Abstract
Recombinant human rhApo2L/TRAIL selectively stimulates apoptosis in various cancer cells through its receptors DR4 and DR5, and is currently in clinical trials. Preclinical studies have established antitumor activity of rhApo2L/TRAIL in models of epithelial cancers; however, efficacy in non-Hodgkin lymphoma (NHL) models is not well studied. Of 7 NHL cell lines tested in vitro, rhApo2L/TRAIL stimulated apoptosis in BJAB, Ramos RA1, and DoHH-2 cells. Rituximab, a CD20 antibody used to treat certain types of NHL, augmented rhApo2L/TRAIL-induced caspase activation in Ramos RA1 and DoHH2 but not BJAB or SC-1 cells, through modulation of intrinsic rather than extrinsic apoptosis signaling. In vivo, rhApo2L/TRAIL and rituximab cooperated to attenuate or reverse growth of tumor xenografts of all 4 of these cell lines. Depletion of natural killer (NK) cells or serum complement substantially reduced combined efficacy against Ramos RA1 tumors, suggesting involvement of antibodydependent cell- and complement-mediated cytotoxicity. Both agents exhibited greater activity against disseminated than subcutaneous BJAB xenografts, and worked together to inhibit or abolish disseminated tumors and increase survival. Moreover, rhApo2L/TRAIL helped circumvent acquired rituximab resistance of a Ramos variant. These findings provide a strong rationale for clinical investigation of rhApo2L/TRAIL in combination with rituximab as a novel strategy for NHL therapy.
Introduction
Effective treatments for non-Hodgkin lymphoma (NHL) remain a serious unmet medical need: The incidence of NHL continues to rise,1 and NHL tumors can relapse in patients who initially respond to treatment. NHL represents a collection of more than a dozen different cancers of lymphocytes. The most prevalent forms of NHL are B-cell malignancies, of which follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) comprise the majority.2 Burkitt lymphoma is a less prevalent form of NHL characterized by translocation of the c-myc oncogene to the Ig heavy chain promoter/enhancer region.3 The monoclonal anti-CD20 antibody rituximab is currently used in the clinic for the treatment of both FL4-7 and DLBCL.8 Expression of the CD20 antigen is restricted to B cells; however, the biologic function of CD20 is not fully understood.9,10 Studies in mice suggest that Fc-mediated effector functions, including antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), are important for the antitumor efficacy of rituximab in vivo.11,12 A valine (V) polymorphism at amino acid 158 in Fc gamma receptor IIIa (Fc gamma RIIIa) confers higher affinity to human IgG1 than the phenylalanine (F) allele, and effector cells bearing the V allele mediate ADCC more effectively.13,14 NHL patients homozygous for the V polymorphism in Fc gamma RIIIa show a better response to rituximab, supporting the importance of ADCC for rituximab activity.15,16 Rituximab has been reported to promote apoptosis in some NHL cell lines in vitro without requirement for ADCC or CDC.17 Furthermore, there is evidence that rituximab can sensitize NHL cells to apoptosis by chemotherapeutic drugs in vitro.18,19
Apo2 ligand/tumor necrosis factor–related apoptosis-inducing ligand (Apo2L/TRAIL) stimulates apoptosis in various cancer cell types by acting through its cognate proapoptotic receptors DR4 and/or DR5 and stimulating the extrinsic pathway.20,21 Upon binding to DR4 and/or DR5 at the cell surface, Apo2L/TRAIL induces recruitment of the cytoplasmic adaptor protein FADD (Fas-associated death domain)22,23 to the intracellular portion of DR4 and DR5. FADD recruits the apical apoptosis-initiating proteases caspases-822,23 and -10,24-26 completing formation of the death-inducing signaling complex (DISC).27 DISC association leads to activation and self-processing of the apical caspases, releasing active caspase molecules into the cytoplasm, where they cleave and activate downstream effector caspases such as caspases-3, -6, and -7, which execute the apoptosis program. In “type I” cells, stimulation of the extrinsic pathway is sufficient for commitment to apoptotic death. In “type II” cells, this commitment requires further signal amplification through the “intrinsic” pathway.28,29 This latter amplification occurs through caspase-8–mediated cleavage of the Bcl-2 homology domain 3 (BH3) protein Bid.30 In turn, truncated Bid binds antiapoptotic Bcl-2 family members such as Bcl-2, Bcl-xL, Bcl-w, and A1.31,32 This frees the proapoptotic Bcl-2 family members Bax and Bak to engage the mitochondria and induce the release of mitochondrial cytochrome c and Smac/Diablo into the cytosol, where these latter factors promote caspase activation. Cytochrome c forms the “apoptosome” complex with the adaptor Apaf-1 and activates the apoptosis-initiating protease caspase-9, which then stimulates effector caspases. Smac/Diablo binds to inhibitor of apoptosis proteins (IAPs), preventing their negative-regulatory binding to caspases-9 and -3 and hence augmenting apoptosis induction.33
There is evidence from mouse models that Apo2L/TRAIL is involved in immunity against certain pathogens as well as in antitumor immune surveillance.34-36 The full-length ligand is a type 2 cell surface protein, but it also can be shed from T cells through proteolytic processing to form a soluble protein.37 We have generated an optimized, nontagged soluble recombinant human (rh) Apo2L/TRAIL based on the protein's extracellular region.38 Crystallographic and biochemical studies reveal that Apo2L/TRAIL is a homotrimeric protein containing an internal zinc molecule that is coordinated by 3 cysteines, located at amino acid position 230 of each subunit.39-41 Zinc coordination is important for the stability of rhApo2L/TRAIL as a soluble homotrimeric molecule; indeed, it is the zinc-bound form of the ligand that displays optimal selectivity for cancer cells over normal cells, including hepatocytes.42-45
Several studies in mice demonstrate that rhApo2L/TRAIL can exert apoptosis-based antitumor activity against human tumor xenografts of several epithelial malignancies including colon, non–small cell lung and pancreatic cancer, as well as in models of nonepithelial cancers such as glioblastoma and multiple myeloma.21,46,47 However, to our knowledge, there are no published reports on rhApo2L/TRAIL activity against NHL xenografts. In the present study, we examined the proapoptotic activity of rhApo2L/TRAIL against a number of NHL cell lines, as well as the ability of this ligand to cooperate with rituximab. Our results suggest that an approach that integrates the proapoptotic activity of rhApo2L/TRAIL with antibody-mediated effector functions of rituximab should be considered for investigation in NHL patients.
Materials and methods
Reagents and cell lines
The BJAB, Ramos RA1, SC-1, Raji, Daudi, and 293 cell lines were obtained from the American Type Culture Collection (Manassas, VA). The DoHH-2, OCI-Ly19, and Colo678 cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Ramos T1 was generated at Genentech (South San Francisco, CA) by inoculation of Ramos RA1 cells into CB 17 ICR severe combined immunodeficient (SCID) mice and allowed to grow to 200 mm3. The mice were treated with 500 mg rituximab (20 mg/kg) intraperitoneally 3 times per week for 3 weeks. The resistant tumors that grew out were similar in size to control-treated tumors and were re-established in tissue culture. This clone was found to be refractory to 10 mg/kg rituximab treatment as a xenograft. rhApo2L/TRAIL was generated at Genentech, and endotoxin levels were determined to be less than 0.06 EU/mg by limulus amebocyte lysate (LAL) assay (BioWhittaker, Walkersville, MD).
Generation of BJAB- and Ramos RA1–transfected cell lines
BJAB-Luc cell line.
The retroviral construct pQCXIH.Luc was generated by cloning the firefly luciferase gene into the pQCXIH retroviral vector (BD Clontech, San Diego, CA). Retroviral production and infection were modified from existing protocols.48,49 Briefly, Phoenix Ampho packaging cells (Orbigen, San Diego, CA) were transfected with pQCXIH.Luc using calcium phosphate. Viral supernatant was collected from these cultures, supplemented with 10 μg/mL polybrene, and added undiluted to BJAB cells plated into 6-well plates. These plates were then centrifuged at room temperature at 300g for 45 minutes. Cells were placed into a 32°C incubator for 11 hours to allow viral infection. This procedure was repeated every 12 hours with fresh virus for 2 additional times. Following the third infection, remaining virus was removed and replaced with fresh medium. Cells were then allowed to recover for 48 hours in a 37°C incubator prior to selection with 400 μg/mL hygromycin for 1 week. The pool of transfected cells was screened for stable luciferase expression for 30 days prior to use in subsequent experiments.
Ramos RA1-hBcl2 cell line.
The coding region of human Bcl-2 was cloned into the pMSCV retroviral vector containing a ZsGreen fluorescence protein marker (BD Clontech). Ramos RA1 cells were infected as described for BJAB-Luc cell line.
Flow cytometry
The analysis of cell surface molecules on lymphoma cell lines was determined using a phycoerythrin (PE)–conjugated mouse monoclonal antibody to human CD20 (BD Biosciences, San Diego, CA); PE-conjugated mouse monoclonal antibodies to human DR4, DR5, DcR1, and DcR2 (eBioscience, San Diego, CA); and fluorescein isothiocynate (FITC)– and PE-conjugated mouse monoclonal antibodies to CD55 and CD59, respectively (Caltag, Carlsbad, CA). The analysis of murine natural killer (NK) cell content was determined using a PE-conjugated antibody to CD49b (DX5; BD Biosciences, San Diego, CA). Active caspase-350 and Bcl-2 were detected by fixation of the cells with Cytofix/Cytoperm buffer, followed by staining with a FITC-conjugated rabbit anti–active caspase-3 or PE-conjugated monoclonal antibody to human Bcl-2 in permeabilization buffer (buffers and stains from BD Biosciences, San Diego, CA). Mitochondrial membrane polarization was detected using DiIC1(5) (Molecular Probes, Eugene, OR). Cells were analyzed on a FACSCalibur machine (BD Biosciences, San Jose, CA).
Cell viability assay
The cells (20 000 cells/well) were treated with rhApo2L/TRAIL alone, rituximab alone, or with the combination of both, and the plates were incubated for 2 days at 37°C. AlamarBlue (Trek Diagnostic Systems, Cleveland, OH), a fluorometric/colorimetric dye, was used as a growth indicator. Fluorescence was read using 96-well fluorometer with excitation at 530 nm and emission of 590 nm. The results are expressed in relative fluorescence units (RFU). For data analysis, the 4-parameter curve-fitting program Kaleidagraph (Synergy Software, Reading, PA) was used.
Immunoblots and DISC assays
Cell lysates were prepared in phosphate-buffered saline (PBS) containing 1% Triton X-100, separated on 4% to 20% Tris-Bis polyacrylamide gels, and transferred to nitrocellulose membrane. Nitrocellulose was blocked with 1% bovine serum albumin/PBS, and proteins were detected with antibodies to human Bcl-XL, Bcl-2, Bax, Bak, Puma, Bim, Bmf, Bad, phospho-Bad, X-linked inhibitor of apoptosis protein (XIAP), and actin (Cell Signaling Technology, Danvers, MA), FADD (BD Transduction Labs, San Diego, CA), or caspase-8 (Immunotech, Fullerton, CA). DISC immune precipitation was performed as previously described.22
Subcutaneous xenograft studies
The studies were conducted in compliance with National Institutes of Health Guide for the Care and Use of Laboratory Animals51 and were approved by the Institutional Animal Care and Use Committee (IACUC) at Genentech. Female CB17 ICR SCID mice (Charles River Laboratories, Hollister, CA) were maintained in accordance with the Guide for the Care and Use of Laboratory Animals.51 Ramos RA1 (5 × 106), Ramos T1 (5 × 106), BJAB (5 × 106), SC-1 (10 × 106), and DoHH-2 (20 × 106) cells were harvested, suspended in PBS, and injected subcutaneously into the right dorsal flank of 6- to 8-week-old mice. When tumors reached 100 to 200 mm3, animals were randomized into groups, and dosed intraperitoneally with vehicle (0.5 M Arg-succinate/20 mM Tris/0.02% Tween 20, pH 7.2), 60 mg/kg Apo2L/TRAIL, and/or 4 mg/kg or 10 mg/kg rituximab (as noted in figure legends). Treatment schedule for rhApo2L/TRAIL was once a day for 5 days, 2 days off, followed by once a day for 5 days. Treatment schedule for rituximab (at all dose levels) was once a week for 2 weeks. Tumor volumes were determined by measuring the length (l) and width (w) and calculating the volume using the following formula: V = lw2/2.
Studies with disseminated xenografts
Female CB17 ICR SCID mice (Charles River Laboratories) were injected intravenously with 5 × 106 BJAB-Luc cells. The mice were imaged weekly using the following procedure to monitor disease progression. Before image acquisition, the tumor-bearing mice were anesthetized using isoflurane and injected intraperitoneally with 200 mg/kg d-luciferin (Invitrogen, Carlsbad, CA). During image acquisition the mice were maintained on isoflurane by nose cone and body temperature was regulated using a warming pad. Bioluminescence images were acquired using a custom-designed cooled intensified charge-coupled device camera system. Image acquisition times varied according to signal intensity but were typically started at 6 seconds and decreased with tumor progression. Tumors were localized by overlaying a reference image of the mouse with the bioluminescence data image. Images were quantified using Image J version 1.37 (developed at the National Institutes of Health, Behesda, MD, and available at http://rsb.info.nih.gov/nih-image/) by evaluating pixel intensities in the bioluminescence data image, applying an appropriate background correction, and scaling the resulting values to account for variations in acquisition time and camera settings to give a mean intensity in relative light units (RLU) that was representative of total disease burden. This approach has been validated by pathology and magnetic resonance imaging (MRI) (data not shown) for other comparable models. Tumor burden as assessed by total luminescence was monitored weekly. At day 13, mice were randomized into 4 groups with equivalent luminescence at which time treatment began. Mice were treated as described for subcutaneous tumors. At day 26, the difference in mean RLU between groups was analyzed using a nonparametric Mann-Whitney test.
Additional methods are in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Results
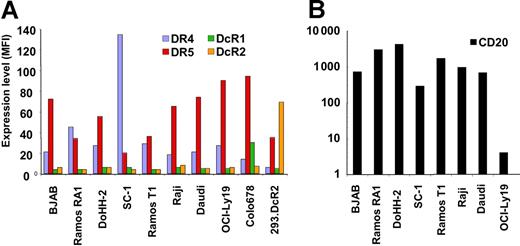
To evaluate the activity of rhApo2L/TRAIL against NHL and the ability of this ligand to cooperate with rituximab, we characterized a panel of NHL cell lines by flow cytometry for surface expression of the proapoptotic Apo2L/TRAIL receptors DR4 and DR5 and the decoy receptors DcR1 and DcR2, as well as for expression of the rituximab antigen CD20. All of the 7 cell lines examined expressed significant levels of DR4 and/or DR5 and had relatively low or undetectable levels of DcR1 and DcR2, as did the rituximab-resistant Ramos T1 variant (Figure 1A; Figure S1). Most of the cell lines, including Ramos T1, also expressed abundant CD20 levels, except OCI-Ly19 cells, which displayed very low CD20 expression (Figure 1B).
Expression of Apo2L/TRAIL receptors and CD20 on cell lines. A panel of NHL cell lines and 2 control cell lines were stained with PE-conjugated antibodies to DR4, DR5, DcR1, and DcR2 (A) and CD20 (B). Data are reported as mean fluorescence intensity (MFI). The background fluorescence for the isotype control antibody on all cell lines was an MFI less than 6.
Expression of Apo2L/TRAIL receptors and CD20 on cell lines. A panel of NHL cell lines and 2 control cell lines were stained with PE-conjugated antibodies to DR4, DR5, DcR1, and DcR2 (A) and CD20 (B). Data are reported as mean fluorescence intensity (MFI). The background fluorescence for the isotype control antibody on all cell lines was an MFI less than 6.
Next, we tested these cell lines in vitro for sensitivity to cell-death induction by rhApo2L/TRAIL (Table 1; Figure 2). Four of the cell lines (BJAB, Ramos RA1, Ramos T1, and DoHH-2) underwent cell death in response to rhApo2L/TRAIL, while the other 4 (SC-1, Raji, Daudi, and OCI-Ly19) did not. Rituximab showed no cytotoxic activity against BJAB, Ramos RA1, or SC-1 cells, but displayed some cytotoxicity against DoHH-2 cells (Figure 2A-D). Rituximab augmented the amount of cell death induced by rhApo2L/TRAIL in Ramos RA1 and DoHH-2 cells, but not in BJAB or SC-1 cells. Further analysis of Ramos RA1 cells indicated dose-dependent activation of caspase-3/7 upon treatment with rhApo2L/TRAIL (Figure 2E,F), confirming the induction of apoptosis. Rituximab by itself did not stimulate caspase-3/7 activity; however, it augmented rhApo2L/TRAIL-induced caspase-3/7 activation (Figure 2E). Flow cytometric analysis of caspase-3 activation at the individual cell level confirmed that rituximab did not directly stimulate caspase-3 and indicated that the number of cells with active caspase-3 increased when rituximab and rhApo2L/TRAIL were used together compared with rhApo2L/TRAIL alone (Figure 2F). Furthermore, pretreatment with rituximab did not change the percentage of cells with active caspase-3 at 24 hours compared with simultaneous treatment, but it did accelerate the rate of caspase-3 cleavage in those cells undergoing apoptosis (Figure S2). Together, these results demonstrated that 3 of 7 NHL cell lines tested were sensitive to rhApo2L/TRAIL, and of those, 2 showed evidence of enhancement by rituximab.
Analysis of NHL cell lines for sensitivity to rhApo2L/TRAIL alone and in combination with rituximab
| Cell lines . | NHL type . | IC50, ng/mL . | |
|---|---|---|---|
| rhApo2L/TRAIL . | rhApo2L/TRAIL + rituximab . | ||
| BJAB | BL | 32 | 26 |
| Ramos RA1 | BL | 38 | 17 |
| DoHH-2 | FL | 14 | —* |
| SC-1 | FL | > 1000 | > 1000 |
| Ramos T1 | BL | 33 | 13 |
| Raji | BL | > 1000 | > 1000 |
| Daudi | BL | > 1000 | > 1000 |
| OCI-Ly19 | DLBCL | > 1000 | > 1000 |
| Cell lines . | NHL type . | IC50, ng/mL . | |
|---|---|---|---|
| rhApo2L/TRAIL . | rhApo2L/TRAIL + rituximab . | ||
| BJAB | BL | 32 | 26 |
| Ramos RA1 | BL | 38 | 17 |
| DoHH-2 | FL | 14 | —* |
| SC-1 | FL | > 1000 | > 1000 |
| Ramos T1 | BL | 33 | 13 |
| Raji | BL | > 1000 | > 1000 |
| Daudi | BL | > 1000 | > 1000 |
| OCI-Ly19 | DLBCL | > 1000 | > 1000 |
BL indicates Burkitt lymphoma.
Rituximab (1000 ng/mL) has single-agent activity, which precludes comparing the IC50 values of the cell viability curves for this cell line.
rhApo2L/TRAIL induces apoptosis and caspase-3/7 activation in NHL cells. BJAB (A), DoHH-2 (B), Ramos RA1 (C), and SC-1 (D) NHL cells were analyzed for cell viability by AlamarBlue staining after 48 hours of treatment with rhApo2L/TRAIL alone (•) or rhApo2L/TRAIL and rituximab (20 μg/mL, ■). Caspase-3/7 activity in Ramos RA1 cells was measured using a fluorescence assay after 24 hours of treatment under the conditions described for panels A-D. (E) Flow cytometric analyses of active caspase-3–positive Ramos RA1 cells as detected by a FITC-conjugated monoclonal antibody to active caspase-3 after 24 hours of treatment as described for panels A-D. (F) In every experiment, rhApo2L/TRAIL and rituximab were added simultaneously.
rhApo2L/TRAIL induces apoptosis and caspase-3/7 activation in NHL cells. BJAB (A), DoHH-2 (B), Ramos RA1 (C), and SC-1 (D) NHL cells were analyzed for cell viability by AlamarBlue staining after 48 hours of treatment with rhApo2L/TRAIL alone (•) or rhApo2L/TRAIL and rituximab (20 μg/mL, ■). Caspase-3/7 activity in Ramos RA1 cells was measured using a fluorescence assay after 24 hours of treatment under the conditions described for panels A-D. (E) Flow cytometric analyses of active caspase-3–positive Ramos RA1 cells as detected by a FITC-conjugated monoclonal antibody to active caspase-3 after 24 hours of treatment as described for panels A-D. (F) In every experiment, rhApo2L/TRAIL and rituximab were added simultaneously.
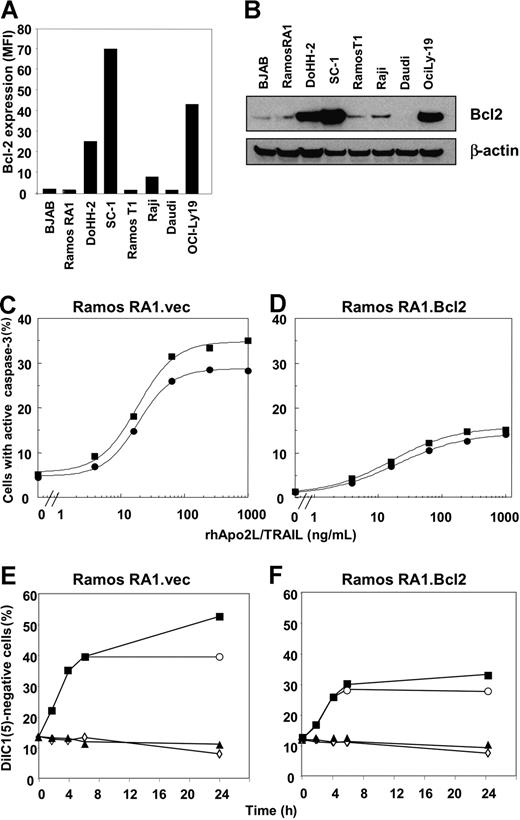
Follicular lymphomas overexpress Bcl-2 due to a characteristic chromosomal translocation t(14:18) that places the Bcl-2 protein coding region under the control of the IgH promoter/enhancer.52,53 Expression of Bcl-2 can render FL cells more resistant to apoptotic stimuli.54 To ascertain if resistance to rhApo2L/TRAIL correlated with Bcl-2 expression, we tested the panel of NHL cells for Bcl-2 expression by intracellular flow cytometry using a Bcl-2–specific antibody (Figure 3A). We also tested for the expression of Bcl-2 by immunoblot analysis (Figure 3B). Whereas the Burkitt lymphoma lines had low or undetectable amounts of Bcl-2, the FL cell lines DoHH-2 and SC-1 and the DLBCL line OCI-Ly19 expressed high Bcl-2 levels. Thus, there was no clear correlation between Bcl-2 expression and resistance to rhApo2L/TRAIL among the 8 cell lines tested.
Modulation of proapoptotic and antiapoptotic Bcl-2 family proteins by rituximab in Ramos RA1 cells. (A) Flow cytometric analysis of Bcl-2 levels in NHL cell lines. Data are reported as MFI. The background fluorescence for the isotype control antibody on all cell lines was an MFI less than 6. (B) Immunoblot analysis of Bcl-2 expression in NHL cell lines. (C,D) Effect of Bcl-2 overexpression in Ramos RA1 cells on caspase-3 activation. Flow cytometric analyses of active caspase-3–positive Ramos RA1 cells treated with rhApo2L/TRAIL alone (•) or rhApo2L/TRAIL and rituximab (20 μg/mL, ■) for 24 hours. (E,F) Effect of Bcl-2 overexpression in Ramos RA1 cells on loss of mitochondrial-membrane integrity. Flow cytometric analyses of DiIC1(5)-negative Ramos RA1 cells treated with vehicle (◇), rhApo2L/TRAIL (1 μg/mL, ○), rituximab (20 μg/mL, ▴), or rhApo2L/TRAIL and rituximab (■). Cells were analyzed for DiIC1(5) at the indicated number of hours after addition of rhApo2L/TRAIL.
Modulation of proapoptotic and antiapoptotic Bcl-2 family proteins by rituximab in Ramos RA1 cells. (A) Flow cytometric analysis of Bcl-2 levels in NHL cell lines. Data are reported as MFI. The background fluorescence for the isotype control antibody on all cell lines was an MFI less than 6. (B) Immunoblot analysis of Bcl-2 expression in NHL cell lines. (C,D) Effect of Bcl-2 overexpression in Ramos RA1 cells on caspase-3 activation. Flow cytometric analyses of active caspase-3–positive Ramos RA1 cells treated with rhApo2L/TRAIL alone (•) or rhApo2L/TRAIL and rituximab (20 μg/mL, ■) for 24 hours. (E,F) Effect of Bcl-2 overexpression in Ramos RA1 cells on loss of mitochondrial-membrane integrity. Flow cytometric analyses of DiIC1(5)-negative Ramos RA1 cells treated with vehicle (◇), rhApo2L/TRAIL (1 μg/mL, ○), rituximab (20 μg/mL, ▴), or rhApo2L/TRAIL and rituximab (■). Cells were analyzed for DiIC1(5) at the indicated number of hours after addition of rhApo2L/TRAIL.
To gain some mechanistic insight into how rituximab might augment rhApo2L/TRAIL-induced effector caspase activation, we examined whether this antibody affects proximal signaling events initiated by rhApo2L/TRAIL in Ramos RA1 cells. We observed no detectable change in cell surface levels of DR4 and DR5 after a 24-hour exposure to 20 μg/mL rituximab (Figure S3A). Rituximab also did not substantially alter the recruitment of FADD and caspase-8 to the rhApo2L/TRAIL DISC (Figure S3B), suggesting that the antibody may modulate events distal to the DISC to augment effector caspase activation. To assess this further, we analyzed Ramos RA1 cells for changes in the levels of the antiapoptotic Bcl-2 homolog Bcl-XL; the proapoptotic BH3-only proteins Bax, Bak, Bad, Puma, Bim, Bmf, Bad, and phospho-Bad; and the inhibitor of apoptosis protein XIAP, by immunoblot (Figure 3C). We observed no major changes in any of the proapoptotic or antiapoptotic proteins tested. To examine the possibility that other proapoptotic modulators not included in our analysis might be altered by rituximab treatment, we generated a Ramos RA1 cell line overexpressing Bcl-2, which inhibits multiple BH3-only Bcl-2 family member proteins.55 We confirmed expression of high Bcl-2 levels by immunoblot analysis (Figure S3D). Bcl-2 overexpression diminished the activation of caspase-3 by rhApo2L/TRAIL alone (Figure 3C,D), consistent with a type II cell phenotype. Importantly, Bcl-2 overexpression also attenuated the ability of rituximab to augment caspase-3 activation. Moreover, Bcl-2 overexpression inhibited rituximab augmentation of rhApo2L/TRAIL-induced mitochondrial permeability transition 24 hours after treatment (Figure 3E,F). Thus, experimental overexpression of Bcl-2 in Ramos RA1 cells reduces the augmentation of rhApo2L/TRAIL-induced apoptosis by rituximab.
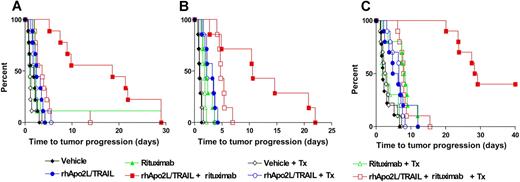
To test the activity of rhApo2L/TRAIL in vivo, we established xenograft models based on several human NHL cell lines in SCID mice. We implanted NHL cells under the skin and allowed subcutaneous tumors to develop to a size of approximately 200 mm3 before beginning therapy. Treatment of mice harboring BJAB tumors with rhApo2L/TRAIL or rituximab caused a clear delay in tumor growth; however, none of the tumors showed partial or complete regression (defined as at least 50% or 100% shrinkage, respectively; Figure 4A; Table 2). In contrast, combined treatment with rhApo2L/TRAIL plus rituximab led to a much more prolonged delay in tumor growth, with 1/7 and 6/7 tumors, respectively, showing partial or complete regression. In mice bearing DoHH-2 xenografts, rhApo2L/TRAIL treatment caused a minimal effect, while rituximab therapy led to a more substantial tumor-growth delay but not tumor regression (Figure 4B; Table 2). The combination of rhApo2L/TRAIL with rituximab was significantly more effective at abating tumor progression, with 1/7 tumors showing partial shrinkage and 1/7 having complete regression. Monotherapy of mice bearing Ramos RA1 xenografts with rhApo2L/TRAIL or rituximab resulted in a minor delay of tumor growth with no partial or complete responses (Figure 4C; Table 2). Again, combined therapy with rhApo2L/TRAIL and rituximab led to a substantially greater delay in tumor growth, with 2/9 (22%) or 7/9 (78%) tumors showing partial or complete regression. Treatment of mice harboring SC-1 xenografts with rhApo2L/TRAIL alone had no measurable effect, while rituximab treatment delayed tumor growth (Figure 4D; Table 2). Combined treatment with rhApo2L/TRAIL plus rituximab caused further growth delay, with 1/6 (17%) partial and 2/6 (33%) complete tumor regressions. In contrast to the latter models, treatment of mice carrying Raji xenografts with rhApo2L/TRAIL did not inhibit tumor growth, and while rituximab modestly slowed tumor growth, the combination showed no added benefit (Figure S4). Likewise, treatment of mice carrying OCI-Ly19 xenografts with rhApo2L/TRAIL or rituximab or both did not inhibit tumor growth (data not shown). These results indicate that rhApo2L/TRAIL and rituximab can cooperate to attenuate or reverse growth of several NHL-based tumor xenografts. This in vivo cooperation applies to cell lines that are sensitive in vitro to cytotoxicity of one or both agents, as well as to some, though not to all, of the cell lines that are resistant to both.
Activity of rhApo2L/TRAIL and rituximab against subcutaneous NHL xenografts. CB17 ICR SCID mice bearing established BJAB (A; n = 7 mice/group), DoHH-2 (B; n = 10 mice/group), Ramos RA1 (C; n = 9 mice/group), and SC-1 (D; n = 6 mice/group) tumors (approximately 200 mm3 in size) were treated with vehicle (black x), 60 mg/kg rhApo2L/TRAIL (open blue circle), 4 mg/kg rituximab (closed green triangle), or rhApo2L/TRAIL and rituximab (closed red square). rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break and a second 5-day treatment as indicated by the blue bar. Rituximab was administered once a week as indicated by the green arrow.
Activity of rhApo2L/TRAIL and rituximab against subcutaneous NHL xenografts. CB17 ICR SCID mice bearing established BJAB (A; n = 7 mice/group), DoHH-2 (B; n = 10 mice/group), Ramos RA1 (C; n = 9 mice/group), and SC-1 (D; n = 6 mice/group) tumors (approximately 200 mm3 in size) were treated with vehicle (black x), 60 mg/kg rhApo2L/TRAIL (open blue circle), 4 mg/kg rituximab (closed green triangle), or rhApo2L/TRAIL and rituximab (closed red square). rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break and a second 5-day treatment as indicated by the blue bar. Rituximab was administered once a week as indicated by the green arrow.
Activity of rhApo2L/TRAIL and rituximab against NHL xenografts
| Cell line/treatment . | PR . | CR . |
|---|---|---|
| BJAB | ||
| Vehicle | 0/7 | 0/7 |
| rhApo2L/TRAIL | 2/7 | 0/7 |
| Rituximab | 2/7 | 0/7 |
| Combination | 1/7 | 6/7 |
| Ramos RA1 | ||
| Vehicle | 0/9 | 0/9 |
| rhApo2L/TRAIL | 0/9 | 0/9 |
| Rituximab | 0/9 | 0/9 |
| Combination | 1/9 | 7/9 |
| DoHH-2 | ||
| Vehicle | 0/10 | 0/10 |
| rhApo2L/TRAIL | 0/10 | 0/10 |
| Rituximab | 0/10 | 0/10 |
| Combination | 1/10 | 1/10 |
| SC-1 | ||
| Vehicle | 0/6 | 0/6 |
| rhApo2L/TRAIL | 0/6 | 0/6 |
| Rituximab | 1/6 | 2/6 |
| Combination | 1/6 | 2/6 |
| Cell line/treatment . | PR . | CR . |
|---|---|---|
| BJAB | ||
| Vehicle | 0/7 | 0/7 |
| rhApo2L/TRAIL | 2/7 | 0/7 |
| Rituximab | 2/7 | 0/7 |
| Combination | 1/7 | 6/7 |
| Ramos RA1 | ||
| Vehicle | 0/9 | 0/9 |
| rhApo2L/TRAIL | 0/9 | 0/9 |
| Rituximab | 0/9 | 0/9 |
| Combination | 1/9 | 7/9 |
| DoHH-2 | ||
| Vehicle | 0/10 | 0/10 |
| rhApo2L/TRAIL | 0/10 | 0/10 |
| Rituximab | 0/10 | 0/10 |
| Combination | 1/10 | 1/10 |
| SC-1 | ||
| Vehicle | 0/6 | 0/6 |
| rhApo2L/TRAIL | 0/6 | 0/6 |
| Rituximab | 1/6 | 2/6 |
| Combination | 1/6 | 2/6 |
CB17 ICR SCID mice bearing established NHL tumors (approximately 200 mm3 in size) were treated with indicated therapeutic. A partial response (PR) is defined as at least 50% tumor shrinkage and a complete response (CR) as 100% shrinkage. rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break. Rituximab was administered once a week. Both therapeutics were administered for 2 weeks. Data are number responding/number in treatment group.
Rituximab exerts antitumor activity in vivo through several mechanisms, including antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). We investigated the ability of rhApo2L/TRAIL to cooperate with rituximab in an NK-dependent ADCC assay in vitro (Figure S5A). We observed a modest enhancement of rituximab mediated ADCC in the presence of rhApo2L/TRAIL; whereas, rhApo2L/TRAIL alone showed no ADCC activity. To investigate whether these activities are important for rituximab's cooperation with rhApo2L/TRAIL in vivo, we used previously established pharmacologic approaches to inhibit specific antibody-mediated effector functions and examined the impact on anti-tumor activity in mice bearing Ramos RA1 tumor xenografts (Figure 5). We depleted NK cells, which can support ADCC by virtue of Fcγ receptor expression, in mice bearing Ramos RA1 tumors with anti–asialo GM1 antibodies before the start of therapy. NK cell depletion was confirmed by analyzing the spleen for NK cells (CD49b+) by flow cytometry (Figure S5B). Consistent with the other Ramos RA1 data (Figure 4B), combined treatment with rituximab and rhApo2L/TRAIL lead to a significant tumor-growth delay (Figure 5A). NK cell depletion did not affect the rate of tumor growth (depicted as a Kaplan-Meier analysis of the time interval for the tumors to reach 2.5-fold their starting volume) in mice treated with vehicle, rhApo2L/TRAIL, or rituximab; however, it substantially reduced therapeutic benefit in mice treated with rituximab plus rhApo2L/TRAIL compared with nondepleted controls (P = .001 by log-rank test, Figure 5A).
Effect of NK cell depletion and complement depletion on cooperation between rituximab and rhApo2L/TRAIL against Ramos RA1 xenografts. CB17 ICR SCID mice bearing subcutaneous Ramos RA1 xenografts were treated (Tx) with rabbit anti–asialo GM1 antibody (aGM1) to deplete NK cells (A; n = 9 mice/group), cobra venom factor (CVF) to deplete complement protein C3 (B; n = 7 mice/group), or aGM1 and CVF (C; n = 10 mice/group) one day prior to the initiation of treatment with vehicle (V), 60 mg/kg rhApo2L/TRAIL (A), 4 mg/kg rituximab (R), or rhApo2L/TRAIL and rituximab (A + R). The data are graphed as a Kaplan-Meier analysis of the time for the tumors to reach 2.5 times their starting size. The aGM1 antibody and CVF were administered 2 times per week for the duration of the experiment. rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break and a second round of 5 days of treatment. Rituximab was administered once a week for 2 weeks.
Effect of NK cell depletion and complement depletion on cooperation between rituximab and rhApo2L/TRAIL against Ramos RA1 xenografts. CB17 ICR SCID mice bearing subcutaneous Ramos RA1 xenografts were treated (Tx) with rabbit anti–asialo GM1 antibody (aGM1) to deplete NK cells (A; n = 9 mice/group), cobra venom factor (CVF) to deplete complement protein C3 (B; n = 7 mice/group), or aGM1 and CVF (C; n = 10 mice/group) one day prior to the initiation of treatment with vehicle (V), 60 mg/kg rhApo2L/TRAIL (A), 4 mg/kg rituximab (R), or rhApo2L/TRAIL and rituximab (A + R). The data are graphed as a Kaplan-Meier analysis of the time for the tumors to reach 2.5 times their starting size. The aGM1 antibody and CVF were administered 2 times per week for the duration of the experiment. rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break and a second round of 5 days of treatment. Rituximab was administered once a week for 2 weeks.
Complement activation by immunoglobulin is dependent on C3. Cobra venom factor (CVF) is a C3 convertase that catalyzes the complete degradation of all circulating C3 protein when administered to mice, preventing complement activation and target-cell killing. To block CDC, we treated Ramos RA1 tumor–bearing mice with CVF one day before commencing treatment with rhApo2L/TRAIL and/or rituximab and every 3 days thereafter (Figure 5B). Immunoblot analysis confirmed essentially complete depletion of serum C3 in CVF-treated animals for the duration of the study (Figure S5C). C3 depletion did not alter the rate of tumor growth in mice treated with vehicle, rhApo2L/TRAIL, or rituximab; however, it substantially prevented therapeutic benefit in mice treated with rituximab plus rhApo2L/TRAIL compared with nondepleted controls (P = .016 by log-rank test, Figure 5B). Last, we simultaneously depleted both NK cells and complement to determine the impact on the efficacy of rhApo2L/TRAIL and rituximab (Figure 5C). As with elimination of either effector alone, elimination of both resulted in a significant loss of the combined activity of rhApo2L/TRAIL and rituximab (P = .02 by log-rank test, Figure 5C). Taken together, these results suggest that both NK- and C3-dependent antibody-mediated mechanisms are important for the cooperation between rituximab and rhApo2L/TRAIL against Ramos RA1 xenografts.
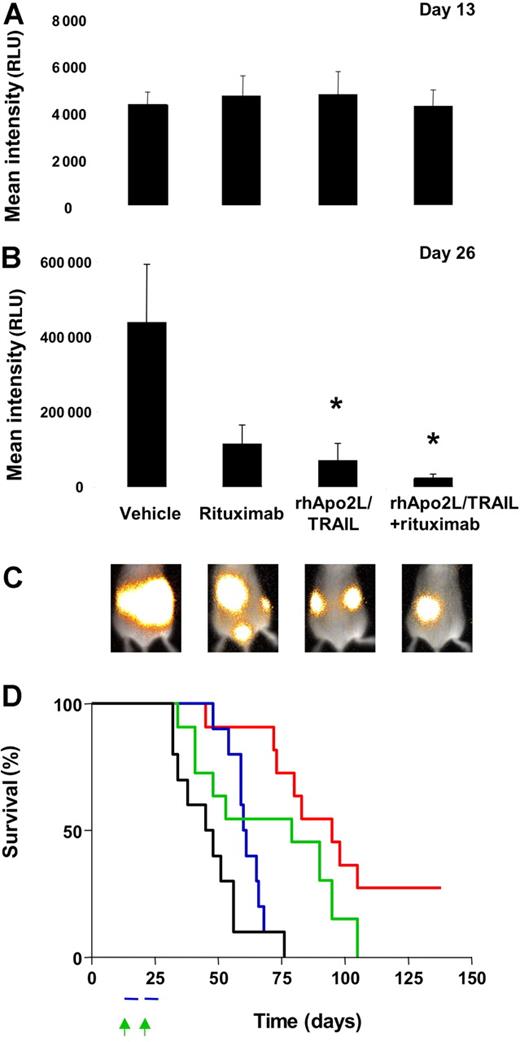
Advanced NHL typically occurs as a disseminated malignancy. To examine the activity of rhApo2L/TRAIL against disseminated NHL tumors, we developed a model based on BJAB cells stably transfected with firefly luciferase (BJAB-Luc cells), which can be detected in vivo by luminescence after injection of a synthetic luciferase substrate. Within approximately 2 weeks after intravenous injection of BJAB-Luc cells into SCID mice, detectable tumors were established in various tissue sites including lymph nodes and bone (Figure 6C and data not shown). We distributed mice with established 13-day-old BJAB-Luc tumors into treatment groups with an equivalent tumor burden as assessed by total luminescence (Figure 6A; Figure S6). After 2 weeks of treatment, mice that received vehicle showed a pronounced increase in tumor burden (compare vehicle group in Figure 6B,C; Figure S6). The BJAB-Luc tumors continued to grow in the presence of vehicle; rituximab alone did not appreciably inhibit tumor growth; however, mice treated with rhApo2L/TRAIL alone or in combination with rituximab showed significantly less tumor growth, with evidence of tumor regression or stasis in some animals. Mice treated with rituximab alone had 74% lower tumor burden compared with vehicle-treated mice (P = .062, Mann-Whitney test). Mice treated with rhApo2L/TRAIL alone had 84% less tumor burden (P = .009 compared with vehicle, Mann-Whitney test). Mice treated with rhApo2L/TRAIL and rituximab had a 94% lower tumor burden than vehicle controls (P = .004 compared with vehicle control, P = .057 compared with rituximab alone and P = .275 compared with rhApo2L/TRAIL alone, Mann-Whitney test). We also assessed tumor-associated morbidity (Figure 6D). Mice treated with rituximab or rhApo2L/TRAIL or both showed significant increases in survival compared with vehicle-treated controls (P = .015, P = .045, or P = .001, respectively, by log-rank test). Importantly, while all animals in the monotherapy groups eventually succumbed to their tumor burden, 3 (30%) of 10 mice receiving rhApo2L/TRAIL plus rituximab survived more than 135 days, suggesting that these animals underwent complete tumor remission. These results indicate that both rhApo2L/TRAIL and rituximab exert significant antitumor activity against disseminated BJAB tumors, and the 2 agents can cooperate to attenuate, and in some cases even to eradicate, these tumors, increasing host survival.
Activity of rhApo2L/TRAIL and rituximab against disseminated NHL tumor xenografts. BJAB-Luc cells were injected intravenously into CB17 ICR SCID mice. Tumor burden was determined by bioluminescent imaging and shown as the mean relative light units (RLU) for 4 randomized groups (n = 10 mice/group) with equivalent tumor burden at day 13 (A). Mice were treated with vehicle, 60 mg/kg rhApo2L/TRAIL, 4 mg/kg rituximab, or rhApo2L/TRAIL and rituximab. rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break and a second round of 5 days of treatment as indicated by the blue bar (panel D). Rituximab was administered once a week for 2 weeks as indicated by the green arrow (panel D). On day 26 (B), the tumor burden for the rituximab group was marginally reduced (P = .062 compared with vehicle, Mann-Whitney test), the tumor burden for the rhApo2L/TRAIL group was significantly lower (P = .009 compared with vehicle), and the tumor burden of the rhApo2L/TRAIL and rituximab group was significantly lower than the vehicle control (P = .004). The tumor burden for the rhApo2L/TRAIL and rituximab group was not significantly lower than the rhApo2L/TRAIL group (P = .275); however, the tumor burden for the rhApo2L/TRAIL and rituximab group was significantly lower than the rituximab group (P = .057). * represents statistical significance of result compared to vehicle. Error bars in panels A and B represent SEM. (C) Representative false color images of the mean tumor luminescence for each group are shown as an overlay of the reference image of the whole mouse. (D) Survival analysis of the BJAB-bearing mice; Kaplan-Meyer curves for the experiment are shown. The vehicle-treated (black line), rhApo2L/TRAIL-treated (blue line), rituximab-treated (green line), and rhApo2L/TRAIL and rituximab–treated (red line) mice are shown.
Activity of rhApo2L/TRAIL and rituximab against disseminated NHL tumor xenografts. BJAB-Luc cells were injected intravenously into CB17 ICR SCID mice. Tumor burden was determined by bioluminescent imaging and shown as the mean relative light units (RLU) for 4 randomized groups (n = 10 mice/group) with equivalent tumor burden at day 13 (A). Mice were treated with vehicle, 60 mg/kg rhApo2L/TRAIL, 4 mg/kg rituximab, or rhApo2L/TRAIL and rituximab. rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break and a second round of 5 days of treatment as indicated by the blue bar (panel D). Rituximab was administered once a week for 2 weeks as indicated by the green arrow (panel D). On day 26 (B), the tumor burden for the rituximab group was marginally reduced (P = .062 compared with vehicle, Mann-Whitney test), the tumor burden for the rhApo2L/TRAIL group was significantly lower (P = .009 compared with vehicle), and the tumor burden of the rhApo2L/TRAIL and rituximab group was significantly lower than the vehicle control (P = .004). The tumor burden for the rhApo2L/TRAIL and rituximab group was not significantly lower than the rhApo2L/TRAIL group (P = .275); however, the tumor burden for the rhApo2L/TRAIL and rituximab group was significantly lower than the rituximab group (P = .057). * represents statistical significance of result compared to vehicle. Error bars in panels A and B represent SEM. (C) Representative false color images of the mean tumor luminescence for each group are shown as an overlay of the reference image of the whole mouse. (D) Survival analysis of the BJAB-bearing mice; Kaplan-Meyer curves for the experiment are shown. The vehicle-treated (black line), rhApo2L/TRAIL-treated (blue line), rituximab-treated (green line), and rhApo2L/TRAIL and rituximab–treated (red line) mice are shown.
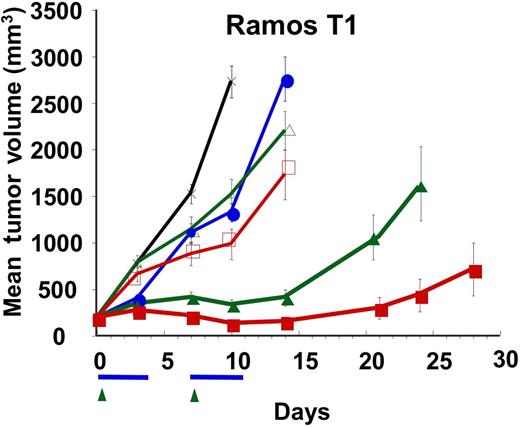
Some NHL tumors can acquire resistance to rituximab in the clinical setting. In an effort to model rituximab resistance, we generated a rituximab-refractory Ramos RA1 cell line (Ramos T1) through multiple rounds of in vivo exposure to rituximab. Monotherapy of mice bearing subcutaneous Ramos T1 tumors with 4 or 10 mg/kg rituximab or with rhApo2L/TRAIL only slightly delayed tumor growth (Figure 7). However, combined treatment with rhApo2L/TRAIL plus either one of the rituximab doses led to a significant delay in tumor growth. At the 4-mg/kg rituximab combination dose, 0 (0%) of 12 mice showed a partial response while 3 (25%) of 12 showed complete tumor regression. At the 10-mg/kg rituximab combination dose, 2 (17%) of 12 mice showed a partial response and another 6 (50%) of 12 showed complete tumor regression. These results suggest that addition of rhApo2L/TRAIL to rituximab may help overcome acquired rituximab resistance of certain NHL tumors.
Activity of rhApo2L/TRAIL and rituximab against a rituximab-refractory Ramos NHL variant. A rituximab-refractory variant of Ramos RA1, designated Ramos T1, was established by treatment of Ramos RA1 in vivo with rituximab followed by harvest of tumors that escaped elimination and subsequent culture of cells. Ramos T1 cells were injected subcutaneously into CB17 ICR SCID mice and allowed to establish approximately 200-mm3 tumors. Mice (n = 12 mice/group) were then treated with vehicle (black x), 60 mg/kg rhApo2L/TRAIL (closed blue circle), 4 mg/kg rituximab (open green triangle), 10 mg/kg rituximab (open red square), rhApo2L/TRAIL and 4 mg/kg rituximab (closed green triangle), or rhApo2L/TRAIL and 10 mg/kg rituximab (closed red square). rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break and a second 5-day treatment as indicated by the blue bar. Rituximab was administered once a week as indicated by the green arrow.
Activity of rhApo2L/TRAIL and rituximab against a rituximab-refractory Ramos NHL variant. A rituximab-refractory variant of Ramos RA1, designated Ramos T1, was established by treatment of Ramos RA1 in vivo with rituximab followed by harvest of tumors that escaped elimination and subsequent culture of cells. Ramos T1 cells were injected subcutaneously into CB17 ICR SCID mice and allowed to establish approximately 200-mm3 tumors. Mice (n = 12 mice/group) were then treated with vehicle (black x), 60 mg/kg rhApo2L/TRAIL (closed blue circle), 4 mg/kg rituximab (open green triangle), 10 mg/kg rituximab (open red square), rhApo2L/TRAIL and 4 mg/kg rituximab (closed green triangle), or rhApo2L/TRAIL and 10 mg/kg rituximab (closed red square). rhApo2L/TRAIL was administered daily for 5 consecutive days followed by a 2-day break and a second 5-day treatment as indicated by the blue bar. Rituximab was administered once a week as indicated by the green arrow.
Discussion
It is now well established that members of the tumor necrosis factor superfamily have important roles in modulating the development and function of the immune system.56 The role of Apo2L/TRAIL and its receptors in immunity has been the focus of several recent studies. Mice deficient in Apo2L/TRAIL or its sole murine proapoptotic receptor DR5 display essentially normal lymphoid development35,57 ; however, they show increased susceptibility to tumor initiation and metastasis.58,59 Consistent with the possibility that Apo2L/TRAIL may be involved in antitumor immune functions, recombinant soluble Apo2L/TRAIL induces apoptosis in various types of cancer cells.38,60-62 Several studies addressing specifically the sensitivity of hematologic malignancies to recombinant Apo2L/TRAIL in vitro show potent proapoptotic activity of the ligand,63-65 often in combination with chemotherapeutic drugs, on Burkitt lymphoma cell lines.66-68 Agonistic monoclonal antibodies to DR4 (TRAIL-R1) or DR5 (TRAIL-R2) have been shown to kill a limited subset of NHL cell lines and primary lymphoid tumors, mostly B-cell chronic lymphocytic leukemia (B-CLL)/small lymphocytic lymphoma (SLL).69 However, none of these studies addresses the anti-NHL activity of rhApo2L/TRAIL in vivo, nor do they assess whether this proapoptotic receptor agonist can cooperate with other targeted agents such as rituximab.
In the present study, we investigated the activity of rhApo2L/TRAIL and its therapeutic interaction with rituximab in xenograft models of several NHL cell lines, including Burkitt, follicular, and diffuse large B-cell lymphomas. We observed proapoptotic activity of rhApo2L/TRAIL on 3 of 7 NHL cell lines in vitro. Consistent with earlier observations in other cancer cell types, the levels of DR4 or DR5 on these cells did not directly correlate with sensitivity to the ligand, suggesting the involvement of downstream mechanisms. Rituximab significantly augmented apoptosis induction by rhApo2L/TRAIL in Ramos RA1, and to a lesser extent, DoHH-2 cells, but not in BJAB or SC-1 cells. The enhancement in Ramos RA1 cell killing was associated with an increase in total caspase-3/7 activation as measured directly by enzymatic activity or indirectly by fluorescence-activated cell sorting (FACS) analysis with an antibody that specifically recognizes activated caspase-3. Rituximab did not alter apical signaling events induced by rhApo2L/TRAIL, suggesting that it may modulate more distal events that lead to activation of effector caspases. Consistent with this possibility, Bcl-2 overexpression attenuated rituximab's enhancement of rhApo2L/TRAIL-induced mitochondrial engagement and effector caspase activation. This observation is consistent with earlier evidence that rituximab can engage cell-intrinsic apoptotic changes even in the absence of effectors that could mediate ADCC and CDC.19 It has been reported that Ramos RA1 cells undergo caspase-independent apoptosis upon treatment with cross-linked rituximab17 ; however, we did not observe evidence of cell death upon treatment of Ramos RA1 cells with non–cross-linked or cross-linked rituximab (Figure 2 and data not shown). Rituximab treatment of NHL cells also can sensitize to apoptosis activation by chemotherapeutic agents,70 further suggesting that rituximab may affect the intrinsic apoptosis pathway.
Given the importance of antibody-mediated effector functions for rituximab's antitumor efficacy,11,15,16,71 we extended our studies into in vivo NHL xenograft models. In keeping with our in vitro findings, rhApo2L/TRAIL and rituximab cooperated against Ramos RA1 and DoHH-2 xenografts. Notably, despite the absence of significant interaction against BJAB cells in culture, we observed a strong cooperation between rhApo2L/TRAIL and rituximab against BJAB xenografts in vivo. Moreover, while SC-1 cells were refractory to either one of the 2 agents in vitro, SC-1 tumor xenografts showed a significant response to rituximab monotherapy and a substantial further response to rituximab plus rhApo2L/TRAIL. Three of the lymphoma lines, BJAB, Ramos RA1, and DoHH-2, were highly responsive to rhApo2L/TRAIL in vitro; however, they showed minimal responses to rhApo2L/TRAIL alone in vivo. Perhaps the rapid clearance of rhApo2L/TRAIL in murine preclinical models contributes to this difference in vivo, as the half-life of rhApo2L/TRAIL in mice is approximately 3 to 5 minutes.72 Certain lymphoma xenografts may require more sustained exposure to rhApo2L/TRAIL, therefore responding mainly in the setting of combination treatment with rituximab. Nonetheless, our results suggest that optimal cooperation between rhApo2L/TRAIL and rituximab is achieved in vivo, where both proapoptotic activity and antibody-mediated effector mechanisms may operate in conjunction. This conclusion is further supported by the observation that depletion of NK cells and complement substantially limited the therapeutic interaction between rituximab and rhApo2L/TRAIL in vivo.
Bcl-2 expression is a hallmark of FL and has been shown to attenuate sensitivity to apoptotic stimuli.54,73 This could be a concern for therapeutic agents designed to target the apoptosis pathway. However, despite relatively high levels of Bcl-2 in the DoHH-2 and SC-1 FL cell lines, rhApo2L/TRAIL and rituximab cooperated effectively in vivo against tumor xenografts derived from these cell lines. Hence, it may be possible to overcome the tumor-protective effect of Bcl-2 by combining therapies that promote apoptosis together with nonapoptotic mechanisms such as ADCC and CDC.
The evidence that Fc gamma RIII polymorphism correlates with differential rituximab responsiveness in NHL patients strongly suggests that ADCC plays a key role in rituximab's efficacy.15,16 Rituximab also has potent complement-fixing activity4 ; however, there is conflicting data on the importance of this function for rituximab's efficacy in NHL patients.74,75 Depletion of either NK cells or serum complement greatly attenuated the combined efficacy of rituximab and rhApo2L/TRAIL against Ramos RA1 xenografts. Thus, NK-mediated functions such as ADCC, as well as complement-mediated cytotoxicity, seem to contribute to the positive therapeutic interaction between the antibody and the ligand in vivo.
Because NHL progresses mainly as a systemic malignancy, we developed a model that allowed investigation of the activity of rhApo2L/TRAIL and rituximab against disseminated tumors. Within 2 weeks after intravenous injection, BJAB-Luc cells formed tumors in various tissue locations, including the lymph nodes and bone. While monotherapy with rituximab or rhApo2L/TRAIL was effective against systemic BJAB tumors, neither agent caused complete tumor regression. However, the combination of both therapies had greater efficacy, as suggested by prolonged survival and by an apparently complete tumor remission in 3 of 10 mice. It is intriguing that both rituximab and rhApo2L/TRAIL exerted stronger antitumor effects in the setting of disseminated compared with subcutaneous NHL tumor xenografts. This difference may be related to better drug accessibility of tumor cells in noncutaneous tissue sites. Alternatively, disseminated tumors may be more susceptible to the cytotoxic mechanisms exerted by these agents. Regardless, this observation is encouraging because disseminated tumors are likely to be better representative of the clinical situation than subcutaneous ones.
Some NHL patients become refractory to rituximab after an initial course of treatment. Of those patients who initially respond to rituximab and then relapse, only approximately 40% respond to a second course of treatment.76 Loss of CD20 expression upon treatment is a rare event, suggesting that other mechanisms may underlie acquired rituximab resistance. We analyzed the expression of the anticomplement receptors CD55 and CD59 (Figure S7), but detected no differences in expression of the receptors between Ramos RA1 and Ramos T1 cells that might account for the resistance to ritaximab. We generated a Ramos subline refractory to rituximab by repeated treatment of Ramos RA1 xenografts with the antibody. Like the majority of rituximab-relapsed human NHL tumors, the Ramos T1 line maintained expression of CD20. We assessed whether it might be possible to harness the positive therapeutic interaction between rhApo2L/TRAIL and rituximab to circumvent the acquired rituximab-resistance of this cell line. Upon reinjection into SCID mice, Ramos T1 tumors were relatively unresponsive to single-agent treatment with either rituximab or rhApo2L/TRAIL; however, these tumors showed substantially attenuated growth in response to treatment with the 2-drug combination, with up to 50% of tumors showing complete regression. These data suggest that the in vivo cooperation between rhApo2L/TRAIL and rituximab may help bypass treatment resistance of certain NHL tumors. Nonetheless, selection of patients for combination therapy based on biomarkers that may help predict response to rhApo2L/TRAIL would be expected to improve the response rate to combined rhApo2L/TRAIL and rituximab therapy. Recent work has identified specific markers that predict sensitivity to rhApo2L/TRAIL in epithelial cancers.77 It will be important to investigate whether similar biomarkers may help guide patient selection in the NHL setting as well.
In conclusion, our studies provide the first evidence that the proapoptotic receptor agonist rhApo2L/TRAIL can exert activity in vivo against certain NHL xenografts. The antitumor activity of rhApo2L/TRAIL was notably greater against disseminated compared with subcutaneous xenografts, suggesting better accessibility and/or greater sensitivity of dissipated tumors. We uncovered a remarkable in vivo cooperation between rhApo2L/TRAIL and rituximab—a targeted antibody that is approved by regulatory agencies and often used to treat certain types of NHL. The strong, positive therapeutic interaction between these 2 agents appears to arise from the integration of several tumor cell–killing mechanisms, including enhanced cell-autonomous apoptosis as well as antibody-mediated effector functions such as ADCC and CDC. The combination of rhApo2L/TRAIL with rituximab showed significant activity also against a rituximab-refractory Ramos tumor variant, suggesting that addition of rhApo2L/TRAIL to rituximab therapy may help overcome acquired resistance to rituximab. These findings provide a strong scientific rationale for clinical investigation of rhApo2L/TRAIL in combination with rituximab as a potential therapeutic strategy for NHL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Heather Maecker for technical advice on retroviral infections and Aaron Calestar for assistance on digital images.
Authorship
Contribution: D.D. designed and performed the experiments and wrote the paper; B.Y., D.A.L., K.T., I.B., W.P.L., A.G., M.J.C., S.F.Y., and S.R. designed and performed the experiments; and A.A. designed the experiments and wrote the paper.
Conflict-of-interest disclosure: All the authors are employees of Genentech and declare competing financial interests.
Correspondence: Avi Ashkenazi, Department of Molecular Oncology, Genentech, Inc, 1 DNA Way, South San Francisco, CA 94080; e-mail: aa@gene.com.