Disease relapse is a major cause of treatment failure after reduced-intensity allografts and while donor lymphocyte infusions (DLIs) can be effective salvage therapy they are associated with severe graft-versus-host disease (GVHD) when administered early after transplantation. We have therefore examined whether imatinib mesylate can delay relapse and postpone the requirement for DLI in 22 patients with chronic myeloid leukemia (CML) allografted using a reduced-intensity regimen. Imatinib was commenced on day + 35 and continued until 1 year after transplantation. Posttransplantation imatinib was well tolerated and abolished the risk of relapse during this period. Twenty-one patients completed 11 months of imatinib therapy, 15 of whom subsequently relapsed and received DLI. Ten patients to date have achieved molecular remission after DLI. Adjunctive targeted therapy allows the kinetics of disease relapse after a reduced-intensity allograft to be manipulated and represents a novel strategy by which outcome may be improved in patients who undergo transplantation for CML and other leukemias.
Introduction
Reduced-intensity conditioning (RIC) regimens have permitted the extension of the potentially curative effect of allogeneic transplantation to older patients in whom allografting was previously contraindicated.1,2 Although effective in limiting immediate transplant toxicity, they have been associated with a high rate of graft-versus-host disease (GVHD) in some series. There has therefore been considerable interest in the use of T-cell depletion strategies, notably the incorporation of alemtuzumab, to reduce the risk of severe acute and chronic GVHD.3 Predictably, such a maneuver is associated with an increased risk of early relapse, which occurs in up to 70% of patients who undergo transplantation for myeloid malignancies.4,–6 Efforts to reduce the relapse rate after RIC allografts have focused on the restoration of a graft-versus-leukemia effect by the use of prophylactic or preemptive DLI. However while DLI has been shown to be an effective and safe salvage therapy in patients who relapse late after a myeloablative allograft, its use in the immediate posttransplantation period is complicated by a high risk of severe, sometimes fatal, GVHD.7,8 Consequently, strategies that either reduce the risk of disease recurrence or postpone the requirement for DLI are required. One such strategy would be to combine RIC allografts with a maintenance therapy with inherent antileukemic properties for a finite period after transplantation until such a time as it is deemed safer to administer DLI.
Although imatinib has obviated the need for up-front allogeneic transplantation in patients with chronic myeloid leukemia (CML),9 allografting remains an important treatment option in some patients.10 However its curative potential is compromised by the toxicity of myeloablative preparative regimens, and the development of an effective RIC regimen would represent an important new treatment option in CML. We have therefore examined whether adjunctive imatinib can improve the outcome of reduced-intensity allografts in CML.
Patients and methods
Patients with CML in first chronic phase who had an HLA-identical sibling donor and in whom a myeloablative allogeneic transplantation was contraindicated on grounds of age, comorbidity, or patient preference were eligible. The protocol was approved by the local Research Ethics Committees at all participating centers. Informed consent was obtained in accordance with the Declaration of Helsinki. All patients underwent transplantation with granulocyte–colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells using a conditioning regimen consisting of fludarabine 25 mg/m2 per day intravenously on days −7 to −3; busulfan 4 mg/kg per day by mouth on days −4 and −3; and alemtuzumab (50 mg over 5 days). Cyclosporin (5 mg/kg per day intravenously) was used as GVHD prophylaxis and tapered by day 90 in the absence of active GVHD. Imatinib was commenced on day +35 after transplantation at a dose of 300 mg daily in engrafted patients (neutrophil count: > 1 × 109/L; platelet count: > 100 × 109/L) and increased to 400 mg within 4 weeks. Imatinib was discontinued at 12 months after transplantation. Lineage-specific chimerism and real-time quantitative reverse-transcription–polymerase chain reaction (RQ-PCR) for the presence of BCR-ABL transcripts were performed as previously described.11,12 The acquisition of molecular negativity was defined as the absence of BCR-ABL transcripts on 2 consecutive peripheral blood samples.
Patients with evidence of molecular or cytogenetic relapse received escalating-dose DLI. The first 5 patients received 107 CD3 cells/kg increasing by semilog increments at 3-month intervals to a maximum dose of 108 CD3 cells/kg as previously published.7 The DLI doses for all subsequent patients were reduced by one log, with no other change in scheduling, because of a high incidence of GVHD in the first cohort. Patients were adjudged to have completed the DLI program once they had achieved molecular remission or completed the defined escalating schedule of DLI.7
Results and discussion
Twenty-two patients (median age, 49 years) underwent transplantation according to the study protocol (Tables 1, 2). The reasons for choice of a RIC regimen were patient age (> 45 years) or comorbidity (n = 14) or patient reluctance to undergo a myeloablative transplantation (n = 8). All patients engrafted and achieved full donor (n = 6) or mixed T-cell (n = 16) chimerism at day +90. The median times to neutrophil (> 0.5 × 109/L) and platelet (> 50 × 109/L) engraftment were 19 days (range: 11-38 days) and 19 days (range: 11-44 days), respectively. Two patients developed secondary graft failure temporally associated with intravenous ganciclovir treatment and required a second infusion of T cell–depleted stem cells from their original donors. One patient engrafted promptly but the other died of sepsis 5 months after transplantation. It will therefore be important to assess the impact of imatinib on engraftment in a larger cohort of patients. The day-100 transplant-related mortality (TRM) was 0%, and the 1-year TRM was 4%. Only one patient developed acute GVHD (grade 3) after stem cell transplantation (SCT), and no patient developed chronic GVHD after SCT.
Demographics of study population (n=22)
| Characteristic . | Quantity . |
|---|---|
| Newly diagnosed, no. (%) | 14 (64) |
| Late CP, no. (%) | 8 (36) |
| Age, y (range) | 49 (25-57) |
| Prior imatinib therapy, no. (%) | 11 (50) |
| Response to imatinib prior to SCT, no. (%) | |
| CCR | 4 (18) |
| MCR | 1 (5) |
| Less than MCR | 5* (23) |
| Loss of CCR | 1† (5) |
| Indications for allograft, no.(%) | |
| Patient choice | 16 (73) |
| Suboptimal response to imatinib | 6 (27) |
| Disease status at time of transplantation, no. (%) | |
| CCR | 4 (18) |
| Less than CCR | 18 (82) |
| CD34+ cells/kg, ×106 (range) | 6.4 (2.79-13.9) |
| Engraftment, d (range) | |
| Neutrophils over 0.5 × 109/L | 19 (11-38) |
| Platelets over 50 × 109/L | 19 (11-44) |
| GVHD after SCT, no. (%) | |
| Acute | 1 (5) |
| Chronic | 0 (0) |
| GVHD after DLI, no. (%) | |
| Acute | 4 (31) |
| Chronic | 1 (8) |
| TRM, no. (%) | |
| At 100 d | 0 (0) |
| At 12 mo | 1 (5) |
| After DLI | 2 (9) |
| Characteristic . | Quantity . |
|---|---|
| Newly diagnosed, no. (%) | 14 (64) |
| Late CP, no. (%) | 8 (36) |
| Age, y (range) | 49 (25-57) |
| Prior imatinib therapy, no. (%) | 11 (50) |
| Response to imatinib prior to SCT, no. (%) | |
| CCR | 4 (18) |
| MCR | 1 (5) |
| Less than MCR | 5* (23) |
| Loss of CCR | 1† (5) |
| Indications for allograft, no.(%) | |
| Patient choice | 16 (73) |
| Suboptimal response to imatinib | 6 (27) |
| Disease status at time of transplantation, no. (%) | |
| CCR | 4 (18) |
| Less than CCR | 18 (82) |
| CD34+ cells/kg, ×106 (range) | 6.4 (2.79-13.9) |
| Engraftment, d (range) | |
| Neutrophils over 0.5 × 109/L | 19 (11-38) |
| Platelets over 50 × 109/L | 19 (11-44) |
| GVHD after SCT, no. (%) | |
| Acute | 1 (5) |
| Chronic | 0 (0) |
| GVHD after DLI, no. (%) | |
| Acute | 4 (31) |
| Chronic | 1 (8) |
| TRM, no. (%) | |
| At 100 d | 0 (0) |
| At 12 mo | 1 (5) |
| After DLI | 2 (9) |
Late CP is defined as greater than 12 months from diagnosis; CCR, complete cytogenetic remission defined as no detectable Ph+ metaphases in the bone marrow; and MCR, major cytogenetic remission defined as 1% to 35% Ph+ metaphases in the bone marrow.
No ABL kinase domain mutation detected.
ABL kinase domain mutation detected.
Whole blood and T-cell chimerism status after transplantation
| . | 90 d after SCT . | 12 mo after SCT . | After DLI . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Whole blood chimerism | ||||||
| Full donor, 95% or higher | 14 | 64 | 12 | 67 | 7 | 88 |
| Mixed chimera, less than 95% | 8 | 36 | 6 | 33 | 1 | 12 |
| T-cell chimerism | ||||||
| Full donor, 95% or higher | 6 | 27 | 1 | 6 | 6 | 75 |
| Mixed chimera, less than 95% | 16 | 73 | 17 | 94 | 2 | 25 |
| . | 90 d after SCT . | 12 mo after SCT . | After DLI . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Whole blood chimerism | ||||||
| Full donor, 95% or higher | 14 | 64 | 12 | 67 | 7 | 88 |
| Mixed chimera, less than 95% | 8 | 36 | 6 | 33 | 1 | 12 |
| T-cell chimerism | ||||||
| Full donor, 95% or higher | 6 | 27 | 1 | 6 | 6 | 75 |
| Mixed chimera, less than 95% | 16 | 73 | 17 | 94 | 2 | 25 |
Twenty-one (95%) patients commenced imatinib on day 35; in one case treatment was delayed until day + 95 because of delayed platelet engraftment. Posttransplantation imatinib was well tolerated in all but 3 patients in whom it had to be temporarily discontinued because of nausea. Commencement of imatinib had no discernible effect on blood levels of cyclosporin. Twenty-one (95%) patients achieved a 3-log reduction in BCR-ABL transcripts with 7 achieving molecular negativity during imatinib therapy. No patient relapsed at a cytogenetic or hematologic level during the period of imatinib administration. One year after transplantation, at the time of discontinuation of imatinib, 7 patients were in molecular remission, while the remaining 14 all had a BCR-ABL/ABL ratio less than 0.5%.
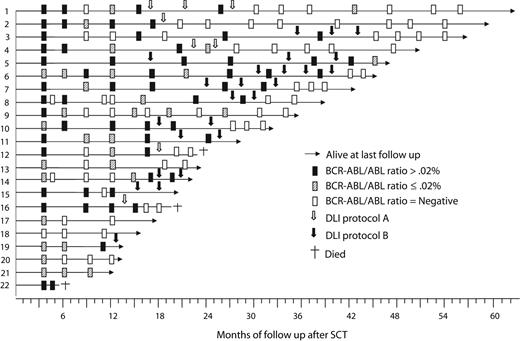
Twenty-one patients are evaluable more than 12 months after transplantation. After discontinuation of imatinib, 5 patients remain in molecular remission (13-40 months after transplantation) and 1 has stable low-level molecular disease (BCR-ABL/ABL ratio: < 0.02%). Fifteen patients relapsed at a median of 17 months (range: 13-29 months) after transplantation. All patients with relapsed disease received DLI, of whom 10 achieved a molecular remission and 5 are midway through a course of DLI (Figure 1). Responding patients required a mean of 2 courses (range: 1-4 courses) of DLI to achieve molecular remission. Two patients developed grade 4 acute GVHD after the first dose of DLI using the initial, higher dose lymphocyte schedule. No patient developed acute or chronic GVHD using the lower dose DLI schedule. With a median follow-up of 36 months (range: 12-64 months), 19 patients are alive with a calculated overall survival of 87%. Fifteen (68%) have achieved molecular remission to date, although this figure is likely to rise with further follow-up as additional patients respond to DLI. There was no correlation between the level of molecular disease at the time of administration of DLI and subsequent response.
Sequential response to treatment as assessed by quantitation of BCR-ABL transcript numbers in the 22 trial patients.
Sequential response to treatment as assessed by quantitation of BCR-ABL transcript numbers in the 22 trial patients.
This study demonstrates that posttransplantation imatinib can be used to manipulate the kinetics of disease relapse after a RIC allograft and abolish the risk of early relapse. These results contrast favorably with the high rate of early relapse reported after T-depleted RIC regimens in CML and other myeloid malignancies.4,5,13 Previously, imatinib has been administered as treatment for disease recurrence after transplantation, but there are no studies addressing the tolerability or activity of adjunctive imatinib after a reduced-intensity allograft although it has recently been shown to be well tolerated in a different setting after a myeloablative conditioning regimen.14,–16 The most important factors determining the risk of GVHD after DLI are time of administration and cell dose, and therefore the ability of imatinib to postpone the requirement for DLI gives the opportunity to administer this important salvage therapy with less toxicity.7,8,17,18 The occurrence of severe GVHD in this study using a higher starting dose of DLI underlines its toxicity and raises the possibility that patients would benefit from a longer period of imatinib administration.
This study demonstrates that the incorporation of adjunctive imatinib into a RIC regimen results in a well-tolerated transplantation strategy with the ability to deliver a high rate of molecular remission with a low risk of GVHD in older patients. The ability of targeted therapy to reduce the risk of early relapse after transplantation has relevance for the design of RIC protocols in other diseases such as acute myeloid leukemia (AML). In this setting, consideration should be given to the use of gemtuzumab ozogamicin or FLT 3 inhibitors, which might also modify relapse kinetics such that DLI, if required, can be delivered with less toxicity.19,20
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Leukemia Research. Imatinib mesylate was supplied by Novartis free of charge.
Authorship
Contribution: E.O. designed the study, recruited patients, and wrote the paper; S.S. collected and analyzed patient data; M.J.G. performed chimerism and minimal residual disease studies; S.A., J.L.B., and A.L.L. recruited patients and collected patient data; K.P.P. performed immunologic studies on patient samples; L.P. collected patient data and constructed figures and tables; J.M.A., J.B.P., and D.O. collected patient data; J.M.G. recruited patients and assisted in data analysis and interpretation; J.F.A. designed the study and recruited patients; C.F.C. designed the study, recruited patients, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles Craddock, Centre for Clinical Haematology, Main Drive, Queen Elizabeth Hospital, Birmingham B15 2TH; e-mail:charles.craddock@uhb.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal