Treatment of acute promyelocytic leukemia (APL) with all-trans retinoic acid (ATRA) is a paradigm of targeted therapy, but identifying the critical downstream targets of ATRA has proven elusive. In this issue of Blood, Hattori and colleagues have used a functional assay to identify such targets. They have identified a gene whose induction by ATRA is necessary, though not sufficient, to induce cell-cycle arrest and allow maturation of ATRA-treated NB4 cells.
RARα functions as a heterodimer with retinoid X receptors (RXR). In the absence of ligand, RAR/RXR recruits a repressor complex to the promoters of target genes; upon ATRA binding, the repressor dissociates, allowing binding of transcriptional activators, induction of histone acetylation, and increased transcription. The PML-RAR fusion protein, formed by the APL-associated t(15;17), displays decreased ATRA binding, leading to corepressor complex association in the presence of physiologic levels of ATRA; this repression can be reversed by pharmacologic ATRA treatment.1
A few ATRA targets have been identified, including c-myc, C/EBPϵ, and p21,2 and microarray analysis revealed over 150 genes that were modulated in response to ATRA treatment of NB4 cells derived from a patient with APL.3 However, as with all microarray studies, the challenge is to single out critical genes deserving of further study. The elegant strategy employed by Hattori and colleagues used a functional read-out to identify genes necessary for the ATRA response. ATRA induction leads to growth inhibition. Exploiting that observation, the authors used RNAi to target the ATRA response: NB4 cells were transduced with a retroviral shRNA library, and resultant clones were screened for ATRA resistance as demonstrated by sustained growth in ATRA-containing medium. The authors thereby identified 26 proteins putatively required for ATRA-induced cell-cycle arrest.
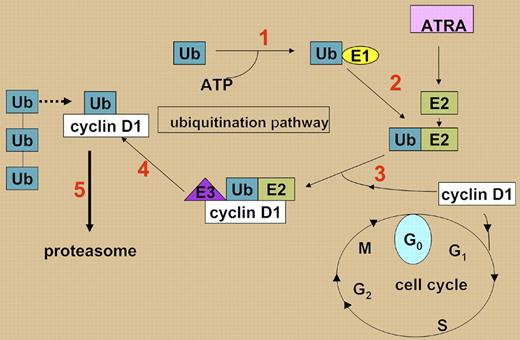
The investigators focused on UBE2D3, a ubiquitin-conjugating enzyme. Their data suggest that down-regulation of UBE2D3 blocks cell-cycle arrest by preventing proteasomal degradation of cyclin D1. Ubiquitination of target proteins requires 3 cooperating enzymes: an E1-activating enzyme, an E2-conjugating enzyme, and an E3 ligase. Substrate specificity is conferred by E3 ligases, and targeted proteins are monoubiquitinated by cooperating E2 and E3 enzymes. Subsequent polyubiquitination destines the protein for proteasomal degradation (see figure).4 Previous studies have highlighted UBE1L, an E1 enzyme, as an ATRA-responsive gene in NB45 ; the new findings by Hattori and colleagues further support an important role for proteasomal degradation in ATRA-induced maturation. The current studies demonstrate that the impact of the UBE2D3 shRNA is mimicked by cyclin D1 overexpression and abrogated by cyclin D1 down-regulation, confirming that cyclin D1 is the important target of UBE2D3.
ATRA up-regulates UBE2D3 (E2) in APL cells, leading to ubiquitination of cyclin D1 (steps 1–4, numbered in red; see text). Monoubiquitinated cyclin D1 is then polyubiquitinated and degraded in the proteasome (step 5). In the absence of UBE2D3, cyclin D1 is not degraded and cells continue to cycle.
ATRA up-regulates UBE2D3 (E2) in APL cells, leading to ubiquitination of cyclin D1 (steps 1–4, numbered in red; see text). Monoubiquitinated cyclin D1 is then polyubiquitinated and degraded in the proteasome (step 5). In the absence of UBE2D3, cyclin D1 is not degraded and cells continue to cycle.
More remains to be defined regarding the maturation of APL cells in response to ATRA. Further experiments are needed to confirm whether UBE2D3 is a direct or indirect transcriptional target of ATRA, which E3 ligase determines cyclin D1 targeting and how it is regulated, and what role other proteins play in the ATRA response. However, the functional screen is a compelling addition to the toolset for addressing these questions, and may provide important therapeutic information along the way. For example, as the authors point out, the importance of proteasomal degradation of cell-cycle proteins to the ATRA response suggests that proteasome inhibitors, which are being used to treat an increasing number of malignancies, may well be contraindicated in APL, at least in combination with ATRA.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal