Heparin-induced thrombocytopenia (HIT) is an antibody-mediated disorder that occurs with variable frequency in patients exposed to heparin. HIT antibodies preferentially recognize large macromolecular complexes formed between PF4 and heparin over a narrow range of molar ratios, but the biophysical properties of complexes that initiate antibody production are unknown. To identify structural determinants underlying PF4/heparin immunogenicity, we characterized the in vitro interactions of murine PF4 (mPF4) and heparin with respect to light absorption, size, and surface charge (zeta potential). We show that PF4/heparin macromolecular assembly occurs through colloidal interactions, wherein heparin facilitates the growth of complexes through charge neutralization. The size of PF4/heparin macromolecules is governed by the molar ratios of the reactants. Maximal complex size occurs at molar ratios of PF4/heparin at which surface charge is neutral. When mice are immunized with complexes that differ in size and/or zeta potential, antibody formation varies inversely with heparin concentration and is most robust in animals immunized with complexes displaying a net positive zeta-potential. These studies suggest that the clinical heterogeneity in the HIT immune response may be due in part to requirements for specific biophysical parameters of the PF4/heparin complexes that occur in settings of intense platelet activation and PF4 release.
Introduction
Heparin-induced thrombocytopenia (HIT) is a drug-dependent immune disorder caused by antibodies to complexes between platelet factor 4 (PF4) and heparin. PF4/heparin antibodies are detected in virtually all patients with HIT, but can also occur in the absence of disease in patients treated with unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or the synthetic pentasaccharide, fondaparinux, and other heparin-like molecules.1,–3
The prevalence of PF4/heparin antibodies varies widely among patient populations and appears to be significantly influenced by the clinical context of drug exposure and heparin formulation. Antibodies are detected most commonly (27%-61%) in patients undergoing cardiopulmonary bypass (CPB) surgery,4,,,,–9 but occur far less frequently in uremic, obstetric, and pediatric patients even after prolonged heparin exposure.10,–12 General medical and surgical patients are reported to have intermediate rates of seroconversion, ranging from 8% to 21%.1
Why only a subset of patients with anti-PF4/heparin antibodies develops HIT is not known. One likely determinant is antigen concentration. Patients undergoing CPB have high circulating levels of both PF4 (560-750 ng/mL13,14 ) and heparin (3-4 Units/mL15 ). Another determinant may be the physical composition of the antigen. We recently reported that when PF4 and UFH associate over a narrow range of molar ratios approximating 1:1, they form ultralarge macromolecular complexes (ULCs, > 670 kDa).16 Assembly of macromolecular complexes is influenced profoundly by small changes in the stoichiometric ratio of PF4/heparin (PF4/heparin ratio or PHR). ULCs are more potent on a molar basis than smaller complexes in mediating the binding of HIT antibodies and causing heparin-dependent platelet activation in vitro.16 These results were recently affirmed by Greinacher et al, who concluded that HIT antibodies preferentially bound to multiple PF4 tetramers approximated by UFH, rather than solely by conformational changes within a single PF4 tetramer induced by heparin.17
Little is also known about why patients become sensitized to heparin, in particular why rates of seroconversion vary among clinical populations, and to what extent macromolecular assembly contributes to antibody formation. The epitopes recognized by antigen-presenting cells and T lymphocytes likely differ from those bound by antibody,18,19 and the properties of PF4/heparin complexes that initiate antibody formation have not been reported. Therefore, we undertook these studies to investigate the biologic determinants that contribute to heparin sensitization in vivo. We examined the influence of 3 discrete properties of PF4/heparin complexes: (1) size, (2) surface charge, and (3) stoichiometric ratios of reactants. To test these variables, we first characterized the interactions of murine PF4 (mPF4) and heparin in vitro, and then, using a previously described murine immunization protocol,20 we determined the relative importance of these parameters in the generation of anti-mPF4/heparin antibodies in vivo. Our studies indicate that PF4-heparin interactions display colloidal properties, wherein heparin serves as a “flocculant,” or a negatively charged polymeric compound,21 to facilitate macromolecular growth and assembly by neutralizing the surface charge of PF4. In addition, our studies indicate that sensitization in vivo is more dependent on the net surface charge on the PF4/heparin complexes than on particle size or distinctive stoichiometric ratios of reactants, suggesting that antigen-presentation and/or T-cell recognition may be influenced by charge interactions.
Materials and methods
Murine PF4 (mPF4) expression and mPF4/heparin ELISA
mPF4 was expressed in a prokaryotic expression system and isolated as previously described.22 Isolated mPF4 migrated as a single band at Mw 7800 Da and was recognized by a polyclonal antihuman PF4 (hPF4) antibody developed in our laboratory (data not shown). Antibodies to mPF4/heparin complexes were measured by enzyme-linked immunosorbent assay (ELISA) using a 96-well microtiter plate (Nunc Maxisorp; Nalge Nunc International, Rochester, NY) coated overnight with 50 μL mPF4 (10 μg/mL) and heparin (0.4 U/mL), followed by blocking unreactive sites with 10% fetal calf serum (FCS) and phosphate-buffered saline (PBS; Invitrogen, Grand Island, NY). The wells were then incubated for 1 hour at room temperature (RT) with murine plasma and then with horseradish peroxidase–labeled goat anti–mouse IgG (1:1000 dilution; Sigma, St Louis, MO). TMB peroxidase substrate (KPL, Gaithersburg, MD) was added, color development was measured at 450 nm using Spectramax 384 PLUS (Molecular Devices, Sunnyvale, CA), and the results were analyzed using SoftMax PRO software (Molecular Devices).
PF4/heparin solutions
Immunizations and in vitro assays were performed using solutions of mPF4 and/or UFH (Heplock; Elkins-Sinn, Cherry Hill, NJ) prepared at various concentrations in Hanks balanced salt solution (HBSS; Invitrogen). To calculate stoichiometry, the mean Mw of the PF4 tetramer was estimated to be 31.2 kDa based on its electrophoretic mobility. Because UFH is biologically heterogeneous,23 with molecular weight of heparin species ranging from 3000 to 30 000, we used previously published estimates of a mean Mw of UFH (15 00023 ) for PHR calculations. We estimated the specific activity of UFH at 140 U/mg and mean Mw was estimated to be 15 000 Daltons.16,23 As only UFH was used in the studies that follow, the term heparin will be used interchangeably with UFH.
Physical characterization of mPF4/heparin complexes
Light absorbance of PF4/heparin solutions was measured in a Molecular Devices Spectrophotometer. Wavelengths from 280 to 750 nm were recorded at increments of 25 nm using Spectramax 384 PLUS. As maximum absorbance occurred at 280 nm, absorbance data are shown only for this wavelength.
Visualization of PF4/heparin particles in solution was performed using an oblique illumination system (CytoViva, Auburn, AL) attached to a bright-field microscope (Olympus America, Melville, NY). The CytoViva provides collimated light at an oblique angle to view particles in suspension. This optical system interfaces with a light microscope to provide high-contrast images and real-time imaging of particles without fixation. Detection limits for particle size are up to 100 nm.
Photon correlation spectroscopy (PCS) and zeta potential
PCS was used to determine the size of particles in solution in the submicron range (> 3 nm).24 PCS measurements were performed with a Zetasizer Nano ZS (Malvern, Worcestshire, United Kingdom) with a fixed 173° scattering angle and external fiber angle, and a 633-nm helium-neon laser. Data were analyzed using the associated Zetasizer software (Dispersion Technology Software 4.2; Malvern). PF4/heparin solutions were prepared in HBSS, and PCS measurements were recorded 30 to 40 minutes after sample preparation. For time-dependent analysis of particle formation, solutions were mixed and serial measurements of particle size were determined over a 3-hour interval or until particle size stabilized.
The zeta potential of mPF4/heparin solutions containing various amounts of PF4 (0-200 μg/mL) and UFH (0-50 U/mL) was measured using the Zetasizer Nano ZS using a zeta disposable clear cuvette. The determination of the zeta potential is based on a measure of the electrophoretic mobility of particles under an applied electric field. Because of the sensitivity of electropotential measurements to electrolytes in HBSS, mPF4/heparin solutions were prepared in deionized water.
Murine immunization model
Immunizations were performed using a previously described model20 with the following modifications. Wild-type C57BL/6 mice (females, aged 10-12 weeks; designated B6) from Charles River Laboratory (Wilmington, MA) were used because they show a more robust antibody response that occurs within 8 to 15 days of immunization compared with 30 to 45 days in BALB/c mice (S.S., G.M.A., unpublished data, 2007). The volume of injected antigen was increased from 50 to 100 μL to optimize delivery. Mice were injected daily for 5 days with sterile antigen in HBSS in a final volume of 100 μL. Where indicated, solutions containing either mPF4 and/or UFH were injected into the retro-orbital complex. Blood samples for ELISA were collected in anesthetized mice from the retro-orbital blood plexus in 3.2% sodium citrate at baseline and at weekly intervals for 4 weeks after the start of immunizations. All studies were performed with the approval of the Institutional Animal Care and Use Committee at Duke University.
Statistical analysis
Antibody responses among animals were compared using the Student t test for comparisons of 2 groups or by a one-way ANOVA analysis for more than 2 groups of animals. ELISA reactivity to various antigens was expressed as mean plus or minus 1 SEM and analyzed for significance using the Student t test. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). Differences were considered significant at a P value less than .05.
Results
Light absorption profiles of PF4/heparin complexes
We have previously shown that the formation of complexes between hPF4 and heparin in solution depends on the stoichiometry of the reactants. When present at approximately equimolar ratios, hPF4 and heparin form ULCs that can be isolated by size separation chromatography.16 ULCs are readily visualized by transmission electron microscopy and measure 7.5 × 13.5 nm on average.16
To determine whether such macromolecular complexes are also formed between mPF4 and heparin, and to determine whether ULC formation could be tracked through changes in light absorption, we performed spectrophotometric assays of mPF4/heparin complex formation. To do so, we mixed various concentrations of mPF4 (10-200 μg/mL) with increasing concentrations of UFH (0-50 U/mL) in HBSS and measured light absorption (A280 nm). mPF4 in solution causes minimal light absorption (Figure 1), consistent with its expected low extinction coefficient due to its low content of phenylalanine and tyrosine residues.25 Similarly, UFH alone did not alter light absorbance (data not shown). When increasing concentrations of UFH were added to a fixed concentration of mPF4 (Figure 1), we noted significant changes in light transmission under conditions that we have previously shown elicit formation of ULCs between hPF4 and heparin.16 The change in light absorption followed an asymmetric, bell-shaped curve that was evident at mPF4 concentrations as low as 10 μg/mL and at all other concentrations studied. At any given concentration of mPF4, the peak light absorption occurred over a narrow range of UFH concentrations (Table 1), similar to those at which ULCs form. Maximal turbidity was seen at or near equimolar ratios of mPF4 to heparin (PHRs from 2.6:1 to 1:2), supporting the notion that light scattering reflects the formation of macromolecular complexes. The magnitude of the change in light transmission was also affected by the concentration of mPF4, consistent with previous observations,26 with turbidity increasing linearly at increasing PF4 concentrations (R2 = 0.98) (Figure 1 inset). Of note, solutions containing hPF4 (10-100 μg/mL) and UFH (0-10 U/mL) displayed similar stoichiometric-dependent changes in light transmission, with peak light absorbance occurring at equivalent molar ratios ranging approximately from 2:1 (10 μg/mL) to 3:1 (50 μg/mL; data not shown).
PF4 and heparin form visible aggregates in a heparin-dependent manner. Solutions containing mPF4 (10-200 μg/mL) were incubated with increasing concentrations of UFH (0-50 U/mL, shown on a log x-axis scale). Optical absorbance was measured at 280nm (y-axis). Encircled UFH concentrations (0.5, 5, and 50 U/mL) and mPF4 at a concentration 200 μg/mL were chosen for further study in subsequent experiments. The inset shows changes in absorbance as a function of PF4 concentration.
PF4 and heparin form visible aggregates in a heparin-dependent manner. Solutions containing mPF4 (10-200 μg/mL) were incubated with increasing concentrations of UFH (0-50 U/mL, shown on a log x-axis scale). Optical absorbance was measured at 280nm (y-axis). Encircled UFH concentrations (0.5, 5, and 50 U/mL) and mPF4 at a concentration 200 μg/mL were chosen for further study in subsequent experiments. The inset shows changes in absorbance as a function of PF4 concentration.
Values of PF4, UFH and PHRs associated with peak light absorbance (A280nm)
| PF4 conc, μg/mL . | UFH conc, U/mL . | Peak A280nm . | PHR . |
|---|---|---|---|
| 200 | 5-20 | 1.38-1.43 | 2.6:1 to 1:2 |
| 100 | 5 | 0.881 | 1.3:1 |
| 50 | 2 | 0.411 | 1.6:1 |
| 25 | 1 | 0.271 | 1.6:1 |
| 10 | 0.25 | 0.068 | 2.6:1 |
| PF4 conc, μg/mL . | UFH conc, U/mL . | Peak A280nm . | PHR . |
|---|---|---|---|
| 200 | 5-20 | 1.38-1.43 | 2.6:1 to 1:2 |
| 100 | 5 | 0.881 | 1.3:1 |
| 50 | 2 | 0.411 | 1.6:1 |
| 25 | 1 | 0.271 | 1.6:1 |
| 10 | 0.25 | 0.068 | 2.6:1 |
Peak light absorption (A280nm) at each dose of PF4 from Figure 1 with calculated PF4-heparin ratios (PHRs) for corresponding PF4 and heparin concentration is shown.
Effect of heparin on PF4/heparin particle size
The findings shown in Figure 1 and Table 1 suggest that heparin-dependent changes in the light transmission of PF4 solutions were likely due to formation of macromolecular complexes between PF4 and heparin. To confirm that peak turbidity was indeed due to the formation of PF4/heparin complexes and to determine the structural basis for changes in the “bell-shaped” curve seen at higher concentrations of heparin, we measured the size of the macromolecular complexes formed at distinct ratios of reactants identified by their positions on the absorbance curve at 280 nm. To do so, we used PCS, also referred to as dynamic light scattering, as a method for sizing submicron particles in solution.
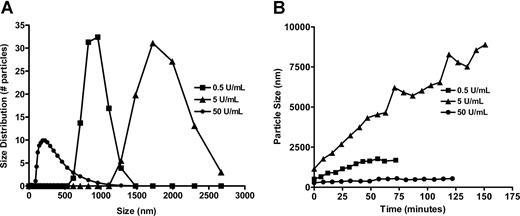
PCS is based on theoretic principles governing particle behavior in solution (Brownian motion), in which motion of a particle in solution is inversely related to its size. To perform PCS, particles are illuminated with a laser beam and particle motion is detected and analyzed as time-dependent fluctuations in light intensity. We quantified changes in particle size using a fixed concentration of mPF4 (200 μg/mL) and tested 3 concentrations of heparin (0.5, 5, and 50 U/mL) equivalent to PHRs of 26:1, 2.6:1, and 1:5, respectively (Figure 1 circled symbols) to determine changes in particle size.
Solutions containing mPF4 alone (200 μg/mL) or heparin alone (5 U/mL) did not produce any signals, indicating the absence of particles larger than 3 nm in size, which is the limit of sensitivity of the instrument. When heparin was added to mPF4, we saw dramatic changes in particle size. The particles formed by PF4 (200 μg/mL) and UFH were extremely large (∼ 900 nm for 0.5 U/mL UFH and ∼ 1800 nm for 5 U/mL UFH; Figure 2A). At higher concentrations of heparin (50 U/mL), particle size fell (< 250 nm), indicating that changes in the turbidity were caused by alterations in the physical composition of the PF4/heparin complexes (Figure 2A). The stability of complexes formed in solution also varied according to the concentration of heparin. PF4/heparin particles formed at a PHR of 2.6:1 enlarged over time (Figure 2B), whereas PF4 solutions containing 0.5 U/mL and 50 U/mL remained stable.
Measurement of PF4/heparin particle size and particle stability over time. (A) PCS measurements were taken of solutions containing mPF4 (200 μg/mL) before and after addition of various concentrations of UFH (0.5, 5, and 50 U/mL). Particle size was largest upon addition of 5 U/mL UFH (PHR, 2.6:1) and smallest with 50 U/mL UFH (PHR, 1:5). (B) Evolution of PF4/heparin particle size over time. UFH (0.5, 5, or 50 U/mL) was added to 200 μg/mL PF4 at time 0, and particle size was measured every 8 minutes over the ensuing 2 to 3 hours until particle size stabilized. Serial measurements of particle sizes are shown.
Measurement of PF4/heparin particle size and particle stability over time. (A) PCS measurements were taken of solutions containing mPF4 (200 μg/mL) before and after addition of various concentrations of UFH (0.5, 5, and 50 U/mL). Particle size was largest upon addition of 5 U/mL UFH (PHR, 2.6:1) and smallest with 50 U/mL UFH (PHR, 1:5). (B) Evolution of PF4/heparin particle size over time. UFH (0.5, 5, or 50 U/mL) was added to 200 μg/mL PF4 at time 0, and particle size was measured every 8 minutes over the ensuing 2 to 3 hours until particle size stabilized. Serial measurements of particle sizes are shown.
The large size of the particles shown in Figure 2 (ranging from 200-2800 nm) permitted them to be visualized by light microscopy using the Cytoviva illumination system (Figure 3). The Cytoviva is an optical system that emits light at an oblique angle to illuminate particles in solution. This system provides high-contrast images and real-time imaging of particles without requiring fixation. The appearance of PF4 alone was comparable with background (Figure 3A). Addition of heparin (0.5 U/mL and 5 U/mL, Figure 3B and 3C, respectively) increased the number and size of the particles visualized in a dose-dependent manner, consistent with the data shown in Figure 2. A greater amount of heparin (50 U/mL) led to the generation of a more uniform population of smaller-sized complexes, which cause less light scattering (Figure 3D). Thus, the biophysical measurements of complex size described in Figures 2-3 are concordant with the asymmetric bell-shaped light absorbance pattern seen in Figure 1 and suggest that they result from the formation of macromolecular complexes that differ in size depending on the molar ratio of reactants.
Light microscopy of PF4/heparin particles. Panels showing solutions containing mPF4 (200 μg/mL) alone (A) or mixed with UFH at 0.5 U/mL (B), 5 U/mL (C), or 50 U/mL (D) and subjected to light microscopy using highly collimated oblique illumination. Microscopic images were obtained using a 10 × magnification with an Olympus microscope (model no. BX41; Olympus America). Images were captured using a Microfibre Optronics camera (Goleta, CA) and analyzed with Picture frame Optronics software.
Light microscopy of PF4/heparin particles. Panels showing solutions containing mPF4 (200 μg/mL) alone (A) or mixed with UFH at 0.5 U/mL (B), 5 U/mL (C), or 50 U/mL (D) and subjected to light microscopy using highly collimated oblique illumination. Microscopic images were obtained using a 10 × magnification with an Olympus microscope (model no. BX41; Olympus America). Images were captured using a Microfibre Optronics camera (Goleta, CA) and analyzed with Picture frame Optronics software.
Heparin-dependent effects on the zeta potential of PF4/heparin complexes
The data shown in Figures 1,Figure 2-3 demonstrate that PF4 and heparin interactions in solution displayed some essential properties of colloidal particles, namely the ability to scatter light (Tyndall effect) and formation of particles of various sizes and “stability” due to changes in the concentration of the reactants. Colloidal particles remain dispersed in solution through stabilizing forces that are mediated via steric and/or repulsive electrostatic interactions. Strongly positive or negative electrostatic forces between particles allow particles to remain stably dispersed in solution, whereas particles manifesting a reduced surface charge, or “neutral” electrostatic potential, show a tendency to aggregate, forming “flocs.”
In colloidal science, charged polymers are often used as “flocculants” to promote electrostatic interactions between particles suspended in solution. To examine the role of heparin in facilitating formation of PF4/heparin complexes, we determined the contribution of surface charge or zeta potential to PF4/heparin macromolecular assembly. The overall surface charge of a particle suspended in a given solution yields a differential accumulation of ions near the surface of the particle. This accumulation of ions gives rise to a potential at the particle surface, which can be approximated by measuring the potential at the particle-liquid interface, or the zeta potential. The zeta potential can be calculated from the particle's electrophoretic mobility in solution. The magnitude of a particle's zeta potential (either positive or negative) is indicative of the repulsive forces arising from electrostatic repulsion between particles and provides a measure of potential energy barrier that may impede particle aggregation. The greater the electrostatic repulsive forces (strongly positive or negative zeta potential), the greater the stability of a particle suspension. On the other hand, reduced or neutral zeta potential is associated with particle instability leading to aggregation.
To assess the contribution of surface charge to PF4/heparin complex formation, we measured the zeta potential of PF4/heparin complexes by performing experiments analogous to the light absorption experiments shown in Figure 1. Increasing amounts of UFH (10-200 μg/mL) were added to fixed concentrations of mPF4. As shown in Figure 4, for any given concentration of mPF4, addition of heparin reduced the zeta potential of the resulting PF4/heparin solution. For each PF4 concentration curve shown in Figure 4, the concentration of UFH that yielded net neutrality (the 0-mV inflection point for each PF4 curve) occurred at approximately the same molar ratio of PF4 and heparin (PHR, ∼ 2.6:1; Table 2).
Heparin-dependent changes in the zeta potential of PF4/heparin particles. The zeta potential of particles formed by solutions of mPF4 (10–200 μg/mL, individual graphs along y-axis) and increasing concentrations of heparin (0.1-50 U/mL along x-axis; log scale).
Heparin-dependent changes in the zeta potential of PF4/heparin particles. The zeta potential of particles formed by solutions of mPF4 (10–200 μg/mL, individual graphs along y-axis) and increasing concentrations of heparin (0.1-50 U/mL along x-axis; log scale).
Values of PF4, UFH and PHRs associated with neutral zeta potential
| PF4 conc, μg/mL . | UFH conc, U/mL . | PHR . |
|---|---|---|
| 200 | 5 | 2.6:1 |
| 100 | 2.5 | 2.6:1 |
| 50 | 1.0 | 3.3:1 |
| 25 | 0.5 | 2.7:1 |
| 10 | 0.2 | 2.4:1 |
| PF4 conc, μg/mL . | UFH conc, U/mL . | PHR . |
|---|---|---|
| 200 | 5 | 2.6:1 |
| 100 | 2.5 | 2.6:1 |
| 50 | 1.0 | 3.3:1 |
| 25 | 0.5 | 2.7:1 |
| 10 | 0.2 | 2.4:1 |
Data for the inflection point, at which surface charge is neutral (0 mV) for each PF4 concentration curve shown in Figure 4. The concentrations of UFH and calculated PHRs are shown for each PF4 concentration. Zeta potential (mV) was zero in all cases.
As would be predicted of colloidal interactions, the zeta potential attained neutrality at the same PHRs (Table 2) at which particle size is maximal (Table 1, Figures 2,3C). Thus, the macromolecular assembly of mPF4/heparin complexes fits a model of colloidal interaction in which stoichiometric ratios are important for determining complex size and charge.
Biophysical determinants of PF4/heparin immunogenicity
These studies show that heparin affects the formation of PF4/heparin complexes in 2 important physical ways, by affecting particle size and surface charge. These 2 parameters correlate with each other and both are influenced by the molar ratios of reactants. These properties allow us to segregate PF4/heparin complexes into discrete populations that differ in size and charge based on the molar ratios of reactants. Specifically, when PF4 and heparin are present at or near equimolar ratios, particles are maximal in size and carry a neutral charge; however, at higher or lower PHRs, smaller particles with net positive or negative surface charges are generated, respectively.
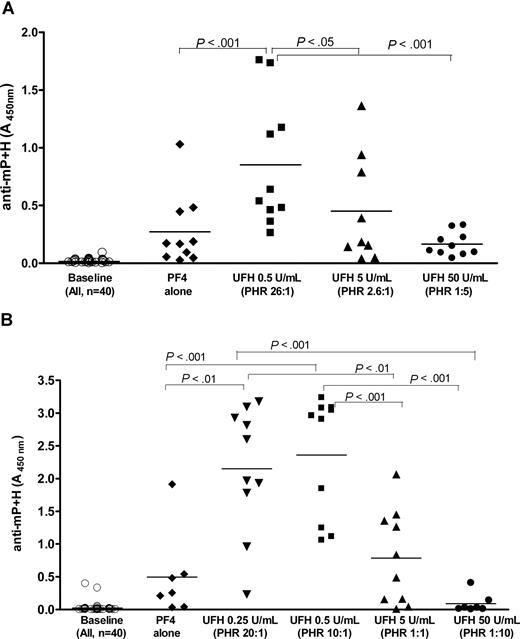
We next examined how size, charge, and stoichiometric ratios contribute to in vivo immunogenicity in a murine immunization model. For these experiments, we selected 2 concentrations of PF4 (200 and 100 μg/mL, Figure 5A,B, respectively). The choice of concentrations was based on prior studies (Figure 1) showing visible particle formation at these concentrations and because of an anticipated 10-fold dilutional effect of injecting material (100 μL) into the approximately 1- to 1.5-mL circulating volume of the mouse. mPF4 was mixed with various concentrations of UFH to yield complexes that correlated with high, low, or near equimolar PHRs. Blood was drawn at baseline and on day 8 (D8) from the start of immunization to measure antibodies to mPF4/heparin (anti-mP + H).
Effect of heparin on the development of anti-mPF4/heparin antibodies in vivo. BL6 mice were injected with mPF4 and UFH at various concentrations and antibodies to mPF4/heparin (mP + H) were assayed at baseline or D8 from the start of immunizations. (A) Cohorts (n = 10/cohort) were injected with mPF4 (200 μg/mL) alone or in combination with heparin at various PHRs (PHRs from left to right after PF4 alone, 26:1, 2.6:1, and 1:5). (B) Cohorts (n = 7/cohort for PF4 alone, and UFH 50 U/mL; n = 10/cohort for other conditions) were injected with mPF4 (100 μg/mL) alone or in combination with heparin at various PHRs (PHRs from left to right after PF4 alone, 20:1, 10:1, 1:1, and 1:5). Significant differences between cohorts are indicated at the top of the figures.
Effect of heparin on the development of anti-mPF4/heparin antibodies in vivo. BL6 mice were injected with mPF4 and UFH at various concentrations and antibodies to mPF4/heparin (mP + H) were assayed at baseline or D8 from the start of immunizations. (A) Cohorts (n = 10/cohort) were injected with mPF4 (200 μg/mL) alone or in combination with heparin at various PHRs (PHRs from left to right after PF4 alone, 26:1, 2.6:1, and 1:5). (B) Cohorts (n = 7/cohort for PF4 alone, and UFH 50 U/mL; n = 10/cohort for other conditions) were injected with mPF4 (100 μg/mL) alone or in combination with heparin at various PHRs (PHRs from left to right after PF4 alone, 20:1, 10:1, 1:1, and 1:5). Significant differences between cohorts are indicated at the top of the figures.
Antibody levels prior to injection were negligible (Figure 5A-B). Mice injected with mPF4 alone (200 or 100 μg/mL) showed a low level of seroconversion, as reflected in the mean level of anti-mPF4/heparin or anti-mP + H (A450nm, mean ± SD: PF4 200 μg/mL = 0.275 ± 0.306; PF4 100 μg/mL = 0.497 ± 0.655). Seroconversion occurred in mice injected with complexes formed over a range of PHRs from 26:1 to 1:1. Surprisingly, immunization with reactants at PHRs that yield the maximal particle size (PHR, 2.6:1 in Figure 5A or PHR, 1:1 in Figure 5B) was not the most potent. Rather, there was a strong inverse relationship between the amount of heparin injected and the intensity of the antibody response. PF4/heparin complexes formed at higher PHRs (PHR, 26:1 in Figure 5A or PHRs, 20:1 or 10:1 in Figure 5B) caused the most robust seroconversion, whereas those formed at low PHRs or high heparin concentrations (PHR, 1:5 in Figure 5A or PHR, 1:10 in Figure 5B) were the least immunogenic. In fact, the levels of antibody in these cohorts were generally lower than those in mice injected with mPF4 alone (P = ns PF4 vs UFH 50 U/mL in Figure 5A,B). When these data are reanalyzed with respect to the zeta potential of the immunizing particles, antibody responses were more robust in mice immunized with PF4/heparin complexes expressing a positive zeta potential (PHR, 26:1 in Figure 5A and PHRs, 20:1 and 10:1 in Figure 5B) and least intense when immunization was performed with complexes expressing a negative zeta potential (PHR, 1:5 in Figure 5A or PHR, 1:10 in Figure 5B).
Discussion
In this paper, we elucidate the mechanisms by which PF4/heparin macromolecules assemble in solution and show that complex growth occurs through colloidal electrostatic interactions. Peak particle size occurs at mPF4 tetramer–heparin molar ratios that approach 1:1, a point at which surface charge is minimized. Solutions in which PF4 or heparin is present in molar excess form small- to intermediate-sized particles. These biophysical properties of macromolecular complexes alter the immunogenicity of PF4/heparin complexes in vivo.
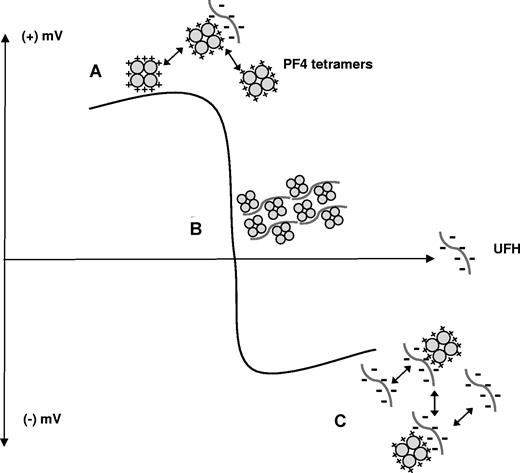
Our studies provide direct experimental support for the concept that the capacity of heparin to neutralize the cationic charge of PF4 is integrally related to macromolecular assembly. Heparin influences colloidal dynamics by decreasing the repulsive forces between PF4 molecules. At any given concentration of PF4, addition of heparin changes the turbidity of the solution, with maximal changes in light scattering occurring at or near equimolar ratios of reactants (Figure 1). Although heparin facilitates aggregate formation, the absolute size of a macromolecular complex appears to be determined primarily by PF4 concentration (Figures 1,Figure 2-3). As shown in Figures 4 and 6, these effects of heparin can be measured through changes in the zeta potential of PF4/heparin complexes. Addition of increasing amounts of heparin lowers the zeta potential of PF4/heparin particles, favoring aggregate formation until molar equivalence or neutral charge is reached. When heparin is present at molar excess, the accumulation of negative charges on the particles increases repulsive interactions favoring formation of smaller-sized complexes.
Zeta potential depiction of PF4 and heparin colloidal interactions. Y-axis represents positive or negative electrostatic potential as a function of increasing concentrations of UFH (on x-axis). (A) Positively charged PF4 molecules remain stably dispersed in the absence of heparin due to repulsive interactions. (B) With increasing amounts of heparin, PF4 charges are neutralized promoting floc or macromolecular assembly. (C) With heparin in molar excess, repulsive interactions once again predominate, leading to stable dispersion of smaller particles.
Zeta potential depiction of PF4 and heparin colloidal interactions. Y-axis represents positive or negative electrostatic potential as a function of increasing concentrations of UFH (on x-axis). (A) Positively charged PF4 molecules remain stably dispersed in the absence of heparin due to repulsive interactions. (B) With increasing amounts of heparin, PF4 charges are neutralized promoting floc or macromolecular assembly. (C) With heparin in molar excess, repulsive interactions once again predominate, leading to stable dispersion of smaller particles.
These observations on PF4/heparin zeta-potential support and extend recent findings by Greinacher et al.17 These investigators used various physical techniques, including PCS and atomic force microscopy, to show that individual PF4 molecules in solution maintained an average distance of 20 nm between molecules. This distance between tetramers was significantly reduced in the presence of low concentrations of UFH. The authors postulated that heparin facilitates complex formation primarily through charge neutralization, allowing close approximation of 2 PF4 tetramers, which increases antibody avidity and is consistent with our previous findings showing HIT antibody binding to ULCs.16
Our studies extend those of Greinacher et al,17 by showing that heparin's influence on the zeta potential of PF4/heparin complexes accounts for the characteristic bell-shaped distribution of PF4-heparin or PF4-glycosaminoglycan (GAG) biologic interactions. The asymmetric bell-shaped pattern was first described by Bock et al, who noted that changes in the stoichiometric ratios of PF4 and heparin alter the size of complexes and that addition of a molar excess of heparin generates complexes that are significantly smaller than complexes formed when PF4 was present in molar excess.26
Subsequent studies of PF4/heparin or GAG interactions not only have corroborated these in vitro findings,16,17,27 but also have demonstrated that similar bell-shaped interactions occur in vivo. In recent studies of transgenic mice underexpressing or overexpressing PF4, we have shown that biologic processes, including HIT antibody binding and platelet-mediated thrombus formation, are governed by charge-dependent interactions. In studies using mPF4 knockout mice (mPF4null), we found that addition of hPF4 to mPF4null platelets supports increased binding of HIT antibody at low but not at high concentrations of hPF4, suggesting that PF4 interacts with cell surface GAGs to form antigenic complexes on cell surfaces.28 In other studies, we noted that mice underexpressing or overexpressing PF4 have defects in platelet-initiated thrombus formation initiated by ferric-chloride arterial injury. In these underexpressing or overexpressing mice, thrombotic defects are corrected through charge neutralization with infusions of either PF4/protamine or heparin, respectively.29 In mPF4null mice, infusions of hPF4 or protamine over a narrow range of concentrations correct thrombotic defects by providing a sufficient number of positively charged PF4 molecules to overcome the negative charges found on resting platelets and endothelial cells. Mice overexpressing hPF4, on the other hand, have excessive amounts of positively charged PF4 molecules on platelets and endothelial cells that interfere similarly with cell-cell interactions that are required for thrombus formation. In these mice, thrombotic defects are overcome through infusions of low doses of heparin, but excess heparin impairs thrombus formation.29 Thus, these in vivo studies show that interactions between PF4 and GAGs, whether in vitro or in vivo, are likely governed by electrostatic interactions that follow a bell-shaped distribution.
For these reasons, we postulated that the immune response to PF4/heparin in vivo may also be influenced by charge-dependent properties of complexes. We hypothesized that the net charge of PF4/heparin particles might contribute more to the immunogenicity of PF4/heparin complexes than particle size or specific molar ratios by facilitating electrostatic interactions between antigen and antigen-presenting cells. Consistent with this concept, PF4/heparin particles exhibiting a strongly negative zeta potential (50 U/mL UFH in Figure 5A,B) were relatively weak immunogens in vivo. PF4/heparin complexes carrying a net positive charge (associated with high PHRs in Figures 4,5), however, were more immunogenic than larger-sized particles expressing neutral charges (PHRs formed at or near molar equivalence in Figures 4,5). The greater immunogenicity of PF4/heparin particles with a positive zeta potential may be mediated in part by the negative zeta potential found on resting cell surfaces, including platelets30 and perhaps, antigen-presenting cells.
Whereas we and others have shown that antibody binding or functional effects occur at PF4-heparin molar ratios of 1:1, our present studies suggest that stoichiometric ratios of 1:1 are not as critical for antibody development. It is likely that the properties of PF4/heparin complexes that initiate antigen presentation and/or T-cell responses may differ from those that mediate antibody binding or antibody-mediated thrombosis. It is also possible that injected PF4/heparin complexes are modified by electrostatic interactions with blood constituents (cells, proteins, and/or electrolytes) or are cleared rapidly from circulation due to expression of certain physical characteristics (charge or size).
Although we could not determine the composition or fate of injected complexes in vivo, several features of the mPF4/heparin immune response were distinctive enough to suggest that critical biophysical characteristics of PF4/heparin complexes were preserved sufficiently for antigen presentation in vivo. It is clear from these studies and our previous work20 that PF4/heparin complex formation is an essential requirement for robust seroconversion. Although animals injected with PF4 alone elicited antibody formation, possibly due to binding of PF4 to endothelial GAGs or trace heparin contamination during protein purification, our studies indicate that injected PF4/heparin complexes are more potent immunogens on a molar basis, either due to increased size or charge density, than complexes that form on the vascular wall. The fact that cohorts receiving the highest heparin doses formed less antibody than mice receiving PF4 alone suggests, notwithstanding the presence of PF4/heparin complexes in both settings, the accompanying negative charges on these soluble complexes has greater influence on dampening the immune response than does particle size on promoting antibody formation. Lastly, in keeping with previous murine studies,16,29 the finding of a bell-shaped distribution in the immune response, rather than an “on/off” response at which the rate of seroconversion remains fixed after a certain threshold level of heparin (or PF4) is reached, suggests that some of the biophysical attributes of PF4/heparin complexes that are being measured in vitro are indeed conserved for immune activation/antigen presentation.
Several limitations of our murine immunization model are recognized. Our immunization studies were performed in wild-type mice, which lack platelet Fc receptors.31 We have previously shown that platelet Fc receptors are essential for the pathogenesis of thrombocytopenia and thrombosis mediated by a HIT-like monoclonal antibody.28,31 However, our current studies demonstrate that the initiation phase of the HIT immune response is preserved in mice lacking platelet Fc receptors. The extent to which platelet Fc receptors augment or help to perpetuate the immune response, once it is initiated, remains to be determined. In addition, our murine studies were performed using passive administration of high doses of antigen. While our approach admittedly does not resemble human heparin sensitization, it does provide an experimental strategy for identifying antigenic features relevant for PF4/heparin antibody formation in vivo.
To the extent that our findings in mice can be generalized to human disease, they offer insights into why certain patient populations appear to be at higher risk for sensitization to PF4/heparin complexes than others. Several criteria must be fulfilled for antibody formation to be initiated. Heparin by itself elicits little or no antibody production in mice under our experimental conditions. On the other hand, elevated levels of PF4, either from endogenous overexpression or from platelet activation, appear critical for sensitization to occur. We have recently shown that approximately 8% of the human blood donor population expresses twice the mean level of platelet PF4.32 In these patients, or those who experience sustained and intense platelet activation, PF4 levels may be sufficiently elevated to bind endogenous GAGs and sensitize some individuals. To what extent these patients become immunologically “primed” to develop pathogenic PF4/GAG antibodies upon subsequent heparin exposure remains to be determined. However, in most settings, the combination of PF4 and heparin is needed and immunization is optimal when PF4 is present in molar excess. These latter conditions, typically seen in patients with underlying atherosclerotic heart disease and after CPB surgery, are the settings most often associated with a high incidence of HIT antibody formation. On the other hand, patient populations with little or no vascular disease (pediatric and obstetric patients) or impaired platelet activation (uremia) may be less likely to develop antibodies due to low levels of circulating PF4.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health HL081395 (G.M.A.); USEPA STAR award and RD83241301 (M.R.W.); HL084006 (M.P.); HL54500 (M.P.); and HL081012 (D.B.C.).
The authors thank Hannah Martin for technical assistance.
National Institutes of Health
Authorship
Contribution: G.M.A., S.S., B.E., and M.R.W. contributed to the study concept and design; S.S., B.E., and R.Q. performed the research and analyzed the data; S.S. and B.E. performed statistical analysis and drafted the paper; M.P., R.L., D.B.C., M.R.W., and G.M.A. analyzed the data and contributed to major revisions of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gowthami M. Arepally, Division of Hematology, DUMC Box 3486, Rm 301 Alex H. Sands Bldg, Research Dr, Durham, NC 27710; e-mail:arepa001@mc.duke.edu.