Graft-versus-host disease (GVHD) is the main complication of allogeneic stem cell transplantation. However, diagnosis of GVHD and evaluation of response to immunosuppressive treatment is sometimes difficult. Since apoptosis is the histopathologic hallmark in GVHD, we investigated whether active GVHD-induced target organ destruction is mirrored by serum levels of the caspase-cleaved neo-epitope of cytokeratin-18 fragments (CK18Fs). Serum CK18F kinetics was monitored by M30 antibody-based enzyme-linked immunosorbent assay (ELISA) in 50 patients who fulfilled histopathologic and/or clinical criteria diagnostic for GVHD. Both intestinal and hepatic GVHD were consistently associated with significant elevations of CK18F levels over baseline. Responses of GVHD to immunosuppressive therapy were paralleled by CK18F decreases, whereas resistant GVHD was characterized by persistent CK18F rises. Clinical conditions that might represent relevant differential diagnoses, such as toxic mucositis, noncomplicated, infection-related diarrhea, and veno-occlusive disease were not associated with CK18F elevations. In conclusion, CK18F monitoring provides a serum marker for quantitative assessment of GVHD-associated apoptotic activity in intestinal and hepatic GVHD. Although apoptosis is not GVHD-specific, CK18Fs may help to distinguish active GVHD from GVHD-unrelated conditions with similar symptoms, and to monitor response to immunosuppressive treatment. Prospective studies are warranted to evaluate how CK18Fs may assist in the diagnosis, grading, and treatment guidance of GVHD.
Introduction
Graft-versus-host disease (GVHD) is the major cause of morbidity and mortality following allogeneic stem cell transplantation (SCT). GVHD is a heterogeneous group of syndromes with autoimmune-like characteristics, which still pose numerous diagnostic and therapeutic challenges. Unresolved clinical issues include—apart from diagnosis and differentiation from infectious or toxic clinical conditions—detection of emerging GVHD, grading, and distinction between ongoing GVHD activity and irreversible end organ damage.
Apoptosis in epidermis and intestinal mucosa is a common finding in GVHD and is used for histologic grading of GVHD.1 Quantification and monitoring of apoptosis might allow distinguishing active GVHD from organ dysfunction due to other causes. However, sequential biopsies are precarious in patients with GVHD, and focal findings might not be representative of the systemic situation. Therefore, an alternative approach might consist of serum assays reflecting ongoing epithelial apoptotic activity.
Cytokeratin-18 (CK18) is an intermediate filament representing about 5% of total protein in most simple epithelia and parenchymal cells.2,3 Induction of apoptosis results in early cleavage of CK18 in position 238VEVD-A mediated by caspases 3, 6, and 7,4 and in position 396DALD-S mediated by caspases 3, 7, and 9.5 Cleavage at the 396DALD-S site gives rise to a neoepitope termed CK18 fragments (CK18Fs), which is recognized by the M30 antibody.5 CK18Fs are stable to proteolysis and are released into tissue culture supernatants6 and into the serum7,8 after plasma membrane disintegration at later stages of apoptosis. Increased serum levels of CK18F have been reported for patients suffering from liver fibrosis9 and are currently evaluated in hepatitis C virus (HCV) as an apoptosis marker to monitor treatment response.10
Given the prominent role of apoptosis in GVHD pathophysiology, the aim of the present study was to investigate if ongoing GVHD activity is reflected by CK18F serum levels. Our data suggest that CK18F monitoring allows quantitative assessment of apoptotic activity associated with intestinal or hepatic GVHD. Therefore, CK18F assessment might be useful to distinguish active GVHD from GVHD-unrelated conditions with similar symptoms, and to monitor response to immunosuppressive treatment.
Patients and methods
Patients
Serum samples were routinely collected after written informed consent was obtained in accordance with the Declaration of Helsinki from 198 consecutive patients who had undergone allogeneic blood or bone marrow stem-cell transplantation between May 2002 and March 2006 at our department at the University of Heidelberg. The sampling period comprised pre-SCT and weekly or second weekly sample collections after SCT for a maximum of 1 year. Informed consent was obtained from all patients and the local ethics committee of the University of Heidelberg had approved sample collection. After blood collection, serum was immediately obtained by centrifugation, transferred into cryotubes, and stored at −80°C until further processing. As serum samples for CK18F measurements were taken together with the normal routine blood sampling, all clinical chemistry examinations (bilirubin, ALAT, AP, γGT, creatinin) were performed at the same day.
For the purposes of this retrospective study, 50 patients were selected representing all who fulfilled the following criteria: (1) one or more episodes of clinical only (skin), clinical and histopathologic (intestinal tract), or clinical and serologic (liver) evidence of acute or chronic GVHD; and (2) availability of longitudinal samples for 1 year after undergoing transplantation or at least for a period flanking the GVHD episode for about 1 month or until death due to GVHD. GVHD was defined by histology, or, if not available, by accepted clinical criteria.11 Samples taken immediately prior to the escalation of immunosuppressive therapy or, in the case of steroid-resistant GVHD, samples taken the day closest to salvage immunosuppressive therapy, were considered as representing maximum GVHD.
CK18F levels were measured in most instances over the full collection period of 360 days. There were 5 additional patients who did not have any episode of GVHD during the observation period and who were included for comparison because of documented infectious diarrhea (n = 4) and early non-GVHD–related hyperbilirubinemia (n = 1). There were 2 patients with severe pseudomembranous colitis following chemotherapy for hematologic diseases without SCT who were also analyzed. Characteristics of the 55 selected patients with SCT are summarized in Table 1 and are shown in detail in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Patient characteristics
| Characteristic . | Patients . |
|---|---|
| Sex, male/female, no. (%) | 32/23 (58/42) |
| Age at SCT, y, median (range) | 44 (17-66) |
| Diagnoses, no | |
| ALL | 3 |
| AML | 11 |
| CIMF | 1 |
| CML | 2 |
| MDS | 5 |
| B-CLL | 1 |
| NHL | 9 |
| HD | 1 |
| MM | 21 |
| PNH | 1 |
| Donor, no. (%) | |
| Matched related | 32 (58) |
| Mismatched related | 2 (4) |
| Matched unrelated | 14 (25) |
| Mismatched unrelated | 7 (13) |
| Conditioning, no. (%) | |
| Standard | 16 (29) |
| Reduced-intensity | 39 (71) |
| Source, PBSC/BM, no. (%) | 54/1 (98/2) |
| Characteristic . | Patients . |
|---|---|
| Sex, male/female, no. (%) | 32/23 (58/42) |
| Age at SCT, y, median (range) | 44 (17-66) |
| Diagnoses, no | |
| ALL | 3 |
| AML | 11 |
| CIMF | 1 |
| CML | 2 |
| MDS | 5 |
| B-CLL | 1 |
| NHL | 9 |
| HD | 1 |
| MM | 21 |
| PNH | 1 |
| Donor, no. (%) | |
| Matched related | 32 (58) |
| Mismatched related | 2 (4) |
| Matched unrelated | 14 (25) |
| Mismatched unrelated | 7 (13) |
| Conditioning, no. (%) | |
| Standard | 16 (29) |
| Reduced-intensity | 39 (71) |
| Source, PBSC/BM, no. (%) | 54/1 (98/2) |
Summary of 55 patients with and without GVHD recruited for longitudinal CK18F measurement (for details see Table S1).
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; CIMF, chronic idiopathic myelofibrosis; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease; MM, multiple myeloma; PNH, paroxysmal nocturnal hemoglobinurea; PBSC, peripheral blood stem cell; and BM, bone marrow.
In addition, samples for the analysis of CK18F levels prior to transplant and during the early posttransplantation period were obtained after written informed consent from 18 consecutive patients who underwent high-dose chemotherapy and autologous SCT and 23 consecutive patients who underwent allogeneic SCT from January through June 2006 (Table S2). Surplus serum of routine blood collections was obtained by centrifugation, transferred into cryotubes, and stored at −80°C until further processing.
For all patients, complete routine clinical and paraclinical data were available. Acute and chronic GVHD was clinically graded using standard criteria.11 Histopathologic grading was performed according to standard criteria.1,12 Results of CK18F monitoring were never available to patients or treating physicians, in order to exclude bias.
ELISA of cytokeratin-18 fragments
Serum levels of cytokeratin-18 fragments (CK18Fs) were measured using the M30-Apoptosense ELISA Kit (Peviva, Bromma, Sweden) according to the manufacturer's instructions. In brief, 25 μL patient serum was transferred into 96-well plates coated with CK18 antibody. All measurements were performed in duplicate wells. After addition of 75 μL directly horseradish peroxidase–conjugated M30 mAB, plates were agitated for 4 hours on a shaker at room temperature. Plates were washed 5 times, 200 μL substrate solution was added to each well, and the plate was incubated in darkness at room temperature for 20 minutes. After adding 50 μL stop solution to each well, 10 seconds agitation and 5 minutes holding time, the absorbance at 450 nm was finally determined using a Sunrise absorbance reader (Tecan, Crailsheim, Germany). The absorbance data were analyzed using Magellan software (Tecan).
Statistics
In order to avoid repeated measures, averages, correlation analyses, and t tests were calculated with the CK18F values of only the first episode of liver and intestinal GVHD of each patient. For the analysis of the relative changes of CK18F levels over time, log-transformed CK18F values were used for the statistical analyses and all averages were calculated from the log-transformed values. To estimate fold changes of the distribution of CK18F levels, all means and 95% confidence intervals (CIs) of the differences of the log-transformed CK18F values were calculated and then back-transformed to obtain the fold change estimate and its corresponding 95% CI. Paired data of log-transformed CK18F values were compared by the paired t-test. Pairwise comparisons of the distribution of CK18F levels between 2 independent groups were done by Welch t test. All statistical tests were 2-sided. An effect was considered statistically significant at a P value smaller than or equal to .05. To check for normality we used the method proposed by Ghosh.13
The correlation of CK18F levels and GVHD intensity was estimated by computing the partial correlation coefficient, denoted by rp, between CK18F levels and GVHD intensity measured by ALAT, AP, γGT, and bilirubin levels, controlling also for time since SCT. Partial correlations were used to account for spurious correlation.
Transplant-related mortality (TRM) was defined as death from any cause without preceding relapse or progression. Since GVHD is a time-dependent event, the Andersen-Gill model was used to model the effect of the GVHD-related value of CK18Fs on TRM.14
Statistical calculations were performed using R, version 2.4.1,15 together with the additional R packages cwhplot, version 1.0.4, and corpcor, version 1.4.4.
Results
Baseline serum concentrations of CK18 fragments
Basal pre-SCT CK18F levels were analyzed in 60 patients (42 allogeneic, 18 autologous) using serum samples obtained immediately prior to conditioning. These individual CK18F baseline levels ranged from 114 U/L to 463 U/L (median 152 U/L).
Serum concentrations of CK18Fs in GVHD of gut, liver and, skin
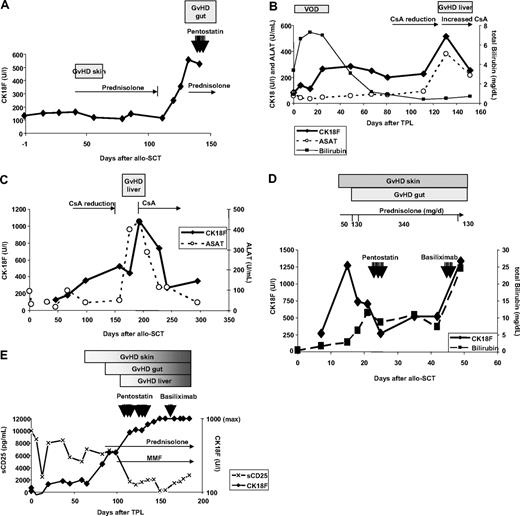
Longitudinal CK18F serum kinetics were measured in 50 patients with documented episodes of GVHD. For 19 patients, biopsy results were available: 13 intestinal, 3 liver, and 3 skin biopsies (Table 2). Highly elevated CK18Fs were observed during biopsy-proven GVHD of liver (480 U/L-6880 U/L; median 1920 U/L) and gut (347 U/L-1276 U/L; median 560 U/L), corresponding to an estimated 5.6-fold change of CK18F levels at intestinal GVHD compared with pre-SCT levels (95% CI 4.2-6.9, P < .001, paired t test). Representative examples are given in Figure 1. Nine intestinal episodes were associated with liver enzyme elevations, suggestive of simultaneous hepatic involvement. However, all 4 episodes of biopsy-proven isolated intestinal GVHD showed increased CK18F levels, too (range 347-561 U/L, median 494 U/L, estimated 3.6-fold change of CK18F levels at GVHD compared with pre-SCT levels, 95% CI 2.5-4.8, P < .001, paired t test). In 9 patients (UPN 178, 192, 196, 205, 211, 225, 235, 271, 275), CK18F increases preceding the clinical diagnosis of GVHD could be documented (Figure 1A,C). All liver and intestinal GVHD episodes were accompanied by mucosal or skin manifestations.
CK18F levels in histologically confirmed GVHD of gut, liver, and skin
| UPN . | Day of onset after TPL . | Histo-grading . | Before SCT CK18F, U/L . | CK18F at symptom maximum, U/L . |
|---|---|---|---|---|
| Gut | ||||
| 301 | 15 | III-IV | 275 | 1276 |
| 284 | 18 | II-III | 81 | 685 |
| 304 | 21 | II | 320 | 718 |
| 210 | 31 | IV | 99 | 568 |
| 271* | 38* | I* | 124 | 435 |
| 363* | 91 | IV* | 115 | 347 |
| 291 | 98 | II-III | 153 | 901 |
| 189 | 107 | III | 31 | 434 |
| 211* | 131* | III* | 132 | 560 |
| 291 | 144 | III-IV | 153 | 1200 |
| 250* | 145* | IV* | 142 | 561 |
| 178 | 163 | I-II | ND | 478 |
| 281 | 210 | I | 123 | 414 |
| Liver | ||||
| 200 | 72 | 18,1† | 159 | 1920 |
| 288 | 163 | 2,5† | 190 | 6880 |
| 221 | 250 | 1,4† | 195 | 480 |
| Skin | ||||
| 279 | 44 | II | 196 | 165 |
| 181 | 61 | IV | 129 | 230 |
| 277 | 77 | I | 296 | 185 |
| UPN . | Day of onset after TPL . | Histo-grading . | Before SCT CK18F, U/L . | CK18F at symptom maximum, U/L . |
|---|---|---|---|---|
| Gut | ||||
| 301 | 15 | III-IV | 275 | 1276 |
| 284 | 18 | II-III | 81 | 685 |
| 304 | 21 | II | 320 | 718 |
| 210 | 31 | IV | 99 | 568 |
| 271* | 38* | I* | 124 | 435 |
| 363* | 91 | IV* | 115 | 347 |
| 291 | 98 | II-III | 153 | 901 |
| 189 | 107 | III | 31 | 434 |
| 211* | 131* | III* | 132 | 560 |
| 291 | 144 | III-IV | 153 | 1200 |
| 250* | 145* | IV* | 142 | 561 |
| 178 | 163 | I-II | ND | 478 |
| 281 | 210 | I | 123 | 414 |
| Liver | ||||
| 200 | 72 | 18,1† | 159 | 1920 |
| 288 | 163 | 2,5† | 190 | 6880 |
| 221 | 250 | 1,4† | 195 | 480 |
| Skin | ||||
| 279 | 44 | II | 196 | 165 |
| 181 | 61 | IV | 129 | 230 |
| 277 | 77 | I | 296 | 185 |
Histologic grading was performed in an academic pathology laboratory according to Kreisel et al.11
ND indicates not done.
Intestinal GVHD without elevated liver enzymes.
Serum bilirubin in mg/dL.
Time course of GVHD and CK18F levels in representative patients. (A) Patient UPN 211: slight increase of basal CK18F levels (132 U/L) during acute GVHD I of the skin (day 40, 162 U/L) responding to steroid treatment. CK18F started to rise immediately after steroid reduction and at least 1 week prior to the onset of clinical symptoms of GVHD grade III of the gut. Steroid resistance of the latter episode was reflected by constantly high levels of CK18F after 7 days of high-dose prednisolone treatment. (B) Patient UPN 297: an episode of veno-occlusive disease (VOD) with high serum bilirubin levels following SCT did not coincide with increases in CK18F levels. Liver GVHD developed during reduction of CsA after day 100, which was accompanied by a brisk CK18F increase. This episode of GVHD immediately responded to the restoration of high CsA levels. (C) Patient UPN 275 developed chronic GVHD (liver and wasting syndrome) after CsA reduction. CK18F levels started to rise early after beginning of CsA tapering, whereas liver enzymes became elevated only 50 days thereafter. This episode of GVHD rapidly responded to renewed CsA treatment as shown by normalization of both CK18F and ALAT levels. (D) Patient UPN 301 developed acute GVHD grade IV of skin, liver, and gut as early as 10 days following SCT. Despite high-dose steroid treatment, GVHD progressed and bilirubin levels rose to 10 mg/dL, requiring second-line immunosuppressive treatment (pentostatin). CK18F fell below 300 U/L following steroid and penostatin therapy, which indicated reduced epithelial apoptotic activity and thereby response to treatment. However, this response was not noticed clinically, as the skin rash, diarrhea, and hyperbilirubinemia went unresolved. Therefore, therapy was switched to anti-CD25 (basiliximab), which could not prevent subsequent deterioration (fatal progress of GVHD in all 3 organs). (E) Patient UPN 363 developed GVHD of the skin around day 60. Intestinal symptoms started after day 90 and bilirubin levels rose after day 100. GVHD grade IV of the gut was histologically confirmed. Prednisolone was started on day 90; however, the GVHD progressed and salvage therapies were given. The patient did not respond to any of these treatments and subsequently died. This lack of clinical response was reflected by persistently high levels of CK18F. Note the reduction in sCD25 levels following steroid treatment.
Time course of GVHD and CK18F levels in representative patients. (A) Patient UPN 211: slight increase of basal CK18F levels (132 U/L) during acute GVHD I of the skin (day 40, 162 U/L) responding to steroid treatment. CK18F started to rise immediately after steroid reduction and at least 1 week prior to the onset of clinical symptoms of GVHD grade III of the gut. Steroid resistance of the latter episode was reflected by constantly high levels of CK18F after 7 days of high-dose prednisolone treatment. (B) Patient UPN 297: an episode of veno-occlusive disease (VOD) with high serum bilirubin levels following SCT did not coincide with increases in CK18F levels. Liver GVHD developed during reduction of CsA after day 100, which was accompanied by a brisk CK18F increase. This episode of GVHD immediately responded to the restoration of high CsA levels. (C) Patient UPN 275 developed chronic GVHD (liver and wasting syndrome) after CsA reduction. CK18F levels started to rise early after beginning of CsA tapering, whereas liver enzymes became elevated only 50 days thereafter. This episode of GVHD rapidly responded to renewed CsA treatment as shown by normalization of both CK18F and ALAT levels. (D) Patient UPN 301 developed acute GVHD grade IV of skin, liver, and gut as early as 10 days following SCT. Despite high-dose steroid treatment, GVHD progressed and bilirubin levels rose to 10 mg/dL, requiring second-line immunosuppressive treatment (pentostatin). CK18F fell below 300 U/L following steroid and penostatin therapy, which indicated reduced epithelial apoptotic activity and thereby response to treatment. However, this response was not noticed clinically, as the skin rash, diarrhea, and hyperbilirubinemia went unresolved. Therefore, therapy was switched to anti-CD25 (basiliximab), which could not prevent subsequent deterioration (fatal progress of GVHD in all 3 organs). (E) Patient UPN 363 developed GVHD of the skin around day 60. Intestinal symptoms started after day 90 and bilirubin levels rose after day 100. GVHD grade IV of the gut was histologically confirmed. Prednisolone was started on day 90; however, the GVHD progressed and salvage therapies were given. The patient did not respond to any of these treatments and subsequently died. This lack of clinical response was reflected by persistently high levels of CK18F. Note the reduction in sCD25 levels following steroid treatment.
Epidermal and oropharyngeal GVHD often coincided with increased liver enzymes, which may have accounted for elevated CK18F levels. However, CK18Fs could be measured in 14 patients with isolated GVHD of skin and oropharynx (acute GVHD I-II, n = 5; acute GVHD III-IV: n = 1; limited chronic (c) GVHD, n = 7; extensive cGVHD n = 1). CK18F values ranged between 102 U/L and 403 U/L (median 192 U/L). Pre-GVHD CK18F levels of the same patients (absence of any epidermal or mucosal symptoms) ranged between 60 U/L and 218 U/L (median 123 U/L; for 2 patients, pre-GVHD values were missing). This corresponds to an estimated 1.7-fold change of CK18F levels at GVHD compared with normal levels (95% CI 0.6-2.8, P = .002, paired t test).
CK18F levels rise during the period preceding the initiation of immunosuppressive therapy
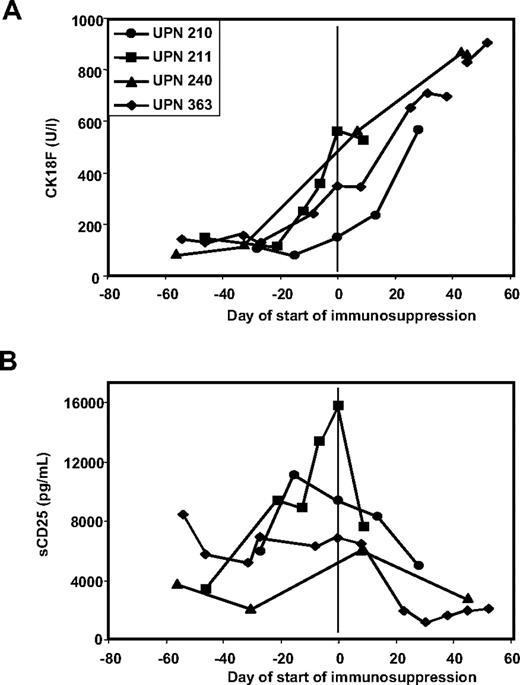
As the exact day of onset of GVHD is often difficult to define, the time of starting/escalating immunosuppressive therapy might be used as a reference that is independent of retrospective interpretation of clinical data. Although the decision to treat GVHD at a certain time also depends on other, non-GVHD–related issues (such as progressive malignant disease that is hoped to be attacked by graft-versus-leukemia effects coinciding with GVHD), this event should correspond to maximum GVHD activity in patients responsive to immunosuppressive treatment. Accordingly, Figure 2A demonstrates that CK18F levels rise in all 20 patients of our cohort with CK18F values available during the period of 50 days preceding start/escalation of immunosuppressive therapy.
CK18F levels rise prior to the start of immunosuppressive treatment of GVHD and decrease with response to therapy. (A) Individual kinetics of CK18F levels are shown for all 20 patients of our cohort who had serum samples available in the period of 50 days preceding the start/escalation of immunosuppressive therapy of hepato-intestinal GVHD. CK18F data were standardized with respect to their value at the start of immunosuppressive therapy (n = 18) or to the day closest to this clinical decision (n = 2). (B) Individual kinetics of all 23 patients with available serum samples responding to immunosuppressive therapy. CK18F data were standardized with respect to their value at the start of immunosuppressive therapy. As the time point for the resolution of GVHD is difficult to fix, we show CK18F levels at the day of steroid withdrawal (or of withdrawal of ciclosporin if this was the only immunosuppressive drug applied •). Alternatively, the last available sample was used if tapering of steroid treatment was not completed at the end of follow-up (○).
CK18F levels rise prior to the start of immunosuppressive treatment of GVHD and decrease with response to therapy. (A) Individual kinetics of CK18F levels are shown for all 20 patients of our cohort who had serum samples available in the period of 50 days preceding the start/escalation of immunosuppressive therapy of hepato-intestinal GVHD. CK18F data were standardized with respect to their value at the start of immunosuppressive therapy (n = 18) or to the day closest to this clinical decision (n = 2). (B) Individual kinetics of all 23 patients with available serum samples responding to immunosuppressive therapy. CK18F data were standardized with respect to their value at the start of immunosuppressive therapy. As the time point for the resolution of GVHD is difficult to fix, we show CK18F levels at the day of steroid withdrawal (or of withdrawal of ciclosporin if this was the only immunosuppressive drug applied •). Alternatively, the last available sample was used if tapering of steroid treatment was not completed at the end of follow-up (○).
CK18F levels correlate with response to immunosuppressive treatment
For 27 episodes of GVHD of liver and/or gut, CK18F levels measured at baseline and time of maximum GVHD were available. Maximum GVHD values (median 729 U/L, range 348 U/L-6880 U/L) were elevated over baseline (median 175 U/L, range 31 U/L-320 U/L) in each individual case, corresponding to an estimated 5.2-fold increase (95% CI 3.8-6.6, P < .001, paired t test). Twenty-three of the 27 GVHD episodes responded to immunosuppressive therapy. Because the time point for the resolution of GVHD is difficult to fix, we show CK18F levels at the day of steroid withdrawal (or of withdrawal of ciclosporin if this was the only immunosuppressive drug applied). Alternatively, the last available sample was used if tapering of steroid/ciclosporin treatment was not completed at the end of follow-up (Figure 2B). Significantly decreased CK18F values were observed when comparing values at the day of de-escalation/the last day of follow-up (median 201 U/L, range 82 U/L-392 U/L) with maximum GVHD values, corresponding to an estimated 3.7-fold decrease (95% CI 2.4-5.1, P < .001, paired t test). Last follow-up values, however, still were significantly higher than baseline levels (estimated 1.4-fold increase, 95% CI 0.1-2.7, P = .010; Figure 2B).
There were 4 patients in the study who had steroid refractory-GVHD. In all 4 patients, CK18F elevations persisted or further increased along with clinical symptoms despite application of high-dose prednisolone (Figure 3A).
CK18F but not sCD25 levels reflect response to immunosuppressive therapy in steroid-resistant disease. (A) Kinetics of CK18F levels in 4 patients with steroid-resistant GVHD: the lack of clinical response to high-dose steroid treatment is reflected in persistent or rising CK18F levels. (B) Kinetics of sCD25 levels in 4 patients with steroid-resistant GVHD: reduced sCD25 levels following steroid treatment despite of the lack of a clinical response.
CK18F but not sCD25 levels reflect response to immunosuppressive therapy in steroid-resistant disease. (A) Kinetics of CK18F levels in 4 patients with steroid-resistant GVHD: the lack of clinical response to high-dose steroid treatment is reflected in persistent or rising CK18F levels. (B) Kinetics of sCD25 levels in 4 patients with steroid-resistant GVHD: reduced sCD25 levels following steroid treatment despite of the lack of a clinical response.
Correlation between CK18F levels and GVHD severity
Due to the low number of patients suffering from isolated intestinal GVHD without an overlap with liver GVHD, correlation analyses between CK18F levels and intestinal GVHD severity were not possible. With regard to liver GVHD, however, we analyzed the correlation of CK18F levels and GVHD intensity during 29 clinically diagnosed episodes of liver GVHD in addition to the 3 histologically confirmed episodes (only the first GVHD episode was evaluated for each patient). Of these 32 GVHD episodes, 6 occurred prior to day 120 after allogeneic SCT and were considered as acute GVHD. Serum CK18F levels clearly correlated with serum bilirubin levels (estimated partial correlation coefficient rp = 0.91) and ALAT levels (rp = 0.96) in these patients. However, this correlation was less stringent for liver GVHD episodes occurring late after SCT. Here, ALAT was the best single marker correlating with CK18F levels (rp = 0.75), whereas bilirubin did not correlate with CK18F levels (rp = 0.08).
Maximum CK18F levels did not correlate with TRM in the 50 patients with GVHD (P = .52, Wald test statistic, Andersen-Gill model). However, it has to be taken into account that only 11 treatment-related deaths were observed.
CK18F levels during toxic hyperbilirubinemia, veno-occlusive disease, infectious enteritis, toxic mucositis, and renal failure
In contrast to liver GVHD, CK18F levels did not rise during early hyperbilirubinemia due to veno-occlusive disease (VOD) or drug-induced toxic liver damage. Figure 1B shows the course of bilirubin and CK18F levels in a patient with VOD and a later episode of chronic GVHD of the liver. This patient (UPN 297) developed hyperbilirubinemia following conditioning with ATG, melphalan, and fludarabine together with typical clinical symptoms of VOD (fluid retention, liver capsular pain). Peak bilirubin values were measured on day 14 after SCT. After treatment with defibrotide, clinical VOD manifestations resolved and bilirubin levels normalized. However, liver enzymes started to rise along with CK18F on day +130 after SCT in the context of liver GVHD. Table 3 summarizes CK18F levels observed during early hyperbilirubinemia in 9 evaluable patients of our cohort, indicating that this condition may not be characterized by CK18F increases in comparison to baseline (P = .46; paired t test).
CK18F levels in early hyperbilirubinemia and VOD
| UPN . | Day after SCT . | CK18F, U/L . | Fold change over baseline, before chemotherapy . | Maximum bilirubin, mg/dL . |
|---|---|---|---|---|
| 340 | 10 | 119 | 0.49 | 2.0 |
| 178 | 11 | 171 | 1.38 | 8.8 |
| 290 | 12 | 114 | 0.93 | 7.3 |
| 210 | 13 | 79 | 0.80 | 6.0 |
| 211 | 14 | 151 | 1.14 | 2.4 |
| 297 | 14 | 113 | 1.31 | 7.3 |
| 200 | 16 | 155 | 0.57 | 2.8 |
| 303 | 17 | 179 | NA | 4.2 |
| 329 | 19 | 180 | NA | 1.5 |
| UPN . | Day after SCT . | CK18F, U/L . | Fold change over baseline, before chemotherapy . | Maximum bilirubin, mg/dL . |
|---|---|---|---|---|
| 340 | 10 | 119 | 0.49 | 2.0 |
| 178 | 11 | 171 | 1.38 | 8.8 |
| 290 | 12 | 114 | 0.93 | 7.3 |
| 210 | 13 | 79 | 0.80 | 6.0 |
| 211 | 14 | 151 | 1.14 | 2.4 |
| 297 | 14 | 113 | 1.31 | 7.3 |
| 200 | 16 | 155 | 0.57 | 2.8 |
| 303 | 17 | 179 | NA | 4.2 |
| 329 | 19 | 180 | NA | 1.5 |
NA indicates no pre-SCT serum samples available.
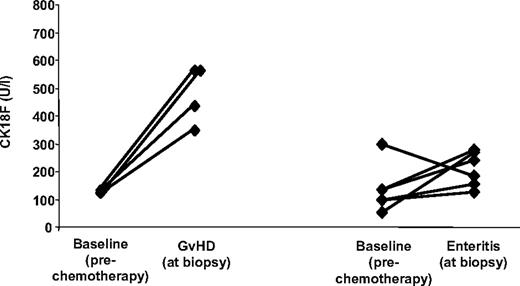
Furthermore, CK18F levels were measured during 11 episodes of documented, noncomplicated, infectious enteritis (Clostridium difficile, n = 4; Clostridium difficile + rotavirus + Norwalk virus, n = 1; rotavirus, n = 1; Norwalk virus, n = 1; astrovirus, n = 1; herpes simplex virus, n = 1; human herpes virus 6 + Epstein-Barr virus, n = 1; and cytomegalovirus, n = 1). CK18F levels associated with these conditions (range 92 U/L-305 U/L; median 185 U/L) were significantly lower than those obtained during episodes of histologically proven isolated intestinal GVHD (4 patients; range 347 U/L-561 U/L, median 494 U/L; P = .008, Welsh t test). In 6 patients with noncomplicated infectious enteritis, paired pre-SCT CK18F levels were available (median 213 U/L vs 117 U/L; corresponding to an estimated fold increase of 1.7 (95% CI −0.1-3.5), P = .12, paired t test) (Figure 4). On the other hand, strongly increased CK18F levels were observed in 3 additional patients suffering from severe Clostridium difficile–associated pseudomembranous colitis, one of them 30 days after undergoing allogeneic transplantation (535 U/L) and 2 in patients following chemotherapy for hematologic diseases without allogeneic transplantation (921 U/L and > 1000 U/L).
CK18F levels in isolated intestinal GVHD and noncomplicated infectious enteritis. Direct comparison of pre-SCT CK18F levels with levels in sera from 6 patients suffering from noncomplicated infectious gastroenteritis after allo-SCT (right), and from 4 episodes of histologically confirmed isolated intestinal GVHD (left).
CK18F levels in isolated intestinal GVHD and noncomplicated infectious enteritis. Direct comparison of pre-SCT CK18F levels with levels in sera from 6 patients suffering from noncomplicated infectious gastroenteritis after allo-SCT (right), and from 4 episodes of histologically confirmed isolated intestinal GVHD (left).
Next, we addressed the impact of high-dose chemotherapy–induced mucositis on CK18F serum levels. For this purpose, CK18F level was quantified before start of conditioning, at the day of the transplantation, on days 6 to 9 after SCT (representing mucositis), and 12 to 24 days after SCT (representing clearance of mucositis) in 41 patients who had undergone autologous (n = 18) or allogeneic SCT (n = 23). Patient characteristics are given in Table S2. Results are summarized in Table 4. There was a small but significant increase in CK18F levels between the day of maximal expected mucositis (days 7-9) compared with baseline (before chemotherapy). However, this increase was not seen when the analysis was restricted to patients suffering from severe mucositis (grade 3-4). Therefore we investigated whether the mild increases of CK18F levels around days 7 to 9 were a result of chemotherapy rather than mucositis. Patients were grouped for conditioning intensity: high-dose melphalan, 200 mg/m2 (high-dose group, n = 18); myeloablative treatment or combinations of fludarabine and alkylating agents with or without antithymocyte globuline (intermediate-dose group, n = 16); fludarabine with 2 Gy total body irradiation (low-dose group, n = 7). Significant albeit moderate alterations of day 0 CK18F levels (corresponding to the second day following chemotherapy or total body irradiation) were seen after high-dose (1.7 fold; 95% CI 1.4-2.0) and intermediate-dose conditioning (1.5 fold; 95% CI 1.2-1.8), implying that chemotherapy might exert limited but measurable systemic proapoptotic effects. (Table 5).
Mucositis and CK18F
| Day . | No mucositis, n=20 . | Mucositis all grades, n=21 . | Mucositis grade 1-2, n=7 . | Mucositis grade 3-4, n=14 . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) . | P . | Mean (95% CI) . | P . | Mean (95% CI) . | P . | Mean (95% CI) . | P . | |
| Baseline, before chemotherapy | 180 (151-216) | .41 | 182 (160-207) | .02 | 175 (132-210) | .04 | 190 (163-222) | .32 |
| Days 6 to 9 after SCT, maximal expected mucositis | 196 (158-244) | NA | 212 (191-236) | NA | 229 (195-280) | NA | 202 (178-229) | NA |
| Days 12 to 24 after SCT, clearance of mucositis | 225 (193-262) | .17 | 213 (183-247) | .97 | 168 (152-176) | .006 | 234 (192-284) | .03 |
| Day . | No mucositis, n=20 . | Mucositis all grades, n=21 . | Mucositis grade 1-2, n=7 . | Mucositis grade 3-4, n=14 . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) . | P . | Mean (95% CI) . | P . | Mean (95% CI) . | P . | Mean (95% CI) . | P . | |
| Baseline, before chemotherapy | 180 (151-216) | .41 | 182 (160-207) | .02 | 175 (132-210) | .04 | 190 (163-222) | .32 |
| Days 6 to 9 after SCT, maximal expected mucositis | 196 (158-244) | NA | 212 (191-236) | NA | 229 (195-280) | NA | 202 (178-229) | NA |
| Days 12 to 24 after SCT, clearance of mucositis | 225 (193-262) | .17 | 213 (183-247) | .97 | 168 (152-176) | .006 | 234 (192-284) | .03 |
CK18F means and 95% CI values are in U/L. P values are in comparison to the day of maximal expected mucositis (days 7-9 after SCT; paired t test).
NA indicates not applicable.
Influence of chemotherapy on CK18F
| Day . | HD-autologous, n=18 . | HD/intermediate-allogeneic, n=16 . | Mini-allogeneic, n=7 . | |||
|---|---|---|---|---|---|---|
| Mean (95% CI) . | P . | Mean (95% CI) . | P . | Mean (95% CI) . | P . | |
| Baseline, before chemotherapy | 171 (147-198) | NA | 179 (158-203) | NA | 203 (131-314) | NA |
| Day 0, day of SCT | 289 (239-349) | <.001 | 268 (221-325) | <.001 | 295 (174-500) | .26 |
| Days 6 to 9 after SCT | 222 (197-250) | .01 | 192 (165-223) | .24 | 172 (103-286) | .36 |
| Days 12 to 24 after SCT | 199 (179-220) | .03 | 224 (184-273) | .008 | 249 (177-350) | .21 |
| Day . | HD-autologous, n=18 . | HD/intermediate-allogeneic, n=16 . | Mini-allogeneic, n=7 . | |||
|---|---|---|---|---|---|---|
| Mean (95% CI) . | P . | Mean (95% CI) . | P . | Mean (95% CI) . | P . | |
| Baseline, before chemotherapy | 171 (147-198) | NA | 179 (158-203) | NA | 203 (131-314) | NA |
| Day 0, day of SCT | 289 (239-349) | <.001 | 268 (221-325) | <.001 | 295 (174-500) | .26 |
| Days 6 to 9 after SCT | 222 (197-250) | .01 | 192 (165-223) | .24 | 172 (103-286) | .36 |
| Days 12 to 24 after SCT | 199 (179-220) | .03 | 224 (184-273) | .008 | 249 (177-350) | .21 |
CK18F means and 95% CI values are in U/L. P values of paired t test are in comparison with baseline (before chemotherapy).
NA indicates not applicable; HD-autologous, high-dose melphalan and autologous SCT; HD/intermediate-allogeneic, all chemotherapy conditioning regimen followed by allogeneic SCT except 2 Gy TBI+/-Fludarabin+/-ATG; and mini-allogeneic, 2 Gy TBI+/-Fludarabin+/-ATG followed by allogeneic SCT.
Finally, CK18F did not accumulate during acute renal failure or chronic renal insufficiency as each observed in one patient (data not shown).
Comparison of CK18F levels with sCD25 and sCD95 serum levels
Sixteen patients were available for measurement of serum levels of sCD95 (representing another apoptosis-related marker) and sCD25 (representing a lymphocyte activation marker) in parallel to CK18F. Results are summarized in Table 6 and shown in detail in Figure S1. Although sCD95 levels tended to increase during active GVHD and to decrease after response to GVHD treatment, interpretation was obscured by high background values. Thus, the fold increase of GVHD versus pre-GVHD levels was low and no changes were seen in 7 of 16 patients (43%). sCD25 levels were always high during active GVHD. However, in particular in the early posttransplantation phase up to day +100, a wide range of sCD25 elevations was observed also in the absence of GVHD, resulting in only modest increases or even decreases of sCD25 levels with the onset of GVHD. Moreover, upon initiation of GVHD treatment, sCD25 levels were reduced by steroid administration even in the absence of a clinical response, contrasting the persisting/rising CK18F levels during steroid-resistant disease (Figures 1E, 3B).
CK18F, sCD25, and sCD95 kinetics during 16 GVHD episodes
| Marker . | CK18F . | sCD25 . | sCD95 . |
|---|---|---|---|
| Individual baseline (before SCT), median (range) | 176 (81-275) | 2832 (1130-10000) | 1142 (677-2666) |
| Maximum during GVHD, median (range) | 773 (427-6880) | 10386 (238-53155) | 1832 (763-6506) |
| Fold increase, estimate (95% CI) | 5.0 (3.5-6.5) | 2.7 (0.4-5.0) | 1.8 (0.5-3.0) |
| P | < .001 | .04 | < .001 |
| Marker . | CK18F . | sCD25 . | sCD95 . |
|---|---|---|---|
| Individual baseline (before SCT), median (range) | 176 (81-275) | 2832 (1130-10000) | 1142 (677-2666) |
| Maximum during GVHD, median (range) | 773 (427-6880) | 10386 (238-53155) | 1832 (763-6506) |
| Fold increase, estimate (95% CI) | 5.0 (3.5-6.5) | 2.7 (0.4-5.0) | 1.8 (0.5-3.0) |
| P | < .001 | .04 | < .001 |
CK18F medians and ranges are in U/L; sCD25 and sCD95 medians and ranges are in pg/mL. P values are in comparison to baseline (before GVHD) using a paired t test.
Discussion
Given the difficulties in assessing diagnosis, severity, and biologic activity of GVHD by clinical means only, objective parameters reflecting GVHD activity are highly desirable. Criteria for appropriate GVHD biomarkers have recently been defined, thereby stating that suitable validated markers for monitoring of (chronic) GVHD are still lacking.16 However, numerous candidate GVHD biomarkers have been studied during the past years, almost all of them belonging to the cytokine cascades driving GVHD effector cells, such as gamma-interferon and the soluble form of its receptor, sTNF-R, interleukin 6 (IL-6), IL-8, IL-18, ICAM-1, IL-1, IL-10, IL-12, IL-2, IL-4, and—investigated best—soluble interleukin-2 receptor (sIL2-R, sCD25).17,,,,,,–24 Although in particular sCD25 and IL-18 showed some association with (acute) GVHD, lack of specificity18,19,25 and poor correlation to GVHD intensity26 have precluded their routine use for GVHD monitoring either alone or in the context of multifactorial grading systems.
In contrast to this plethora of factors that all reflect the activation status of the immune system or its inflammatory activity, CK18F mirrors the pathogenetic end point of GVHD, that is, GVHD-induced apoptotic activity in critical epithelial organs (bowel and liver). Although apoptosis is clearly not GVHD-specific, it is the direct cause of GVHD-mediated end organ damage, and therefore should be a much better indicator of clinically relevant GVHD sequelae than the general immune activation status. This study demonstrates that epithelial apoptosis induced by GVHD of gut and liver can indeed be assessed by serum CK18F measurement. Without exception, CK18F levels were strongly elevated during uncontrolled clinical hepato-intestinal GVHD in comparison to baseline. CK18F values obtained after clinical response of GVHD to immunosuppressive therapy were always lower than those determined at the start of immunosuppressive treatment. On the other hand, refractory GVHD was consistently associated with persisting or increasing CK18F serum levels. The only exception was patient UPN 301 (Figure 1D), who showed a temporary decline of CK18F despite ongoing symptoms of severe hepato-intestinal GVHD. A possible explanation might be that in this case symptoms were due to irreversible end organ destruction rather than continuing GVHD activity.
CK18 is cleaved by caspases and the resulting proteolytic fragments (CK18F) are specifically recognized by the M30 mAB.5 These fragments are stable and are not further proteolytically degraded in culture supernatants and in human serum.6,–8 Although little is known about the renal or biliary clearance of CK18F, the decreases of CK18F levels in response to successful immunosuppressive therapy suggest that accumulation of these fragments requires persistent epithelial apoptosis. Furthermore, CK18F kinetics was not affected by renal failure. This suggests that CK18F serum levels reflect active and persistent apoptosis in epithelial organs expressing CK18.
Since GVHD is a clinical diagnosis relying on largely unspecific criteria, a critical problem of this kind of study in patients having undergone allo-SCT is to define “absence of GVHD” and “start of GVHD” if sequential histology results are not available. Therefore we did not consider post-SCT samples as baseline for CK18F fold-change estimates in order to avoid possible bias induced by subclinical GVHD. Instead, only preconditioning values were used. With that approach, we did not observe a single case of clinical liver or gut GVHD without CK18F increases, suggesting a high sensitivity of this assay for recognizing hepato-intestinal GVHD.
It is intriguing that CK18F often started to rise long before the indication for GVHD-specific immunosuppressive treatment was reached, in several patients even before clinical manifestations of GVHD became apparent. Although amount and frequency of available samples in this study did not allow a structured analysis, these observations suggest that CK18F monitoring might be a suitable tool for both early detection of emerging GVHD and diagnosis of subclinical GVHD. Thus, longitudinal CK18F measurement might turn out to be valuable for triggering preemptive treatment of GVHD. On the other hand, it is tempting to speculate that CK18F could also be useful for recognizing and guiding graft-versus-tumor effects after allo-SCT, since GVHD and graft-versus-leukemia activities are closely correlated in a number of transplant indications.27,–29
Taking into account its retrospective, event-driven character, this study was not designed to systematically determine the specificity of CK18F measurement for diagnosis of GVHD. Although CK18F serum levels correlate with GVHD-induced epithelial apoptosis, apoptosis is not GVHD-specific and may also be observed in viral hepatitis and infectious gastroenteritis.30 Critically ill patients have also been reported to have somewhat increased levels of CK18F.31 Furthermore, severe pseudomembranous colitis coincided with high CK18F levels in our hands. However, extended investigation of patients in different clinical situations, including those who have undergone autologous SCT, shows that CK18F seems to be very helpful to distinguish active GVHD from clinically relevant GVHD-unrelated conditions with similar symptoms, such as mucositis and VOD during the early posttransplantation phase, and noncomplicated infectious enteritis during longer follow-up.
Moreover, epithelial damage due to infectious agents may trigger intestinal GVHD. Therefore it might be justified to hypothesize that after allogeneic SCT the level of epithelial apoptosis (independent of its cause) rather than the actual diagnostic proof of GVHD should guide immunosuppressive treatment of enteritis.
Given the lack of CK18 expression in skin, the increases in CK18F levels observed during skin GVHD cannot be attributed to epidermal apoptosis. We rather hypothesize that coinciding apoptosis of glandular epithelial cells (eg, GVHD of salivary glands) and/or subclinical involvement of parenchymatous organs might have caused the slightly elevated CK18F levels in isolated skin and oropharyngeal GVHD. Therefore, for monitoring of skin GVHD, other markers of apoptosis such as cytokeratin 15–related peptides could be more appropriate.32
In a limited number of patients, we compared CK18F to 2 parameters previously examined as candidate markers for GVHD: sCD95 as another apoptosis-related biomarker,33,34 and sCD25. In comparison to CK18F, sCD95 appeared to be of only limited diagnostic value due to high background levels with consecutive sensitivity restrictions, whereas sCD25 lacked GVHD specificity particularly during the early posttransplantation period. Moreover, sCD25 does not appear to be suitable for monitoring of treatment response, as this marker seems to be correlated to steroid administration rather than to GVHD activity.
Taken together, CK18F monitoring provides a promising tool for sensitive assessment of GVHD-associated apoptotic activity in both intestinal and hepatic GVHD. Although apoptosis is not GVHD-specific, CK18F may be useful to distinguish active GVHD from GVHD-unrelated conditions with similar symptoms commonly observed after transplantation, and to monitor response of GVHD to immunosuppressive treatment. Prospective studies are warranted to prove its suitability for diagnosis, grading, prognosis, and treatment guidance of this major complication of SCT.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (LU830/1-3), and the Tumorzentrum Heidelberg-Mannheim.
We thank Dr Frank Neumann (BIOAXXESS Technology Development, Malvern, United Kingdom) for constructive discussions.
Authorship
Contribution: T.L. designed and performed research, analyzed data, and wrote the paper; M.C. performed research and analyzed the data; A.B. analyzed the data; M.R., M.H., U.S., and U.H. performed research; M.G. designed research; A.D.H. wrote the paper; and P.D. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Luft, University of Heidelberg, Department of Medicine V, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail:thomas_luft@med.uni-heidelberg.de.