Abstract
Currently availabel staging systems for non-Hodgkin lymphomas are not useful for clinical staging classification of most primary cutaneous lymphomas. The tumor, node, metastases (TNM) system used for mycosis fungoides (MF) and Sézary syndrome (SS) is not appropriate for other primary cutaneous lymphomas. A usable, unified staging system would improve the communication about the state of disease, selection of appropriate management, standardization of enrollment/response criteria in clinical trials, and collection/analysis of prospective survival data. Toward this goal, during the recent meetings of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC), the representatives have established a consensus proposal of a TNM classification system applicable for all primary cutaneous lymphomas other than MF and SS. Due to the clinical and pathologic heterogeneity of the cutaneous lymphomas, the currently proposed TNM system is meant to be primarily an anatomic documentation of disease extent and not to be used as a prognostic guide.
Introduction
The goals of a staging system
Staging of cancer is important in the appropriate and effective care of patients. The concept of staging was formalized internationally by the establishment of the International Union Against Cancer (UICC)1 in 1950 and in the United States by the American Joint Commission on Cancer (AJCC)2 in 1959. The goals of a staging system for cutaneous lymphoma are to offer a clinically reasonable basis for appropriate management, to predict prognosis, and to facilitate comparison of results among different therapies and different institutions.
Currently used staging systems for cutaneous lymphomas
The most widely used staging system for lymphoma is the Ann Arbor system, which was first introduced as a staging system for Hodgkin disease (HD) in 1971.2 Although its utility for the staging of other lymphomas has been challenged, it is still the primary means for classifying patients with non-Hodgkin lymphomas (NHL). The Ann Arbor system is primarily an anatomical assessment of disease with modifications by presence or absence of systemic symptoms. However, it does not distinguish consistently between patients with different prognoses. Thus, in 1993, the International Prognostic Index was established to supplement the Ann Arbor staging to aid in the treatment decision or stratification of patients in clinical trials for patients with lymphoma.3 However, the Ann Arbor system has a number of shortcomings, especially when staging lymphomas that arise primarily in extranodal sites such as the skin. In primary cutaneous lymphomas, the initial stage according to the Ann Arbor system would either be IE (if single skin site) or IVD + (if multiple skin sites), thus disproportionately or inappropriately placing high number of patients in the highest stage, resulting in unnecessarily aggressive treatments.
The TNM classification system is the most widely used means for classifying the extent of nonlymphoid malignant disease and is endorsed by the UICC and the AJCC. The TNM + B (blood) system has been used to stage patients with mycosis fungoides (MF) and Sézary syndrome (SS),4 which together constitute the majority of patients with primary cutaneous lymphomas. This TNMB classification is converted to a clinical staging classification that has been in use for nearly 30 years for prediction of survival and stratification of therapy. Overall, the TNMB classification and clinical staging system has provided clinically useful prognostic information5 and has been widely used for treatment selection and stratification in clinical trials. However, this system for MF/SS has some shortfalls and is currently undergoing modification. The TNMB designations and descriptions helpful in MF/SS are not applicable for non-MF/SS primary cutaneous lymphomas. The clinical presentations and treatment approaches in non-MF/SS cutaneous lymphomas are different from those in MF/SS,6 thus, a new staging classification scheme that is more appropriate and useful for the non-MF/SS cutaneous lymphomas is required.
Primary cutaneous lymphomas other than MF/SS
The term “primary cutaneous lymphomas” refers to those lymphomas that present in the skin where there is no evidence of extracutaneous involvement at the time of diagnosis and completion of initial staging evaluation. The new classification system for primary cutaneous lymphomas is a product of a consensus effort of the EORTC and the World Health Organization (WHO) and is referred to as the WHO-EORTC classification for cutaneous lymphomas.6 The TNM system proposed in this document addresses the non-MF/SS primary cutaneous lymphomas described in this new classification. The representative primary cutaneous lymphomas other than MF/SS are listed in Table 1.
WHO-EORTC classification of primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome
| Cutaneous T-cell and NK-cell lymphomas |
| Primary cutaneous CD30+ lymphoproliferative disorders |
| Primary cutaneous anaplastic large-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Extranodal NK/T-cell lymphoma, nasal-type |
| Primary cutaneous peripheral T-cell lymphoma, unspecified |
| Primary cutaneous aggressive epidermotrophic CD8+ T-cell lymphoma, provisional |
| Cutaneous γ/δ T-cell lymphoma, provisional |
| Primary cutaneous CD4+ small/medium pleomorphic T-cell lymphoma, provisional |
| Primary cutaneous peripheral T-cell lymphoma, unspecified, other |
| Cutaneous B-cell lymphomas |
| Primary cutaneous marginal zone B-cell lymphoma |
| Primary cutaneous follicle center lymphoma |
| Primary cutaneous diffuse large B-cell lymphoma, leg-type |
| Primary cutaneous diffuse large B-cell lymphoma, other |
| Intravascular large B-cell lymphoma |
| Precursor hematologic neoplasm: CD4+/CD56+ hematodermic neoplasm, blastic NK-cell lymphoma |
| Cutaneous T-cell and NK-cell lymphomas |
| Primary cutaneous CD30+ lymphoproliferative disorders |
| Primary cutaneous anaplastic large-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Extranodal NK/T-cell lymphoma, nasal-type |
| Primary cutaneous peripheral T-cell lymphoma, unspecified |
| Primary cutaneous aggressive epidermotrophic CD8+ T-cell lymphoma, provisional |
| Cutaneous γ/δ T-cell lymphoma, provisional |
| Primary cutaneous CD4+ small/medium pleomorphic T-cell lymphoma, provisional |
| Primary cutaneous peripheral T-cell lymphoma, unspecified, other |
| Cutaneous B-cell lymphomas |
| Primary cutaneous marginal zone B-cell lymphoma |
| Primary cutaneous follicle center lymphoma |
| Primary cutaneous diffuse large B-cell lymphoma, leg-type |
| Primary cutaneous diffuse large B-cell lymphoma, other |
| Intravascular large B-cell lymphoma |
| Precursor hematologic neoplasm: CD4+/CD56+ hematodermic neoplasm, blastic NK-cell lymphoma |
Reports of staging in individual studies for cutaneous lymphomas other than MF/SS
Individual centers and consortia have published clinical experiences with non-MF/SS cutaneous lymphomas using a variety of criteria for defining type/extent/location of skin involvement, stage, or prognostic factors.7-9 These have included type (papule, plaque, nodule, tumor), number, size, or location of skin lesions or number of body regions involved, and other parameters to describe and categorize the type and extent of cutaneous lymphoproliferative disease. Since the criteria are unclear and have varied from one publication to another, it is difficult to compare the results of different experiences. Despite the lack of consistent criteria, the extent of skin involvement may be an important factor in the selection of treatment and thus warrants development of a reproducible, well-defined system to document the extent of skin involvement in the cutaneous lymphomas other than MF/SS.
The ISCL/EORTC proposal on TNM classification of cutaneous lymphomas other than MF/SS
Recognizing the need of a new staging classification system for the non-MF/SS primary cutaneous lymphomas, the ISCL held conferences in Washington, D.C. (February 5, 2004), New Orleans (February 17, 2005), and San Francisco (March 2, 2006) to define a working staging classification system. The intent was that this system would be simple, effective, and reproducible. It would be employed by the international community involved in the treatment of these diseases, that it would be tested with respect to its ease of application, reproducibility and correlation with clinical outcome for each subtype of the cutaneous lymphomas defined in the latest WHO-EORTC classification.6 As more data were derived from clinical experience, it could be modified by international consensus. It is recognized that the system may prove applicable to some of the cutaneous lymphomas more than others, and that revision may be required to accommodate the unique behavior of some lymphomas. Endorsement would be sought from the AJCC and the UICC.
Due to the clinical and pathologic heterogeneity of the cutaneous lymphomas, the currently proposed staging system is meant to be purely an anatomic documentation of disease extent. The ISCL/EORTC proposal uses the TNM nomenclature for classification. Future revisions may include supplementation of the TNM designation with additional clinical, histological, immunophenotypic, molecular, or other biologic information of prognostic relevance including features described in the joint WHO-EORTC cutaneous lymphoma classification. The ISCL/EORTC proposes to defer any stage groupings of the TNM classification until further information is availabel to validate specific stage grouping strategies. Furthermore, the ISCL/EORTC supports using a single staging system for all cutaneous lymphomas, regardless of biologic aggressiveness.
T classification
Consistent with the definition of “T classification” by the AJCC/UICC, the T classification for cutaneous lymphoma reflects the extent/distribution of primary cutaneous involvement (Table 2). A circular area is selected to define size/area criteria of T categories since a circular area (vs. a square area) may be more biologic and relevant to radiation therapy planning. The size criteria of 5 cm, 15 cm, and 30 cm were chosen arbitrarily to distinguish small or limited tumor involvement from the greater or more extensive involvement within T1 and T2 categories, respectively. The T1 (solitary lesion) category is intended for the skin involvement that is a single discrete tumor without morphologic appearance of coalescence (merging of more than one lesion). The definitions of body regions are given in the footnote of Table 2 and depicted in Figure 1. For extremities, left and right serve as separate body sites to define the specific regions. Thus, involvement of left and right arms defines 2 non-contiguous body regions (T3a). The “regional, T2” designation is intended to describe the skin presentations where the tumors are confined in 1 or 2 contiguous body regions, whereas the “T3” designation is intended for skin presentations that are more generalized with either extensive (3 or more body regions) or distant (2 non-contiguous body regions) skin involvement. In cases of T2 and T3 designations, the morphologic distribution of tumor lesions can be either discrete/separate or clustered/grouped/coalescent. It is recommended to track the type of morphologic distribution and the specific body regions that are involved for prospective analysis of survival and treatment outcome.
ISCL/EORTC proposal on TNM classification of cutaneous lymphoma other than MF/SS
| Classification . |
|---|
| T |
| T1: Solitary skin involvement |
| T1a: a solitary lesion <5 cm diameter |
| T1b: a solitary >5 cm diameter |
| T2: Regional skin involvement: multiple lesions limited to 1 body region or 2 contiguous body regions* |
| T2a: all-disease-encompassing in a <15-cm-diameter circular area |
| T2b: all-disease-encompassing in a >15- and <30-cm-diameter circular area |
| T2c: all-disease-encompassing in a >30-cm-diameter circular area |
| T3: Generalized skin involvement |
| T3a: multiple lesions involving 2 noncontiguous body regions |
| T3b: multiple lesions involving ≥3 body regions |
| N |
| N0: No clinical or pathologic lymph node involvement |
| N1: Involvement of 1 peripheral lymph node region that drains an area of current or prior skin involvement |
| N2: Involvement of 2 or more peripheral lymph node regions or involvement of any lymph node region† that does not drain an area of current or prior skin involvement |
| N3: Involvement of central lymph nodes |
| M |
| M0: No evidence of extracutaneous non–lymph node disease |
| M1: Extracutaneous non–lymph node disease present |
| Classification . |
|---|
| T |
| T1: Solitary skin involvement |
| T1a: a solitary lesion <5 cm diameter |
| T1b: a solitary >5 cm diameter |
| T2: Regional skin involvement: multiple lesions limited to 1 body region or 2 contiguous body regions* |
| T2a: all-disease-encompassing in a <15-cm-diameter circular area |
| T2b: all-disease-encompassing in a >15- and <30-cm-diameter circular area |
| T2c: all-disease-encompassing in a >30-cm-diameter circular area |
| T3: Generalized skin involvement |
| T3a: multiple lesions involving 2 noncontiguous body regions |
| T3b: multiple lesions involving ≥3 body regions |
| N |
| N0: No clinical or pathologic lymph node involvement |
| N1: Involvement of 1 peripheral lymph node region that drains an area of current or prior skin involvement |
| N2: Involvement of 2 or more peripheral lymph node regions or involvement of any lymph node region† that does not drain an area of current or prior skin involvement |
| N3: Involvement of central lymph nodes |
| M |
| M0: No evidence of extracutaneous non–lymph node disease |
| M1: Extracutaneous non–lymph node disease present |
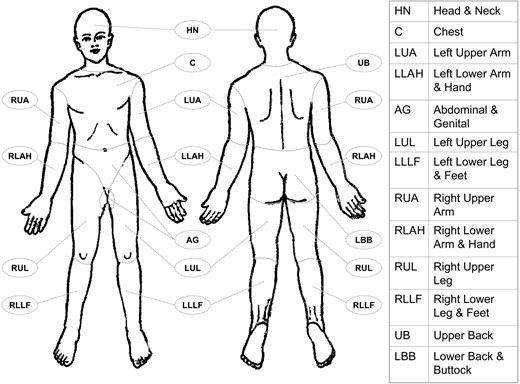
Definition of body regions (see Figure 1): Head and neck: inferior border—superior border of clavicles, T1 spinous process. Chest: superior border—superior border of clavicles; inferior border—inferior margin of rib cage; lateral borders—mid-axillary lines, glenohumeral joints (inclusive of axillae). Abdomen/genital: superior border—inferior margin of rib cage; inferior border—inguinal folds, anterior perineum; lateral borders—mid-axillary lines. Upper back: superior border—T1 spinous process; inferior border—inferior margin of rib cage; lateral borders—mid-axillary lines. Lower back/buttocks: superior border—inferior margin of rib cage; inferior border—inferior gluteal fold, anterior perineum (inclusive of perineum); lateral borders—mid-axillary lines. Each upper arm: superior borders—glenohumeral joints (exclusive of axillae); inferior borders—ulnar/radial-humeral (elbow) joint. Each lower arm/hand: superior borders—ulnar/radial-humeral (elbow) joint. Each upper leg (thigh): superior borders—inguinal folds, inferior gluteal folds; inferior borders—mid-patellae, mid-popliteal fossae. Each lower leg/foot: superior borders—mid-patellae, mid-popliteal fossae.
Definition of lymph node regions is consistent with the Ann Arbor system: Peripheral sites: antecubital, cervical, supraclavicular, axillary, inguinal-femoral, and popliteal. Central sites: mediastinal, pulmonary hilar, paraortic, iliac.
Body regions as defined in the proposed TNM system for the designation of T (skin involvement) category. Left and right extremities are assessed as separate body regions. The designation of these body regions are based on regional LN drainage patterns.
Body regions as defined in the proposed TNM system for the designation of T (skin involvement) category. Left and right extremities are assessed as separate body regions. The designation of these body regions are based on regional LN drainage patterns.
N classification
Since it is implicit in the definition of primary cutaneous lymphoma that extracutaneous disease (lymph node or visceral) is absent, all patients are N0 at presentation. However, to permit application of the staging system at the time of relapse (as a relapse-stage designation) or disease progression, the N classification is defined as in Table 2. It is required that lymph node involvement be documented by histologic evaluation (fine needle aspiration [FNA] or biopsy) from at least one site of clinical involvement whenever feasible. The definition of peripheral and central lymph node regions is consistent with the Ann Arbor system.2
M classification
Since it is implicit in the definition of primary cutaneous lymphoma that extracutaneous disease (lymph node or visceral) is absent, all patients are at M0 presentation. However, to permit application of the staging system at the time of relapse (as a relapse-stage designation) or disease progression, the M-classification is defined as in Table 2. It is strongly recommended that visceral involvement be documented by histologic evaluation from at least one site of clinical involvement whenever feasible.
Descriptors for the proposed TNM system
The initial clinical TNM, designated as cTNM or TNM, should be assigned at the completion of initial staging evaluation and prior to any primary therapy. The clinical TNM is not changed on the basis of subsequent information. As defined by the AJCC/UICC, prefix descriptors “r” or “a” can be used with a given TNM designation to further classify the origin or state of tumor involvement.1,2 The prefix “r” designates recurrent or relapsed state of disease when staged after a disease-free interval. The prefix “a” designates the TNM classification determined at autopsy.
Clinical examples of the proposed TNM classification system
Clinical examples of T1–3 categories demonstrating the application of the proposed TNM classification system are shown in Figures 2,Figure 3,Figure 4,Figure 5–6.
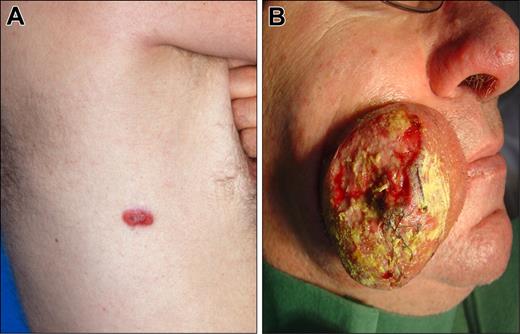
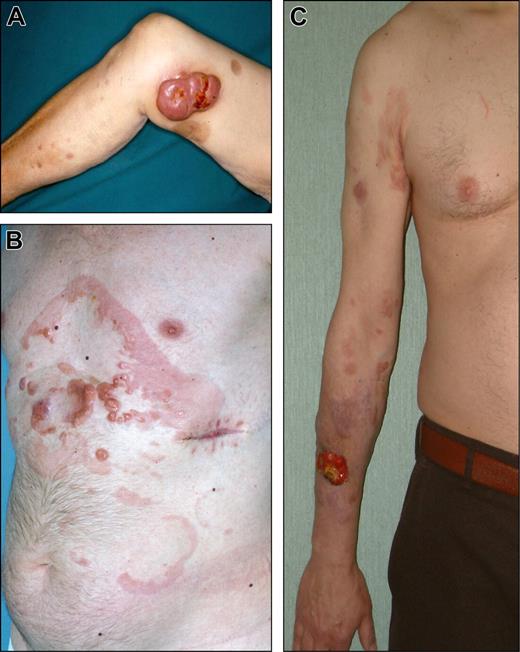
Clinical examples of proposed T1 (discrete solitary lesion) category of primary cutaneous lymphomas. (A) T1a (solitary lesion equal or less than 5 cm), CD30 + anaplastic large cell lymphoma. (B) T1b (solitary lesion greater than 5 cm), NK/T-cell, nasal-type, lymphoma.
Clinical examples of proposed T1 (discrete solitary lesion) category of primary cutaneous lymphomas. (A) T1a (solitary lesion equal or less than 5 cm), CD30 + anaplastic large cell lymphoma. (B) T1b (solitary lesion greater than 5 cm), NK/T-cell, nasal-type, lymphoma.
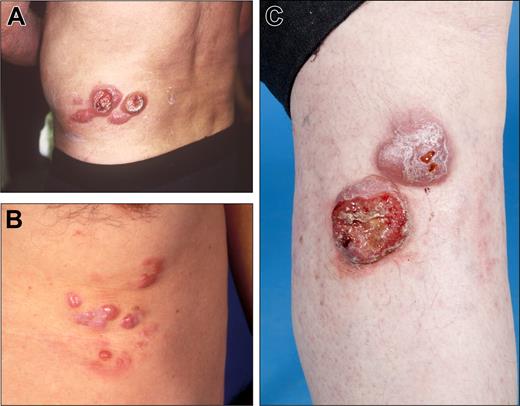
Clinical examples of proposed T2a (regional involvement with lesions within a 15 cm diameter area) category of primary cutaneous lymphomas. (A) CD30 + anaplastic large cell lymphoma. (B) Follicle center lymphoma. (C) Diffuse large B-cell lymphoma, leg-type.
Clinical examples of proposed T2a (regional involvement with lesions within a 15 cm diameter area) category of primary cutaneous lymphomas. (A) CD30 + anaplastic large cell lymphoma. (B) Follicle center lymphoma. (C) Diffuse large B-cell lymphoma, leg-type.
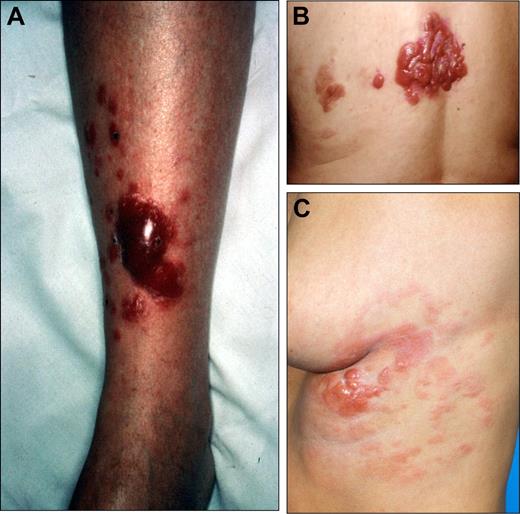
Clinical examples of proposed T2b (regional involvement with lesions in an area between 15 cm and 30 cm diameter) category of primary cutaneous lymphomas. (A) diffuse large B-cell lymphoma, leg-type. (B) Follicle center lymphoma. (C) Follicle center lymphoma.
Clinical examples of proposed T2b (regional involvement with lesions in an area between 15 cm and 30 cm diameter) category of primary cutaneous lymphomas. (A) diffuse large B-cell lymphoma, leg-type. (B) Follicle center lymphoma. (C) Follicle center lymphoma.
Clinical examples of proposed T2c (regional involvement with lesions more than 30 cm in diameter) category of cutaneous lymphomas. (A) Diffuse large B-cell lymphoma, leg-type. (B) Follicle center lymphoma. (C) Diffuse large B-cell lymphoma, leg-type.
Clinical examples of proposed T2c (regional involvement with lesions more than 30 cm in diameter) category of cutaneous lymphomas. (A) Diffuse large B-cell lymphoma, leg-type. (B) Follicle center lymphoma. (C) Diffuse large B-cell lymphoma, leg-type.
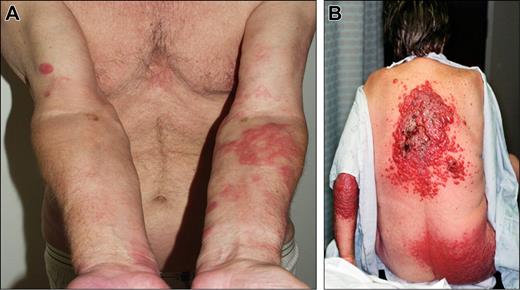
Clinical examples of proposed T3 (generalized) category of cutaneous lymphomas. (A) T3a (multiple lesions involving 2 noncontiguous body regions), follicle center lymphoma. (B) T3b (multiple lesions involving 3 or more body regions), CD30 + anaplastic large-cell lymphoma.
Clinical examples of proposed T3 (generalized) category of cutaneous lymphomas. (A) T3a (multiple lesions involving 2 noncontiguous body regions), follicle center lymphoma. (B) T3b (multiple lesions involving 3 or more body regions), CD30 + anaplastic large-cell lymphoma.
The ISCL/EORTC recommendation for staging evaluation in cutaneous lymphomas other than MF/SS
Accurate staging in non-MF/SS cutaneous lymphomas serves multiple purposes including confirmation of a primary cutaneous process at diagnosis and provision of prognostic and anatomical information for optimal selection of treatment. The ISCL and EORTC are committed to provide the clinicians with a guideline of recommended staging evaluations in non-MF/SS cutaneous lymphomas (Table 3). Proper clinical staging evaluations should begin with a complete history/review of systems (eg, +/− B-symptoms, organ-specific signs) and a thorough physical examination. The laboratory studies should include a complete blood count with differential and a comprehensive blood chemistry measurement including lactate dehydrogenase (LDH). Appropriate imaging studies should be obtained to assess at least the chest, abdomen and pelvis. If there is extensive skin disease or clinically significant lymph node findings of the head and neck areas, the imaging should include the neck for evaluation of the cervical lymph nodes. Whenever possible, tissue sampling should be obtained of any suspicious extracutaneous sites to confirm or exclude involvement. In order to optimize the yield of any invasive biopsy procedures it would be critical to select the most informative imaging study that would best aid in the selection of the site and technique of the invasive procedure. Bone marrow biopsy and aspirate should be performed in patients at risk of involvement and is required in cutaneous lymphomas with intermediate to aggressive clinical behavior (eg, natural killer (NK)/T-cell, aggressive CD8 + T-cell and γ/δ T-cell lymphoma and diffuse large B-cell lymphoma, leg type) as categorized in the WHO-EORTC classification.6 In cutaneous lymphomas with indolent clinical behavior such as cutaneous marginal zone, follicle center, or CD30 + anaplastic large cell lymphoma (WHO-EORTC), bone marrow evaluation should be considered, but not required unless indicated by other staging assessments. A negative marrow involvement would further confirm that the skin involvement is primary and not secondary to a primary extracutaneous presentation. Patients at high risk for central nervous involvement (eg, NK/T-cell lymphoma) should receive a lumbar puncture and spinal fluid assessment and appropriate imaging studies to evaluate any involvement of the central nervous system.
ISCL/EORTC recommendations for staging evaluation in cutaneous lymphomas other than MF/SS
| Complete history/review of systems and physical examination |
| Laboratory studies |
| Complete blood count, comprehensive serum chemistries, serum LDH |
| Whenever indicated, relevant flow cytometric studies of peripheral blood mononuclear cells |
| Imaging studies* |
| CT of chest, abdomen and pelvis with contrast alone or with whole-body PET (18F-FDG); include CT or ultrasound of neck if clinically indicated |
| Whole-body integrated PET/CT (as alternative imaging study to the standard contrast-enhanced CT) |
| Bone marrow biopsy and aspirate† |
| Required in cutaneous lymphomas with intermediate to aggressive clinical behavior as categorized in the WHO-EORTC classification |
| Should be considered in cutaneous lymphomas with indolent clinical behavior, but not required unless indicated by other staging assessments |
| Additional studies as indicated clinically |
| Complete history/review of systems and physical examination |
| Laboratory studies |
| Complete blood count, comprehensive serum chemistries, serum LDH |
| Whenever indicated, relevant flow cytometric studies of peripheral blood mononuclear cells |
| Imaging studies* |
| CT of chest, abdomen and pelvis with contrast alone or with whole-body PET (18F-FDG); include CT or ultrasound of neck if clinically indicated |
| Whole-body integrated PET/CT (as alternative imaging study to the standard contrast-enhanced CT) |
| Bone marrow biopsy and aspirate† |
| Required in cutaneous lymphomas with intermediate to aggressive clinical behavior as categorized in the WHO-EORTC classification |
| Should be considered in cutaneous lymphomas with indolent clinical behavior, but not required unless indicated by other staging assessments |
| Additional studies as indicated clinically |
Lymph nodes that are >1.0 cm in short axis and/or have significantly increased PET activity should be sampled for tissue examination (an excisional biopsy is preferable whenever possible).
At the time of this proposal, there is not a unified standard for bone marrow examination as part of the staging evaluation in cutaneous lymphomas with indolent clinical behavior. The clinician should follow the standard of care of his or her regional practice.
Several factors should be considered in selecting the optimal imaging study. The typical body imaging study used to assess involvement has been contrast-enhanced computerized tomography (CT) scan which is the standard imaging tool for anatomic/structural information. There is increasing evidence for added value in obtaining functional/metabolic information using positron emission tomography (PET) technique for staging and monitoring response to treatment in lymphoma.10,11 More recently, the integrated PET/CT scanners have become availabel allowing the clinician to have simultaneous anatomic and functional/metabolic information of tumor spread/involvement12,13 . In patients unable to safely undergo a CT scan, magnetic resonance imaging (MRI) studies are often substituted. As in the standardized criteria for NHLs,14 lymph node(s) with longest transverse diameter (short axis) of less than 1.0 cm by imaging study or significantly high PET activity should be assessed with tissue sampling. An excisional biopsy of lymph node(s) is preferable whenever possible.
Strengths and shortcomings of the proposed ISCL/EORTC TNM classificaiton system
The proposed staging classification system does have its advantages; one of which is in its simplicity in the documentation of disease and ease of communication and comparison of patient data. Furthermore, it provides a potentially reproducible, standardized classification of tumor extent/distribution and can be used to select appropriate treatment and/or stratify patients entering clinical trials. In addition to describing the initial disease extent, this TNM system can be used to track the status of disease at relapse, with progression, or with treatment initiation.
However, the proposed TNM-based system has its shortfalls that need to be re-emphasized. The proposed TNM classification gives only anatomic/structural information of disease extent and does not by itself provide helpful prognostic information at this time. The joint WHO-EORTC cutaneous lymphoma classification serves as the primary guide for the clinician in understanding the clinical behavior and prognostic variables in the heterogeneous group of cutaneous lymphomas.6 The proposed TNM system that documents disease extent should serve as an additional guide in the appropriate management of patients. This TNM classification system will be tested and evaluated for its prognostic value as more data are obtained with its use.
Possible future modifications
In order to address the limitations and shortcomings of the proposed TNM classification system, we would need to establish a body of additional prognostic information that can be used in conjunction with the proposed TNM system. This would be analogous to the International Prognostic Index (IPI) which supplements the Ann Arbor anatomic staging information in the optimal assessment and management of patients with NHLs.3,15 As we learn more about the prognostic parameters in the non-MF/SS primary cutaneous lymphomas, we can design a prognostic index system that can supplement the proposed TNM system for the cutaneous lymphomas categorized in the WHO-EORTC classification.6 The added prognostic information may be very important in the cutaneous lymphomas categorized as having intermediate-aggressive clinical behavior in the WHO-EORTC classification to help guide management decisions. The parameters (predicting worse outcome) of such prognostic index system can potentially include patient age less than 60 years, elevated serum LDH, extensive regional (T2b) or generalized skin involvement (T3), disease involving unfavorable body regions (if there is such an association), and other biologic/molecular/genetic markers/patterns (yet to be determined or confirmed) of aggressive disease. The index score and risk category can be assigned depending on how many of the unfavorable prognostic features are associated with the diagnosed cutaneous lymphoma according to the WHO-EORTC classification. Then, the proposed anatomical TNM classification and the supplemental prognostic (clinical/biologic/molecular/other markers) information systems can be both applied for the final assessment of prognosis, selection of treatment, and stratification for clinical trials.
The online version of this article contains a data supplement.
Authorship
Contribution: All authors participated in the meetings from which the proposal for the TNM Classification for Primary Cutaneous Lymphomas other than MF/SS was generated and/or contributed to the preparation of this manuscript.
Complete lists of the members of the International Society for Cutaneous Lymphomas and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer are provided as Document S1 and Document S2, respectively, availabel on the Blood website; see the Supplemental Documents link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Youn H. Kim, M.D., Department of Dermatology, Stanford Comprehensive Cancer Center, 900 Blake Wilbur Drive, #W0069, Stanford, CA 94305; E-mail: younkim@stanford.edu

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal