figure 6C). These results suggest that Tyk2 expression in DCs is involved in the induction of Th1-cell differentiation presumably by producing a Th1-promoting cytokine IL-12 (and subsequently IFN-γ).
The expression of Tyk2 in DCs is required for the optimal Th1-cell differentiation. (A) Splenic CD4+ T cells (1 × 106 cells) from DO11.10+ mice were stimulated with OVA323–339 peptide (0.2 μg/mL) in the presence of WT or Tyk2−/− CD11c+ DCs (2 × 105 cells). Where indicated, IL-12 (10 ng/mL) or anti–IFN-γ antibody (15 μg/mL) was added to the culture. Five days later, intracellular staining for IL-4 and IFN-γ was performed and analyzed by FACS. Representative FACS profiles of IL-4 versus IFN-γ gated on CD4+ CD11c− cells are shown from 5 independent experiments. (B) DO11.10+ CD4+ T cells were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs with or without IL-12 (10 ng/mL). Five days later, the levels of IFN-γ, IL-4, and IL-5 in the culture supernatants were determined by ELISA. Data are means (± SD) from 5 independent experiments. *Significantly different from the corresponding mean values of WT DCs, *P < .05. (C) DO11.10+ CD4+ T cells (2 × 105 cells) were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs (4 × 104 cells) with or without IL-12 in a 96-well microtiter plate for 72 hours, with 1 μCi (0.037 MBq) [3H] thymidine added for the final 12 hours. Data are means (± SD) of [3H] thymidine uptake from 4 independent experiments. (D) DO11.10+ CD4+ T cells were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs. Where indicated, IL-12 or anti–IFN-γ antibody was added to the culture. Five days later, the levels of IL-17 in the culture supernatants were determined by ELISA. Data are means (± SD) from 5 independent experiments. *P < .05.
The expression of Tyk2 in DCs is required for the optimal Th1-cell differentiation. (A) Splenic CD4+ T cells (1 × 106 cells) from DO11.10+ mice were stimulated with OVA323–339 peptide (0.2 μg/mL) in the presence of WT or Tyk2−/− CD11c+ DCs (2 × 105 cells). Where indicated, IL-12 (10 ng/mL) or anti–IFN-γ antibody (15 μg/mL) was added to the culture. Five days later, intracellular staining for IL-4 and IFN-γ was performed and analyzed by FACS. Representative FACS profiles of IL-4 versus IFN-γ gated on CD4+ CD11c− cells are shown from 5 independent experiments. (B) DO11.10+ CD4+ T cells were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs with or without IL-12 (10 ng/mL). Five days later, the levels of IFN-γ, IL-4, and IL-5 in the culture supernatants were determined by ELISA. Data are means (± SD) from 5 independent experiments. *Significantly different from the corresponding mean values of WT DCs, *P < .05. (C) DO11.10+ CD4+ T cells (2 × 105 cells) were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs (4 × 104 cells) with or without IL-12 in a 96-well microtiter plate for 72 hours, with 1 μCi (0.037 MBq) [3H] thymidine added for the final 12 hours. Data are means (± SD) of [3H] thymidine uptake from 4 independent experiments. (D) DO11.10+ CD4+ T cells were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs. Where indicated, IL-12 or anti–IFN-γ antibody was added to the culture. Five days later, the levels of IL-17 in the culture supernatants were determined by ELISA. Data are means (± SD) from 5 independent experiments. *P < .05.
Given that the production of IL-23, an important cytokine that enhances the survival of IL-17–producing CD4+ T cells (Th17 cells),32 was decreased in Tyk2−/− DCs (Figure 5B), we next investigated the ability of Tyk2−/− DCs for IL-17 production from CD4+ T cells. However, unexpectedly, the levels of IL-17 were rather increased when CD4+ T cells were stimulated with Tyk2−/− DCs (Figure 6D). Importantly, the addition of anti–IFN-γ antibody canceled the difference of IL-17 induction by Tyk2−/− DCs and WT DCs (Figure 6D), suggesting that the enhanced IL-17 induction by Tyk2−/− DCs may result from the lower levels of IFN-γ in the culture (Figure 6B).
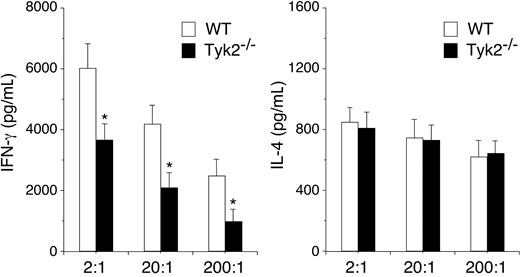
Finally, we examined the role of Tyk2 expression in DCs in vivo using a system established by Lugo-Villarino et al.27 Purified CD4+ T cells from DO11.10+ mice were transferred intraperitoneally into recipient BALB/c mice and 2 days later, OVA323-339–pulsed WT or Tyk2−/− CD11c+ DCs were injected into footpad of the recipient mice. Five days later, the popliteal lymph nodes (LNs) were harvested from the recipient mice, and cytokine production by LN cells stimulated with OVA323-339–pulsed WT CD11c+ DCs was evaluated. As shown in Figure 7, IFN-γ production was significantly decreased in LN cells that were primed in vivo with Tyk2−/− CD11c+ DCs compared with that of WT CD11c+ DCs (n = 4, P < .05). In contrast, IL-4 production was similarly induced in LN cells that were primed with Tyk2−/− CD11c+ DCs and WT CD11c+ DCs (Figure 7). These results indicate that the expression of Tyk2 in DCs is required for Th1 cell differentiation but not for Th2 cell differentiation.
The expression of Tyk2 in DCs is required for in vivo priming of Th1 cells. DO11.10+ CD4+ T cells (1 × 106 cells/mouse) were injected intraperitoneally into recipient BALB/c mice. Two days later, OVA323–339–pulsed WT or Tyk2−/− CD11c+ DCs (3.5 × 105 cells/mouse) were injected into footpad of the recipient mice. Five days later, popliteal lymph node cells were harvested from the recipient mice and cultured with OVA323–339–pulsed WT CD11c+ DCs at the indicated ratios for 96 hours. The amounts of IL-4 and IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 4 independent experiments. *P < .05.
The expression of Tyk2 in DCs is required for in vivo priming of Th1 cells. DO11.10+ CD4+ T cells (1 × 106 cells/mouse) were injected intraperitoneally into recipient BALB/c mice. Two days later, OVA323–339–pulsed WT or Tyk2−/− CD11c+ DCs (3.5 × 105 cells/mouse) were injected into footpad of the recipient mice. Five days later, popliteal lymph node cells were harvested from the recipient mice and cultured with OVA323–339–pulsed WT CD11c+ DCs at the indicated ratios for 96 hours. The amounts of IL-4 and IFN-γ in the supernatants were measured by ELISA. Data are means (± SD) from 4 independent experiments. *P < .05.
Discussion
In the present study, we show that the expression of Tyk2 in DCs is required for the production of IL-12 and IFN-γ upon activation and thereby for the induction of Th1-cell differentiation. We found that while the differentiation and maturation of DCs was normal in Tyk2−/− mice (Figure 1), IL-12–induced Stat4 phosphorylation was diminished in Tyk2−/− DCs (Figure 2). Accordingly, IL-12–induced IFN-γ production was significantly diminished in Tyk2−/− DCs to levels similar to those in Stat4−/− DCs (Figure 3). CpG ODN–induced IL-12 production and subsequent IFN-γ production were also significantly diminished in Tyk2−/− DCs (Figures 4–5). Moreover, Tyk2−/− DCs were impaired in their ability to induce Th1-cell differentiation (Figures 6–7). These results indicate that Tyk2 expression in DCs is crucial for the regulation of T helper cell differentiation by producing Th1 cell–promoting cytokines such as IL-12 and IFN-γ upon activation.
We show that Tyk2-Stat4 pathway is required for IL-12–induced IFN-γ production in DCs. We found that Stat4 phosphorylation (Figure 2) and IFN-γ production (Figure 3) in response to IL-12 were impaired in Tyk2−/− DCs. IFN-γ production in response to IL-12 was also impaired in Stat4−/− DCs (Figure 3). Therefore, analogous to T cells and NK cells,15,16 Tyk2-Stat4 pathway is essential for transducing IL-12 signaling for gene expression of IFN-γ in DCs. In contrast, we found that, interestingly, IL-23–induced IFN-γ production was impaired in Tyk2−/− DCs but not in Stat4−/− DCs (Figure 3A). These results indicate that both IL-12 and IL-23 induce IFN-γ production through Tyk2 activation in DCs, but IL-23–induced IFN-γ production in DCs is Stat4 independent. This is consistent with the finding that Stat3 is predominantly activated by IL-23 signaling.22,30
We also found that CPG ODN–induced IFN-γ production was severely decreased in Tyk2−/− DCs to levels similar to those in Stat4−/− DCs (Figure 4). Consistently, it has been demonstrated that Tyk2−/− macrophages are defective in LPS-induced IFN-β and IFN-γ production.33,34 Moreover, we found that a blocking antibody against IL-12 inhibited CpG ODN–induced IFN-γ production in WT DCs (Figure 5C), suggesting that the activation of Tyk2-Stat4 pathway by endogenously produced IL-12 is crucial for CpG ODN–induced IFN-γ production in DCs.
We unexpectedly found that Tyk2−/− DCs were impaired in the ability to produce IL-12 and IL-23 upon CPG ODN or LPS stimulation (Figure 5). On the other hand, we could not detect the activation of Tyk2 by CPG ODN stimulation in DCs (data not shown), although some tyrosine kinases such as Btk have been shown to be directly involved in the signaling pathways under TLRs.35 Therefore, it is suggested that Tyk2-activating cytokines produced by CPG ODN–stimulated DCs are involved in IL-12 and IL-23 production from DCs. In this regard, it has been shown that IL-12 acts directly on DCs and induces IL-12 production from DCs.31 We also found that IL-12 induced IL-12 p35 mRNA expression in DCs (data not shown). These results suggest that IL-12 is a candidate for Tyk2-activating cytokines that are involved in IL-12 production from CPG ODN–stimulated DCs and that this Tyk2-activating cytokine cascade in DCs could be involved as an amplification loop in the production of Th1 cell–promoting cytokines by paracrine and/or autocrine mechanisms.
Consistent with the impaired IL-12 and IFN-γ production in Tyk2−/− DCs, we demonstrate that Tyk2 expression in DCs is crucial for inducing the differentiation of Th1 cells but not of Th2 cells upon antigen stimulation (Figures 6–7). Our findings that the addition of IL-12 could overcome the impaired Th1-cell–promoting ability of Tyk2−/− DCs (Figure 6) suggest that the defective IL-12 production in Tyk2−/− DCs is responsible in part for their impaired Th1-cell–promoting ability. Because Th1 cells are required for counterbalancing Th2-cell differentiation,1,2 the impaired Th1-cell–promoting ability of Tyk2−/− DCs may also provide a possible explanation for our previous finding36 that antigen-induced Th2-cell differentiation and subsequent allergic airway inflammation are enhanced in Tyk2−/− mice. Discovery of a Tyk2-deficient patient who suffers from severe atopic dermatitis with elevated serum IgE and is diagnosed with hyper-IgE syndrome37 also supports the notion that the impaired Tyk2 function may be involved in the pathogenesis of allergic diseases.
It has recently been shown that Th17 cells are a distinct lineage from Th1 cells and Th2 cells, and that IL-23 enhances the survival of Th17 cells.32 However, we show here that while Tyk2−/− DCs produces less IL-23 compared with WT DCs (Figure 5B), IL-17 production is enhanced when CD4+ T cells are cultured with Tyk2−/− DCs (Figure 6D). Interestingly, the enhanced IL-17 production from CD4+ T cells stimulated with Tyk2−/− DCs was cancelled by the addition of anti–IFN-γ antibody (Figure 6D). Because IFN-γ has been shown to repress the differentiation of Th17 cells,32 it is suggested that the enhanced IL-17 induction may result from the decreased ability of Tyk2−/− DCs for IFN-γ production.
Recently, accumulating evidence suggests that Tyk2 is required for the protective immune response against a number of pathogens. It has been shown that Tyk2 is required for harboring efficient CTL responses upon lymphocytic choriomeningitis virus infection24 and clearance of murine cytomegalovirus.38 In addition, Tyk2−/− mice have been shown to be susceptible to Toxoplasma gondii39,40 and Leishmania major.41 Moreover, it has recently been shown that Tyk2−/− mice exhibit increased susceptibility to an infection with Listeria monocytogenes.42 In humans, a hyper-IgE syndrome patient who lacks Tyk2 also shows unusual susceptibility to various microorganisms including viruses, fungi, and mycobacteria.37 These results indicate that Tyk2 plays a nonredundant role in the protective immune responses against pathogens in both mice and humans.
Although the precise mechanism underlying Tyk2-mediated protective immune responses is still unclear, the production of IFN-γ through Tyk2-mediated signaling could participate in this process. It is well established that IFN-γ plays a crucial role in the development of effective adaptive immune responses in controlling intracellular pathogens.43-46 In Listeria monocytogenes infection, it has been reported that Tyk2-mediated signaling in DCs is responsible in part for the induction of MHC class I–restricted, IFN-γ–producing CD8+ T cells.42 In this context, IFN-γ production by DCs offers a plausible and attractive explanation for the induction of antigen-specific T-cell responses. Because DCs are on the front lines of the defense against pathogens, the local production of IFN-γ by DCs even in small amounts could influence Th1-cell differentiation at the time of antigen presentation and could contribute significantly to the initial control of infections. Our data support the notion that the defective IFN-γ production in Tyk2−/− DCs could be involved in the impaired immune responses against pathogens in Tyk2−/− mice presumably by involving a positive-feedback regulation for Th1 cell differentiation.
In conclusion, we have shown that the expression of Tyk2 in DCs is required for IL-12 and IFN-γ production upon activation and contributes to the induction of Th1-cell differentiation. Although further studies are required to address the physiological significance of Tyk2 expression in DCs, our results would give a new insight into the crosstalk between DCs and T cells for the induction of Th1-cell differentiation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government.
We thank Drs D. Y. Loh and K. M. Murphy for DO11.10 mice and Dr K. I. Nakayama for Tyk2−/− mice.
Authorship
Contribution: N.T. performed research; A.S. designed the research; S.K. performed research; S.F. performed research; K.H. designed the research; N.W. analyzed data; Y.S. designed the research; K.S. contributed vital new reagents; I.I. wrote the paper; H.N. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiroshi Nakajima, Department of Molecular Genetics, Graduate School of Medicine, Chiba University, 1-8-1 Inohana, Chiba City, Chiba 260-8670, Japan; e-mail: nakajimh@faculty.chiba-u.jp.


![Figure 6. The expression of Tyk2 in DCs is required for the optimal Th1-cell differentiation. (A) Splenic CD4+ T cells (1 × 106 cells) from DO11.10+ mice were stimulated with OVA323–339 peptide (0.2 μg/mL) in the presence of WT or Tyk2−/− CD11c+ DCs (2 × 105 cells). Where indicated, IL-12 (10 ng/mL) or anti–IFN-γ antibody (15 μg/mL) was added to the culture. Five days later, intracellular staining for IL-4 and IFN-γ was performed and analyzed by FACS. Representative FACS profiles of IL-4 versus IFN-γ gated on CD4+ CD11c− cells are shown from 5 independent experiments. (B) DO11.10+ CD4+ T cells were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs with or without IL-12 (10 ng/mL). Five days later, the levels of IFN-γ, IL-4, and IL-5 in the culture supernatants were determined by ELISA. Data are means (± SD) from 5 independent experiments. *Significantly different from the corresponding mean values of WT DCs, *P < .05. (C) DO11.10+ CD4+ T cells (2 × 105 cells) were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs (4 × 104 cells) with or without IL-12 in a 96-well microtiter plate for 72 hours, with 1 μCi (0.037 MBq) [3H] thymidine added for the final 12 hours. Data are means (± SD) of [3H] thymidine uptake from 4 independent experiments. (D) DO11.10+ CD4+ T cells were stimulated with OVA323–339 peptide in the presence of WT or Tyk2−/− CD11c+ DCs. Where indicated, IL-12 or anti–IFN-γ antibody was added to the culture. Five days later, the levels of IL-17 in the culture supernatants were determined by ELISA. Data are means (± SD) from 5 independent experiments. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-11-059246/4/m_zh80130704250006.jpeg?Expires=1768514153&Signature=RerSeEa-gRItUPoQbF3s7lACyvyLOXZ1XqbQ4yv2vdNmUu1FO0Z3JZkb33XyjNpQ~Pn5HVGV1eL7ICTSh8mAD5bk5vaWx~aiOqUzJB0jkm2BPJ6BP~8ywGlJ5QStHJg-B-XFf1d8TL4ujbA~17po3XjGgyPKgV-KZBKBPCOFPjL09-9fYNmJpX983op8UvEf4aGJWXn5g8l5CO0jq4OrD~ktiMeO8Y8kYkmgtn35ylMLa8rzixeDb9l4kf630mZwRrVgr~bn~MIqwgFt9xGUfRzkYblRIYco55NCOWDt1LVPIrsuefCFPUswlY6flDZTOqTy1-Er2gb7nAcGCtOqiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal