Abstract
Coronary artery thrombosis is often initiated by platelet activation on collagen-rich subendothelial layers in the disrupted atherosclerotic plaque. The activating platelet collagen receptor glycoprotein VI (GPVI) noncovalently associates with the Fc receptor γ-chain (FcRγ), which signals through its immunoreceptor-tyrosine–based activation motif (ITAM) via the adaptor LAT leading to the activation of phospholipase Cγ2 (PLCγ2). GPVI is a promising antithrombotic target as anti-GPVI antibodies induce the irreversible loss of the receptor from circulating platelets by yet undefined mechanisms in humans and mice and long-term antithrombotic protection in the latter. However, the treatment is associated with transient but severe thrombocytopenia and reduced platelet reactivity to thrombin questioning its clinical usefulness. Here we show that GPVI down-regulation occurs through 2 distinct pathways, namely ectodomain shedding or internalization/intracellular clearing, and that both processes are abrogated in mice carrying a point mutation in the FcRγ-associated ITAM. In mice lacking LAT or PLCγ2, GPVI shedding is abolished, but the receptor is irreversibly down-regulated through internalization/intracellular clearing. This route of GPVI loss is not associated with thrombocytopenia or altered thrombin responses. These results reveal the existence of 2 distinct signaling pathways downstream of the FcRγ-ITAM and show that it is possible to uncouple GPVI down-regulation from undesired side effects with obvious therapeutic implications.
Introduction
Arterial thrombosis is often initiated by abrupt disruption of the atherosclerotic plaque and deposition and activation of platelets on the subendothelial layers that are enriched in highly thrombogenic collagens.1-3 Glycoprotein VI (GPVI)4-6 is an essential receptor for collagen-induced platelet activation, adhesion, and thrombus growth.7 It noncovalently associates with the Fc receptor γ-chain (FcRγ-chain)8,9 and the complex signals through sequential activation of Src and Syk family tyrosine kinases. The Src kinases Fyn and Lyn stimulate tyrosine phosphorylation of 2 conserved tyrosines in the FcRγ-chain immunoreceptor tyrosine–based activation motif (ITAM).10,11 This leads to engagement of Syk via its 2 SH2 domains and its subsequent activation. Syk orchestrates a downstream signaling cascade that is regulated through the interaction of several adaptor proteins, including LAT, and SLP-76, and leads to activation of effector enzymes, including phosphatidylinositol 3-kinases (PI3-kinases) and phospholipase Cγ2 (PLCγ2).12
GPVI-deficient patients suffer from a mild bleeding diathesis and their platelets show severely impaired responses to collagen.4,13,14 Similarly, platelets from GPVI-deficient mice15,16 or FcRγ-chain–deficient mice, which also lack GPVI,17 do not aggregate to collagen, but no major bleeding has been observed in those animals, making GPVI a potential target for safe antithrombotic therapy. In vivo treatment of mice with the anti-GPVI antibody JAQ1 induces the rapid and quantitative down-regulation of the receptor in circulating platelets resulting in a prolonged “GPVI knockout”–like phenotype18 and profound antithrombotic protection in various thrombosis models.19,20 Antibody-induced loss of GPVI has also been reported in autoimmune patients who had developed anti-GPVI antibodies resulting in clearing of the receptor from their platelets.4,14 Furthermore, it has been demonstrated that GPVI can be down-regulated in human platelets by anti-GPVI antibodies in vivo in a newly established nonobese diabetic severe combined immunodeficient (NOD/SCID) mouse model,21 confirming that anti-GPVI treatment may be a powerful strategy to specifically shut off one central activation pathway in platelets for a prolonged period of time while preserving other functions.
Importantly, however, anti-GPVI antibodies cause severe transient thrombocytopenia in mice,18,22 and, in the early phase after anti-GPVI treatment, platelets are not only GPVI-deficient but also display a diminished response to thrombin.23 Moreover, similar effects are observed with human platelets.21 Although these undesired side effects are fully reversed after 2 to 3 days, they are potentially problematic because they lead to a transient but dramatic increase in bleeding times during the early phase after anti-GPVI treatment in mice.23
GPVI down-regulation can occur through internalization/intracellular clearing18 as well as ectodomain shedding by an unidentified platelet-derived metalloproteinase.24-26 It is currently unclear by which route antibody-induced down-regulation of the receptor occurs in vivo and whether signaling downstream of the receptor is involved in these processes. We here show that antibody-induced down-regulation of GPVI occurs through internalization and shedding and that both pathways, as well as transient thrombocytopenia, the defect in thrombin responses, and increase in tail bleeding time, are absent in mice carrying a point mutation in the ITAM motif of the FcRγ-chain (FcRγ-YF). Using mice deficient in PLCγ2 or LAT, we identify a novel signaling pathway downstream of GPVI that bypasses these 2 molecules and show that the “classical” GPVI signaling is responsible for GPVI shedding, but not for internalization. Antibody-induced thrombocytopenia and increase in bleeding time were absent in LAT-deficient mice, showing that it is possible to uncouple the associated side effects from the down-regulation process.
Materials and methods
All experiments and animal care were approved by the local Animal Care and Use Committee.
Animals
NMRI and C57BL/6 wild-type mice 6 to 10 weeks of age were obtained from Charles River (Sulzfeld, Germany) and Jackson Laboratory (Bar Harbor, ME), respectively, and kept in our animal facilities. FcRγ−/−, FcRγ-YF (TG), LAT−/−, and PLCγ2−/− mice have been described previously.27-31
Reagents
JAQ1, JAQ3 (anti-GPVI), and EDL1 (anti-GPIIIa) were generated in our laboratory and have been described.17,22,32 JON/A-PE and anti–P-selectin-FITC were from Emfret Analytics (Würzburg, Germany). EZ-Link sulfo-NHS-LC-biotin (Pierce, Rockford, IL), PGI2, high-molecular-weight heparin, and streptavidin-HRP (DAKO, Glostrup, Denmark) were purchased.
Platelet preparation
Mice were bled from the retroorbital plexus. Blood was collected in a tube containing 10 U/mL heparin, and platelet-rich plasma (prp) was obtained by centrifugation at 300g for 10 minutes at room temperature (RT). For washed platelets, prp was centrifuged at 1000g for 5 minutes and the pellet was resuspended twice in modified Tyrode-Hepes buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM Hepes, 5 mM glucose, 0.35% bovine serum albumin, pH 6.6) in the presence of prostacyclin (0.1 μg/mL) and apyrase (0.02 U/mL). Platelets were then resuspended in the same buffer (pH 7.0, 0.02 U/mL apyrase) and incubated at 37°C for at least 30 minutes before analysis.
GPVI ELISA shedding assay
Plasma from mice treated with 20 μg biotinylated JAQ1 or vehicle was incubated at serial dilutions on JAQ3-coated (10 μg/mL) enzyme-linked immunosorbent assay (ELISA) plates for 1 hour at 37°C. After extensive washing, plates were incubated with HRP-conjugated streptavidin for 45 minutes at 37°C and developed using 3,3,5,5-tetramethylbenzidine (TMB). The reaction was stopped by addition of 2 N H2SO4 and absorbance at 450 nm was recorded on a Multiskan MCC/340 (Labsystems, Quickborn, Germany).
Flow cytometry
Whole blood samples were incubated with fluorophore-conjugated mAbs at saturating concentrations for 15 minutes at RT and directly analyzed on a FACScalibur (Becton Dickinson, Heidelberg, Germany). Platelets were gated by FSC/SSC characteristics.
In vivo experiments
Mice were injected intravenously with biotinylated JAQ1 (20 μg; in 200 μL sterile PBS) and blood was collected at different time points. The blood was centrifuged at 1000g for 5 minutes. The platelet-poor plasma was then centrifuged at 3000g for 10 minutes to obtain platelet-free plasma. Plasma was then incubated on JAQ3-coated ELISA plates and ELISA was performed as mentioned in “GPVI ELISA shedding assay.” For bleeding time experiments, mice were anesthetized and 3 mm of tail tip was amputated with a scalpel. The tail was then blotted with filter paper every 15 seconds until the paper was no longer blood stained.33 Where necessary, bleeding was manually stopped at the 20-minute time point to prevent death. Experiments were conducted in accordance with the regulations of the local authorities.
Immunoblotting
Washed platelets were solubilized in 1 mL lysis buffer (Tris-buffered saline containing 20 mM Tris/HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1% Nonidet P-40), and whole-cell extract was run on a SDS–polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. The membrane was then incubated with different antibodies, followed by HRP-labeled secondary Ig and proteins were visualized by enhanced chemiluminescence (ECL).
Results
JAQ1 induces GPVI shedding in vivo
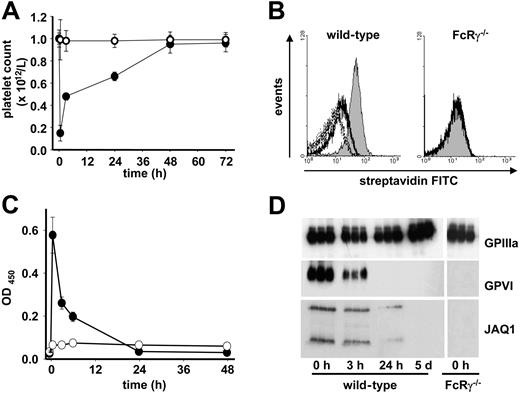
To study the mechanisms underlying antibody-induced GPVI depletion in vivo, wild-type mice were injected with biotinylated JAQ1 (20 μg; intravenously), and platelets were analyzed at various time points after antibody administration. As a control, FcRγ-chain–deficient (FcRγ−/−) mice were used, which also lack GPVI.17 Consistent with previous results,18,23 JAQ1 injection induced strong and transient thrombocytopenia with a maximum drop in platelet count of 80% after 30 minutes and a return to almost normal after 48 hours (Figure 1A). No effect of JAQ1 on platelet counts was observed in FcRγ−/− mice. JAQ1 was undetectable on the surface of circulating platelets (Figure 1B) but present in lysates at early but not late time points after antibody injection (Figure 1D), suggesting that the receptor, at least in part, had been internalized and cleared intracellularly. To test whether receptor shedding occurs in parallel, we used a newly established ELISA system that detects the soluble GPVI/JAQ1 complex in plasma. Indeed, high levels of JAQ1/GPVI complex were found in the plasma of antibody-treated wild-type mice that peaked after 30 minutes and rapidly decreased over time such that it was only weakly detectable after 24 hours. In contrast, JAQ1/GPVI was undetectable in the plasma of FcRγ−/− mice at any time point, confirming the specificity of the ELISA system (Figure 1C). As previously described, platelets from JAQ1-treated wild-type mice were unresponsive to collagen and displayed transiently impaired aggregation and secretion responses to thrombin, a combined defect that resulted in a severe bleeding time prolongation at early but not late time points after treatment as described23 (not shown). In contrast, JAQ1 had no effect on thrombin responses or bleeding times in FcRγ-chain–deficient mice, which lack GPVI (not shown). These data demonstrate that binding of JAQ1 to platelet GPVI in vivo induces rapid proteolytic cleavage of the receptor and indicate that internalization/intracellular clearing may occur in parallel. This down-regulation is associated with transient thrombocytopenia, defective platelet thrombin responses, and severely increased bleeding times.
GPVI down-regulation through ectodomain shedding. Wild-type (•) or FcRγ−/− (○) mice received 20 μg biotinylated JAQ1 intravenously and (A) platelet counts were determined. (B) Surface-bound JAQ1 was detected by flow cytometric measurement of streptavidinFITC binding after 10 minutes (black line) and 30 minutes (dotted line). The shaded area represents maximal binding (t = 0 minutes) as determined after in vitro incubation of platelets with 20 μg/mL JAQ1biotin followed by streptavidinFITC. (C) Detection of sGPVI/JAQ1biotin complex in plasma of wild-type (•) or FcRγ−/− (○) mice at the indicated time points after antibody injection. (D) Western blot analysis of platelets at the indicated time points. (A,C) Results are expressed as the mean platelet count ± SD for groups of 6 mice each. (B,D) The results are representative of 3 individual experiments.
GPVI down-regulation through ectodomain shedding. Wild-type (•) or FcRγ−/− (○) mice received 20 μg biotinylated JAQ1 intravenously and (A) platelet counts were determined. (B) Surface-bound JAQ1 was detected by flow cytometric measurement of streptavidinFITC binding after 10 minutes (black line) and 30 minutes (dotted line). The shaded area represents maximal binding (t = 0 minutes) as determined after in vitro incubation of platelets with 20 μg/mL JAQ1biotin followed by streptavidinFITC. (C) Detection of sGPVI/JAQ1biotin complex in plasma of wild-type (•) or FcRγ−/− (○) mice at the indicated time points after antibody injection. (D) Western blot analysis of platelets at the indicated time points. (A,C) Results are expressed as the mean platelet count ± SD for groups of 6 mice each. (B,D) The results are representative of 3 individual experiments.
Signaling through the FcRγ-chain is essential for GPVI down-regulation
Previous studies on GPVI regulation in mouse and human platelets have failed to answer the question whether signaling downstream of the receptor is required to induce its down-regulation.14,18,22,34 To address this directly, we used mice that express a mutant variant of the FcRγ-chain in which the tyrosines at position 65 and 76 in the ITAM motif have been replaced by phenylalanine (FcRγ-YF). Platelets from these mice are completely unresponsive to collagen stimulation due to defective GPVI signaling.29 FcRγ-YF and littermate control mice were injected with JAQ1-biotin (20 μg; intravenously) and analyzed as above. In contrast to the controls, FcRγ-YF mice did not develop detectable thrombocytopenia, demonstrating an essential role of GPVI signaling in this process (Figure 2A). Furthermore, maximal levels of JAQ1 were found on the surface of circulating platelets in FcRγ-YF mice for 24 hours, showing that GPVI was not down-regulated in these animals (Figure 2B). Consistent with this, no JAQ1/GPVI complex was detected in the plasma of the mutant animals, while the expected peak was measured in control mice (Figure 2C). Western blot analysis confirmed the presence of maximal amounts of both JAQ1 and GPVI in the platelet lysate of FcRγ-YF mice for up to 5 days, whereas only reduced amounts were found at early time points in wild-type mice (Figure 2D). Importantly, platelets isolated from FcRγ-YF mice 24 hours after JAQ1 injection showed unaltered activation responses to thrombin as determined by flow cytometric analysis of integrin αIIbβ3 activation and surface expression of P-selectin (a marker for α-degranulation) (not shown). Together, these results demonstrated that signaling through the FcRγ-chain ITAM is essential for antibody-induced GPVI down-regulation, thrombocytopenia, and the observed transient impairment of thrombin responses.
Signaling through the FcRγ-ITAM is essential for all JAQ1-induced effects. Wild-type (•) or FcRγ-YF (○) mice received 20 μg biotinylated JAQ1 intravenously and (A) platelet counts were determined. (B) Surface-bound JAQ1biotin was detected by flow cytometric measurement of streptavidinFITC binding and is given as percentage of maximal binding determined in vitro. (C) Detection of sGPVI/JAQ1biotin complex in plasma at the indicated time points after antibody injection. (A-C) Results are expressed as the mean platelet count ± SD for groups of 6 mice each. (D) Western blot analysis of platelets at the indicated time points. The results are representative of 3 individual experiments.
Signaling through the FcRγ-ITAM is essential for all JAQ1-induced effects. Wild-type (•) or FcRγ-YF (○) mice received 20 μg biotinylated JAQ1 intravenously and (A) platelet counts were determined. (B) Surface-bound JAQ1biotin was detected by flow cytometric measurement of streptavidinFITC binding and is given as percentage of maximal binding determined in vitro. (C) Detection of sGPVI/JAQ1biotin complex in plasma at the indicated time points after antibody injection. (A-C) Results are expressed as the mean platelet count ± SD for groups of 6 mice each. (D) Western blot analysis of platelets at the indicated time points. The results are representative of 3 individual experiments.
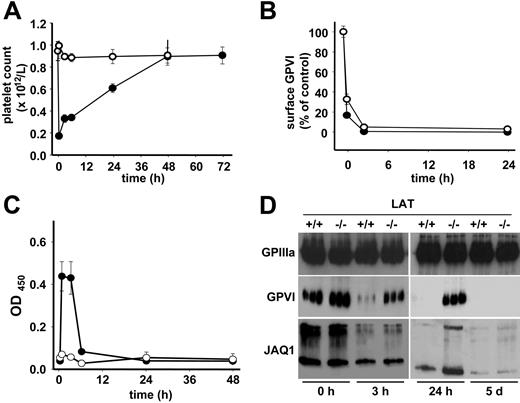
LAT is essential for JAQ1-induced GPVI shedding, but not internalization
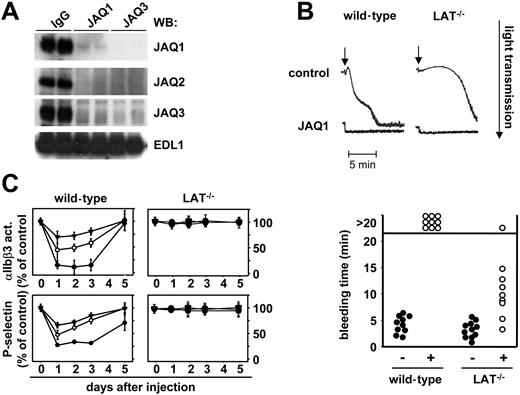
The above findings demonstrated that binding of anti-GPVI antibodies to their target in vivo evokes a number of distinct responses in platelets, but it remained to be determined whether this involves different signaling events within the cells. To address this directly, we used mice deficient in the adaptor molecule LAT. Platelets from these mice show severely impaired, but not abrogated, activation by collagen or GPVI-specific agonists demonstrating the existence of LAT-dependent and -independent processes downstream of GPVI.35,36 LAT−/− and control mice received JAQ1-biotin (20 μg; intravenously) and platelets were analyzed at different time points. Interestingly, LAT−/− mice did not show any alterations in platelet counts in response to this treatment (Figure 3A), but GPVI down-regulation occurred to the same extent and with the same kinetics as in the wild-type controls as shown by flow cytometric analysis (Figure 3B). In contrast to wild-type controls, this down-regulation did not involve ectodomain shedding as the JAQ1/GPVI complex was not detected in plasma of the mutant mice at any time point (Figure 3C), suggesting that quantitative internalization of the GPVI/JAQ1 complex had occurred in the mutant animals. This was confirmed by the detection of maximal levels of both GPVI and JAQ1 in platelet lysates of LAT−/− mice 3 hours after antibody injection, whereas only low amounts were detected in the controls. Both GPVI and JAQ1 levels decreased over time in LAT−/− mice, suggesting an intracellular clearing mechanism (Figure 3D). To discriminate whether the loss of GPVI signals was due to a selective loss of the JAQ1-binding epitope or the entire receptor, we used JAQ2 and JAQ3, which are known to bind to different, nonoverlapping epitopes on mouse GPVI than JAQ1.22 As shown in Figure 4A, both antibodies, like JAQ1, failed to detect GPVI in platelets from LAT−/− mice on day 5 after injection of JAQ1 or JAQ3. In contrast, at early time points (6 hours), GPVI was lost from the surface of JAQ1- and JAQ3-treated LAT−/− mice but still found in platelet lysates (not shown). This showed that anti-GPVI–induced internalization occurs irrespective of the exact binding epitope of the antibody and is followed by intracellular clearing of the receptor.
LAT is required for shedding but not for internalization/intracellular clearing of GPVI. Wild-type (•) or LAT−/− (○) mice received 20 μg biotinylated JAQ1 intravenously and (A) platelet counts were determined. (B) Surface-bound JAQ1biotin was detected by flow cytometric measurement of streptavidinFITC binding and is given as percentage of maximal binding determined in vitro. (C) Detection of sGPVI/JAQ1biotin complex in plasma at the indicated time points after antibody injection. (A-C) Results are expressed as the mean platelet count ± SD for groups of 6 mice each. (D) Western blot analysis of platelets at the indicated time points. The results are representative of 3 individual experiments.
LAT is required for shedding but not for internalization/intracellular clearing of GPVI. Wild-type (•) or LAT−/− (○) mice received 20 μg biotinylated JAQ1 intravenously and (A) platelet counts were determined. (B) Surface-bound JAQ1biotin was detected by flow cytometric measurement of streptavidinFITC binding and is given as percentage of maximal binding determined in vitro. (C) Detection of sGPVI/JAQ1biotin complex in plasma at the indicated time points after antibody injection. (A-C) Results are expressed as the mean platelet count ± SD for groups of 6 mice each. (D) Western blot analysis of platelets at the indicated time points. The results are representative of 3 individual experiments.
LAT−/− mice are protected from JAQ1-induced bleeding. (A) LAT−/− mice received 100 μg JAQ1 or JAQ3 intraperitoneally, and platelets were isolated on day 5 and tested in Western blot analysis for the presence of GPIIIa (EDL1) or GPVI (JAQ1, JAQ2, JAQ3). (B) Wild-type or LAT−/− mice received vehicle or 20 μg JAQ1 intravenously and platelets were isolated on day 5 followed by aggregometric analysis of convulxin (cvx)–induced aggregation. The arrows indicated the addition of the agonist (5 μg/mL). The results are representative of 6 individual experiments. (C) JAQ1 does not alter thrombin responses in LAT−/− mice. Washed platelets were prepared at the indicated time points after JAQ1 treatment and stimulated with 0.1 (▾), 0.01 (○), or 0.001 (•) U/mL thrombin. Activation of integrin αIIbβ3 and surface expression of P-selectin were determined by flow cytometry and are given as percentage ± SD of the values obtained with untreated controls (n = 6 per group). Platelets were gated by FSC/SSC characteristics and FL-4 positivity (anti–GPIbα-Cy5). (D) Tail bleeding times in wild-type and LAT−/− mice 24 hours after treatment with vehicle or 20 μg JAQ1.
LAT−/− mice are protected from JAQ1-induced bleeding. (A) LAT−/− mice received 100 μg JAQ1 or JAQ3 intraperitoneally, and platelets were isolated on day 5 and tested in Western blot analysis for the presence of GPIIIa (EDL1) or GPVI (JAQ1, JAQ2, JAQ3). (B) Wild-type or LAT−/− mice received vehicle or 20 μg JAQ1 intravenously and platelets were isolated on day 5 followed by aggregometric analysis of convulxin (cvx)–induced aggregation. The arrows indicated the addition of the agonist (5 μg/mL). The results are representative of 6 individual experiments. (C) JAQ1 does not alter thrombin responses in LAT−/− mice. Washed platelets were prepared at the indicated time points after JAQ1 treatment and stimulated with 0.1 (▾), 0.01 (○), or 0.001 (•) U/mL thrombin. Activation of integrin αIIbβ3 and surface expression of P-selectin were determined by flow cytometry and are given as percentage ± SD of the values obtained with untreated controls (n = 6 per group). Platelets were gated by FSC/SSC characteristics and FL-4 positivity (anti–GPIbα-Cy5). (D) Tail bleeding times in wild-type and LAT−/− mice 24 hours after treatment with vehicle or 20 μg JAQ1.
The complete absence of GPVI in both wild-type and LAT−/− mice on day 5 resulted in abrogated activation of the cells by the strong GPVI agonist convulxin (Figure 4B). Together, these results demonstrated that LAT is essential for JAQ1-induced thrombocytopenia and ectodomain shedding of GPVI, whereas it is dispensable for the induction of internalization/ intracellular clearing of the receptor.
Next, we tested whether JAQ1 treatment affects platelet thrombin responses and thus the ability to arrest bleeding in LAT−/− mice. Clearly, platelet activation in response to thrombin was unchanged at all doses and time points in the mutant animals, whereas a profound transient defect occurred in the controls as determined by flow cytometric analysis of integrin αIIbβ3 activation and surface expression of P-selectin (Figure 4C). This full preservation of thrombin responses and the absence of thrombocytopenia in LAT−/− mice resulted in only a moderate increase in tail bleeding times 24 hours after antibody injection, whereas none of the control animals arrested bleeding within the 20-minute observation period, confirming previous results23 (Figure 4D). These results revealed the existence of a signaling pathway downstream of GPVI that bypasses LAT and leads to efficient GPVI down-regulation while circumventing the undesired transient side effects of anti-GPVI treatment on normal hemostasis.
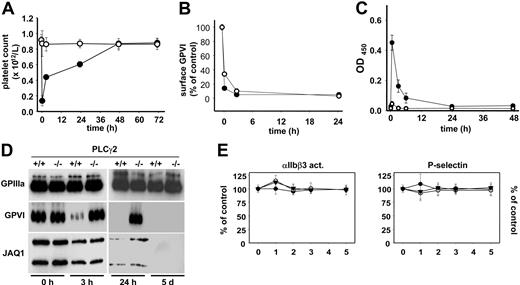
GPVI signals in a LAT- and PLCγ2-independent manner
We and others have previously provided evidence for the existence of LAT-dependent and -independent pathways, both of which culminate in the activation of PLCγ2, a central effector molecule in the GPVI signaling cascade.35-37 Analysis of platelets from PLCγ2−/− mice31 showed no38 or very minor39 responses to GPVI-specific agonists, demonstrating the mandatory role of this enzyme for classical platelet activation through GPVI. To test the involvement of PLCγ2 in antibody-induced GPVI down-regulation, PLCγ2−/− mice were analyzed. As expected, JAQ1 treatment had no effect on platelet counts in the mutant animals, whereas severe transient thrombocytopenia was observed in the wild-type littermate controls (Figure 5A). Remarkably, however, GPVI was completely down-regulated from the platelet surface in PLCγ2−/− mice, and this occurred within the same time frame as in the controls (Figure 5B). Like in LAT−/− mice, this GPVI loss did not involve ectodomain shedding (Figure 5C), but appeared to be mediated exclusively by internalization/intracellular clearing of the receptor/antibody complex (Figure 5D). As before, this route of GPVI down-regulation was not associated with defects in thrombin responses of the cells at any time point (Figure 5E). Effects on bleeding times were not determined in PLCγ2−/− mice as they are markedly increased also in the absence of anti-GPVI treatment. These results demonstrate for the first time the existence of a signaling pathway downstream of GPVI that bypasses both LAT and PLCγ2 and efficiently leads to internalization and irreversible loss of GPVI.
A PLCγ2-independent pathway of GPVI-down-regulation. Wild-type (•) or PLCγ2−/− (○) mice received 20 μg biotinylated JAQ1 intravenously and (A) platelet counts were determined. (B) Surface-bound JAQ1biotin was detected by flow cytometric measurement of streptavidinFITC binding and is given as percentage of maximal binding determined in vitro. (C) Detection of sGPVI/JAQ1biotin complex in plasma at the indicated time points after antibody injection. (A-C) Results are expressed as the mean platelet count ± SD for groups of 6 mice each. (D) Western blot analysis of platelets at the indicated time points. The results are representative of 3 individual experiments. (E) JAQ1 does not alter thrombin responses in PLCγ2−/− mice. Washed platelets were prepared at the indicated time points after JAQ1 treatment and stimulated with 0.1 (▾), 0.01 (○), or 0.001 (•) U/mL thrombin. Activation of integrin αIIbβ3 and surface expression of P-selectin were determined by flow cytometry and are given as percentage ± SD of the values obtained with untreated controls (n = 6 per group). Platelets were gated by FSC/SSC characteristics and FL-4 positivity (anti–GPIbα-Cy5).
A PLCγ2-independent pathway of GPVI-down-regulation. Wild-type (•) or PLCγ2−/− (○) mice received 20 μg biotinylated JAQ1 intravenously and (A) platelet counts were determined. (B) Surface-bound JAQ1biotin was detected by flow cytometric measurement of streptavidinFITC binding and is given as percentage of maximal binding determined in vitro. (C) Detection of sGPVI/JAQ1biotin complex in plasma at the indicated time points after antibody injection. (A-C) Results are expressed as the mean platelet count ± SD for groups of 6 mice each. (D) Western blot analysis of platelets at the indicated time points. The results are representative of 3 individual experiments. (E) JAQ1 does not alter thrombin responses in PLCγ2−/− mice. Washed platelets were prepared at the indicated time points after JAQ1 treatment and stimulated with 0.1 (▾), 0.01 (○), or 0.001 (•) U/mL thrombin. Activation of integrin αIIbβ3 and surface expression of P-selectin were determined by flow cytometry and are given as percentage ± SD of the values obtained with untreated controls (n = 6 per group). Platelets were gated by FSC/SSC characteristics and FL-4 positivity (anti–GPIbα-Cy5).
Discussion
GPVI is the central collagen receptor on platelets and may serve as a powerful target for antithrombotic therapy.2 This hypothesis was first supported by Konishi et al who demonstrated that mice deficient in the FcRγ-chain, which fail to express GPVI in their platelets, are protected against arterial thrombosis and subsequent neointima formation40 but do not show a major bleeding defect. A similar protective effect can be observed in JAQ1-treated mice on day 5 or later (ie, time points when GPVI is absent in platelets but the responses to all other tested agonists, including thrombin, are normal19,20 ), clearly documenting the profound antithrombotic potential of this regimen. One advantage of anti-GPVI agents could be the selective inhibition of platelet activity in distinct etiologies of arterial thrombosis (eg, on atherosclerotic plaque rupture) while preserving the ability of the cells to fully respond in other situations. This notion is supported by recent elegant studies indicating that in models that favor thrombin-driven thrombosis, the antithrombotic effect of GPVI deficiency is reduced or overcome.41,42 This agrees with the observation that GPVI inhibition or loss has a relatively mild effect on normal hemostasis in humans and mice,4,13-17 a process also known to be highly thrombin dependent. Thus, anti-GPVI treatment could be preferable to inhibitors of pathways that are crucial for thrombus formation, irrespective of the initial stimulus, and are also powerful antithrombotics (but by definition also bear a bleeding risk).
Our data provide evidence for 2 distinct routes of GPVI down-regulation that are controlled by different branches of the signaling cascade downstream of the receptor. Both pathways require a functional ITAM in the FcRγ-chain, demonstrating that signal transduction through GPVI/FcRγ complex is essential to stimulate receptor shedding as well as internalization. Ectodomain shedding of GPVI appears to be mediated by a yet unidentified metalloproteinase,24-26 and our data indicate that this process occurs in a LAT/PLCγ2-dependent manner (Figure 2). The same route is involved in JAQ1-induced reduction of PAR4 activity and the resulting hemostatic defect (Figure 4), raising the interesting possibility that the activated GPVI-cleaving metalloproteinase may also affect PAR4 or associated signaling molecules. Further studies will be required to test this hypothesis. Finally, the “classical” GPVI signaling pathway is also responsible for the sharp drop in platelet counts after antibody injection, suggesting transient integrin αIIbβ3 activation and trapping of the cells in the reticuloendothelial system (Figures 3 and 5). We have previously shown that binding of JAQ1 to GPVI induces subliminal signaling through the receptor as revealed by low levels of tyrosine phosphorylation of signaling molecules, including LAT and PLCγ2.37 Although this subliminal signaling is not associated with any signs of cellular activation in vitro,43 it probably contributes to the down-regulation of the receptor and the associated side effects in vivo. On the other hand, monovalent Fab fragments of JAQ1 do not induce any detectable changes in tyrosine phosphorylation in mouse platelets in vitro, suggesting that dimerization of the receptor is required for this to occur.37 However, JAQ1 Fab fragments do induce GPVI down-regulation in circulating mouse platelets,22 and others have demonstrated GPVI down-regulation by anti-GPVI Fab in human platelets in a NOD/SCID mouse model.21 This raises the interesting possibility that monovalent anti-GPVI agents, such as Fab fragments of antibodies, fail to induce “classical GPVI signaling” and may therefore induce GPVI down-regulation through a different pathway than agents that cluster the receptor, but no evidence in support of this has been provided to date.
In contrast to the above-described processes, internalization and subsequent intracellular clearing of GPVI are fully functional in the absence of LAT or PLCγ2. This finding reveals the existence of an uncharacterized signaling pathway downstream of the GPVI/FcRγ complex that may have a role in dampening FcRγ-ITAM–dependent activation processes in platelets by targeting the complex for degradation. Interestingly, a mechanism of down-regulation of the T cell receptor that is dependent on the Src family kinase, Lck, but neither the Syk family kinase, ZAP-70, nor the adapter SLP-76, has been described.44 This pathway is mediated by Lck-dependent phosphorylation of the T-cell receptor ζ-chain and subsequent recruitment of the adapter protein, SLAP, and the ubiquitin ligase c-Cbl.44 Ubiquitination of the T-cell receptor leads to internalization and degradation. This pathway plays an important role in T-cell development by ensuring that the mature cells have the right levels of the T-cell receptor and appropriate range of avidities for MHC complex. Importantly, a similar pathway has been described for down-regulation of the B-cell receptor, which is also mediated through SLAP and c-Cbl.45 c-Cbl is expressed in platelets, and platelets from mice deficient in the ubiquitin ligase show an increase in response to the GPVI-specific collagen-related peptide (CRP), which is associated with increased ubiquitination of Syk.46,47 Further, platelets also express high levels of the related adapter, SLAP-2 (M. Tomlinson and S.P.W., July 2006, unpublished), raising the possibility that a similar mechanism may underlie internalization and degradation of the GPVI receptor complex.
Taken together, we have shown for the first time that it is possible to mechanistically uncouple the targeted down-regulation of an ITAM-coupled receptor from undesired cellular activation. This finding may serve as a basis for the development of safe anti-GPVI agents but also agents that target immunoreceptors to suppress uncontrolled inflammatory reactions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 688 A1 to B.N.) and the Rudolf Virchow Center.
The authors would like to thank V. Schulte and S. Hartmann for help with antibody preparation and flow cytometry.
Authorship
Contribution: T.R., D.V.S., M.B., and R.P. performed research and analyzed data; F.L., T.S., and S.P.W. contributed vital new reagents; and B.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Versbacher Str. 9, 97078 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal