Increased expression of CD30 is associated with a variety of hematologic malignancies, including Hodgkin disease (HD) and anaplastic large cell lymphoma (ALCL). The anti-CD30 monoclonal antibody SGN-30 induces direct antitumor activity by promoting growth arrest and DNA fragmentation of CD30+ tumor cells. In this study, we investigated the contributions of Fc-mediated effector cell functions to SGN-30 activity. We determined that antibody-dependent cellular phagocytosis, mediated by macrophages, to contribute significantly to antitumor activity in vitro. To delineate the identity of the host effector cells involved in mediating antitumor activity in vivo, we studied the effects of effector cell ablation in a disseminated model of HD (L540cy). Depletion of macrophages markedly reduced efficacy of SGN-30, demonstrating that macrophages contribute significantly to SGN-30 efficacy in this model. These findings may have implications for patient stratification or combination treatment strategies in clinical trials conducted with SGN-30 in HD and ALCL.
Introduction
Increased expression of CD30 was observed on Reed-Sternberg cells in Hodgkin disease (HD),1 on neoplastic cells in ALCL, and on a subset of non-Hodgkin lymphomas (NHLs).2 Expression of CD30 on normal cells is restricted to activated T and B cells, because CD30 is not expressed by resting lymphocytes. Such highly restricted expression of CD30 to activated lymphocytes and neoplastic tumor cells led to the investiga-tion of this cell surface antigen as a target for immunotherapy in hematologic malignancies.3 Previous reports demonstrated that SGN-30, a therapeutic antibody binding to CD30, elicits direct effects on tumor cell intrinsic signaling by promoting growth arrest and DNA fragmentation of CD30+ tumors, including HD and anaplastic large cell lymphoma (ALCL), ultimately resulting in cell death.4,5 However, it remained unknown whether SGN-30 engages Fc-mediated effector cell activities. In this report, we demonstrate that macrophages contribute significantly to the anti-tumor activity of SGN-30 in vitro by inducing cellular cytotoxicity via ADCP. In vivo, depletion of macrophages reduced survival of tumor bearing mice treated with SGN-30. In contrast, ablation of natural killer (NK) cells did not significantly affect efficacy in this model. Combined, these data suggest that the interaction between SGN-30 and host macrophages contributes significantly to the mechanisms by which SGN-30 interferes with tumor growth. The newly defined role of macrophages in mediating antitumor activity of SGN-30 identified here may be relevant for the clinical activity of SGN-30, which is currently being developed for HD and ALCL.
Materials and methods
Cells and reagents
CD30+ HD lines: L540cy was provided by Dr Phil Thorpe (University of Texas, Southwestern Medical School, Dallas, TX), and HDLM-2 was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). The CD30+ lymphoblast B-cell line WIL2-S and CD30− acute promyelocytic leukemia line HL60 were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in RPMI [Roswell Park Memorial Institute] 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum. For in vivo depletion studies, rabbit anti-asialo-GM-1 antibody was obtained from Wako Pure Chemical Industries (Richmond, VA), and rat anti-mouse-Gr-1 antibody was obtained from BD Biosciences (San Jose, CA). Liposome-encapsulated clodronate (CEL) was prepared as described previously.6 Clodronate was a gift of Roche Diagnostics (Mannheim, Germany). SGN-30 was engineered at Seattle Genetics, Inc (Seattle, WA)4 and does not cross-react with rodent CD30.
Antibody-dependent cellular phagocytosis assay
This assay was performed as described previously.7 In brief, once CD30+ target cells were labeled, they were precoated with SGN-30 and then incubated with monocyte-derived macrophages. The monocytes represent the adherent cell components of peripheral blood mononuclear cells (PBMCs) cultured for 10 to 15 days in X-VIVO 15 medium (Lonza BioScience, Walkersville, MD) containing 500 U/mL recombinant human granulocyte macrophage–colony-stimulating factor (PeproTech, Rocky Hill, NJ). Monocytes harvested from these cultures were CD3/CD19-negative and expressed CD14, CD11b, CD16, CD32, and CD64 as determined by flow cytometry or by fluorescence microscopy (Zeiss Microscope, Thornwood, NY). The purity of the macrophage preparations was routinely more than 95% (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Xenograft models
C.B-17 SCID mice were injected with 5 × 106 L540cy cells intravenously on day 0. Mice were monitored at least twice weekly and were terminated when exhibiting signs of disease as described previously.4 Tumor-bearing mice were depleted of effector cells using specific antibody or CEL as described previously.6,–8 Natural killer cells were depleted by intraperitoneally injection of anti-asialo-GM 1 (1.25 mg/kg). Mice were given a total of 3 doses once every 5 days, beginning on the day of tumor cell implantation. Macrophages were depleted by intraperitoneally injection of CEL (100 μL/10 g) on the day of tumor injection and every 3 days thereafter for a total of 5 doses. Cell depletion was confirmed by flow cytometric analysis of splenocytes, lymph nodes, and blood (data not shown). Statistical analysis was conducted using the log-rank test provided in the Prism software (version 4.01; GraphPad Software, San Diego, CA). All animal experiments were conducted under Seattle Genetics' Institutional Animal Care and Use Committee (IACUC) guidelines and approval.
Slides were viewed with a Leitz Orthoplan Research Microscope (Leica Microsystems, Wetzlar, Germany) using a PL FLUOTAR oil-immersion lens at 100×/1.32 NA and Slowfade antifade reagent (Invitrogen, Carlsbad, CA). Images were acquired with a Nikon Coolpix 990 digital camera (Nikon, Tokyo, Japan) and processed using Adobe Photoshop Elements 2.0 (Adobe Systems, San Jose, CA).
Results and discussion
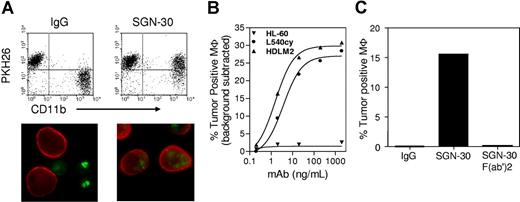
In this report, we investigated the ability of SGN-30 to engage Fc-mediated effector cell functions in vitro and in vivo. When tested in an antibody-dependent cellular phagocytosis (ADCP) assay, as described previously,7 SGN-30 induced ADCP against a panel of cell lines including L540cy, HDLM-2, and WIL2-S (Figure 1A,B). As expected, ADCP activity was not observed in the presence of an F(ab′)2 fragment of SGN-30 lacking the Fc domain or when a CD30-negative tumor cell line (HL-60) was tested. These findings demonstrate that antitumor activity of SGN-35 was antigen-specific, and that Fc-Fc receptor interactions were necessary for phagocytic uptake of antibody labeled target cells (Figure 1C). The degree of ADCP induced by SGN-30 was comparable with the levels obtained for similar human IgG1 subtype antibodies, targeting cell surface antigens expressed on hematologic tumor cells, including an anti-CD70 antibody.7 SGN-30 was further tested for its ability to induce cell lysis of CD30+ tumor cells via complement fixation, a process termed complement-dependent cytotoxicity (CDC)7 and/or via antibody-dependent cellular cytotoxicity (ADCC), mediated by NK cells. For the determination of ADCC, an assay described previously7 using PBMCs as a source for NK cells was used. In neither assay, we were able to detect significant levels of CDC or ADCC by SGN-30 when tested on a large variety of CD30+ cell lines (data not shown). These findings support the notion that therapeutic antibodies can vary in their potency to engage effector cells, depending on their IgG isotypes and other, less well-characterized characteristics, including antigen binding.
SGN-30 mediates ADCP activity in vitro. (A) Representative flow cytometry analysis and fluorescence microscopy of SGN-30-mediated phagocytosis. For flow cytometry, L540cy, HDLM-2, Wil-2S, and HL-60 target cells were labeled with PKH26 lipophilic dye for tracking purposes, and treated with SGN-30 or non-binding control IgG and mixed with monocyte-derived macrophages (MΦ). MΦ were stained with PE-conjugated anti-CD11b. Cells present in the upper right quadrant (PKH26+CD11b+) are MΦ that internalized tumor cells. For microscopy, tumor cells were labeled with PKH67 (green), and the macrophages were detected with Alexa Fluor 568-conjugated antibody specific for CD11b (red). (B) Tumor targets were treated with various concentrations of SGN-30 before incubation with MΦ. The levels of MΦ that engulfed tumor cells were determined by flow cytometry (C). WIL2-S cells were incubated with SGN-30, SGN-30 F(ab′)2, or nonbinding control IgG before incubation with MΦ and analyzed by flow cytometry.
SGN-30 mediates ADCP activity in vitro. (A) Representative flow cytometry analysis and fluorescence microscopy of SGN-30-mediated phagocytosis. For flow cytometry, L540cy, HDLM-2, Wil-2S, and HL-60 target cells were labeled with PKH26 lipophilic dye for tracking purposes, and treated with SGN-30 or non-binding control IgG and mixed with monocyte-derived macrophages (MΦ). MΦ were stained with PE-conjugated anti-CD11b. Cells present in the upper right quadrant (PKH26+CD11b+) are MΦ that internalized tumor cells. For microscopy, tumor cells were labeled with PKH67 (green), and the macrophages were detected with Alexa Fluor 568-conjugated antibody specific for CD11b (red). (B) Tumor targets were treated with various concentrations of SGN-30 before incubation with MΦ. The levels of MΦ that engulfed tumor cells were determined by flow cytometry (C). WIL2-S cells were incubated with SGN-30, SGN-30 F(ab′)2, or nonbinding control IgG before incubation with MΦ and analyzed by flow cytometry.
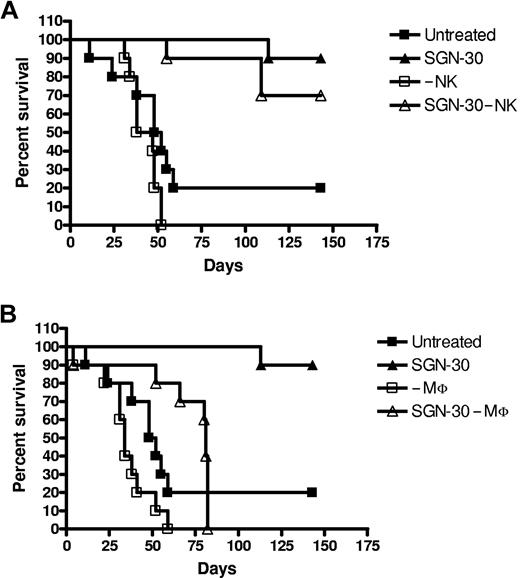
Having demonstrated that SGN-30 induces tumor cell killing via ADCP in vitro, we sought to define the nature of the FcγR-expressing cells that are responsible for SGN-30 targeted cell clearance in vivo. For this purpose, control or SGN-30 treated mice were depleted of NK cells or macrophages as described in “Xenograft models.” As shown in Figure 2, depletion of either NK cells or macrophages in control mice did not significantly affect survival of mice implanted with L540cy tumors (Figure 2A,B). In SGN-30–treated mice, macrophage depletion reduced survival of mice significantly, resulting in a median survival of 81 days compared with a 90% survival at 143 days in the control, non–macrophage-depleted group (Figure 2B). However, despite the profound level of macrophage depletion in most organs achieved in our experiments (data not shown), SGN-30–treated mice survived significantly longer compared with control, untreated mice. These observations are consistent with our previous observations, suggesting that SGN-30 can induce direct tumor cell killing in the absence of effector cells via direct effects on tumor cell signaling, resulting in apoptosis.4
Macrophages are involved in antitumor activity of SGN-30 in vivo. L540cy-bearing mice were depleted of NK cells (A) and macrophages (MΦ) (B) as described in “Xenograft models.” Mice were either left untreated (control) or treated with 10 mg/kg SGN-30 on day 1 after L540cy challenge (n = 10 per group). (A) Untreated vs. SGN-30 (P = .007), –NK vs SGN-30 –NK (P < .001), SGN-30 vs SGN-30 –NK (P = .245). (B) Untreated vs SGN-30 (P = .007), –MΦ vs SGN-30 –MΦ (P = .002), SGN-30 vs SGN-30 –MΦ (P < .001).
Macrophages are involved in antitumor activity of SGN-30 in vivo. L540cy-bearing mice were depleted of NK cells (A) and macrophages (MΦ) (B) as described in “Xenograft models.” Mice were either left untreated (control) or treated with 10 mg/kg SGN-30 on day 1 after L540cy challenge (n = 10 per group). (A) Untreated vs. SGN-30 (P = .007), –NK vs SGN-30 –NK (P < .001), SGN-30 vs SGN-30 –NK (P = .245). (B) Untreated vs SGN-30 (P = .007), –MΦ vs SGN-30 –MΦ (P = .002), SGN-30 vs SGN-30 –MΦ (P < .001).
As demonstrated here, Fc-dependent effector-cell functions contribute significantly to the antitumor activity of SGN-30 when tested in a preclinical model of HD. Additional evidence for ADCP contributing to the effects of some therapeutic monoclonal antibodies targeting cell surface markers on hematopoietic cells was provided by other reports.9,10 Even though the role of macrophages in tumor progression is controversial,11 it is tempting to speculate that therapeutic strategies aimed at enhancing host immune cell functions, in particular myeloid cell lineages, may improve anti-tumor activity.
Response to SGN-30 treatment in patients may be less affected by the Fcγ polymorphism if efficacy is predominantly mediated by cells of macrophages/monocyte origin. In contrast, the naturally occurring sequence polymorphisms in the Fcγ receptor IIIa (FcγRIIIA;158V/F) is shown to correlate with therapeutic efficacy of rituximab in clinical trials in NHL,12,13 supposedly by affecting ADCC activity mediated by NK cells.14,–16 It is noteworthy that the efficacy of the same therapeutic antibody may not correlate with FcyRIIIA polymorphism in CLL trials, indicating that a different mechanism of action may be operative in different indications.17,18 Macrophages express all 3 subtypes (FcγRIIIa/CD16, FcγRIIa/CD32 and FcγRI/CD64) and FcγRI is involved in ADCP activity, whereas NK cells, which are predominantly involved in ADCC activities, express exclusively FcγRIIIa.14,–16 For FcγRI, no sequence polymorphisms were reported so far. Based on these observations, it is plausible that ADCP may not be affected by the sequence polymorphisms within the FcγRIIIa alleles. These findings may have implications for patient stratification or combination treatment strategies in clinical trials conducted with SGN-30 in HD and ALCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.O. designed and conducted research, analyzed data, and wrote the manuscript. K.A.G. and I.S. conducted research. N.v.R. contributed vital reagents. I.S.G. and C.-L.L. contributed to the design of research. H. G. designed research and wrote the manuscript.
Conflict-of-interest disclosure: E.O., I.S., K.A.G., I.S.G., C.-L.L., and H.G. are full-time employees of Seattle Genetics.
Correspondence: Hans-Peter Gerber, Seattle Genetics, 21823 30th Drive SE, Bothell, WA 98021; e-mail:hgerber@seagen.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal