Abstract
Few investigators have evaluated the usefulness of the JAK2 V617F mutation for explaining the phenotypic variations and for predicting the risk of major clinical events in primary myelofibrosis (PMF). In a transversal survey we assayed by allele-specific polymerase chain reaction (PCR) the JAK2 V617F mutational status in 304 patients with PMF. Multiple DNA samples were collected prospectively from 64 patients, and a highly sensitive quantitative PCR was used as a confirmatory test. In a longitudinal prospective study we determined the progression rate to clinically relevant outcomes in 174 patients who had JAK2 mutation determined at diagnosis. JAK2 V617F was identified in 63.4% of patients. None of the V617F-negative patients who were sequentially genotyped progressed to become V617F positive, whereas progression rate from heterozygous to homozygous mutation was 10 per 100 patient-years. JAK2 V617F mutation contributed to hemoglobin, aquagenic pruritus, and platelet count variability, whereas homozygous mutation was independently associated with higher white blood cell count, larger spleen size, and greater need for cytoreductive therapies. Adjusting for conventional risk factors, V617F mutation independently predicted the evolution toward large splenomegaly, need of splenectomy, and leukemic transformation. We conclude that JAK2 V617F genotype should be considered in any future risk stratification of patients with PMF.
Introduction
The Philadelphia chromosome-negative chronic myeloproliferative disorders consist of 3 main diseases: polycythemia vera, essential thrombocythemia, and primary myelofibrosis.1 Primary myelofibrosis is characterized by a shortened life expectancy and a progressive clinical course. Heterogeneity of the disease presentations with frequent development of severe anemia, large splenomegaly, and acute leukemia is the hallmark of the disease.2 Established prognostic factors, including age, hemoglobin level, and white blood cell count, have been incorporated into algorithms for risk assessment,3-8 but these characteristics do not fully explain the risk of death or major clinical events.9
A somatically acquired Janus kinase 2 (JAK2) mutation (V617F) has been reported in half of the patients with primary myelofibrosis, in nearly all of those with polycythemia vera, and in more than half of those with essential thrombocythemia.10-14 The V617F mutation lies in the autoinhibitory JH2 domain of the JAK2 gene and therefore increases JAK2 kinase activity, with the potential to affect phenotype and clinical outcomes of mutated subjects. Few studies directly faced the clinical relevance of JAK2 V617F in primary myelofibrosis.15,16 Patients with V617F mutants usually have higher white blood cell counts and a history of thrombosis or pruritus and were less likely to require transfusion during follow-up. Divergent results were obtained as far as the effect of JAK2 V617F mutation on survival15,16 and evolution to acute leukemia.17-19 However, the relatively small sample size of studied cohorts hampered a precise dissection of genotype-phenotype associations, and the retrospective design of risk assessment studies reduced the strength of the evidence.
We therefore evaluated the usefulness of JAK2 V617F mutation for explaining the phenotypic variability using a large series of patients and for predicting death and major clinical events using a prospective design in a suitable subset of the whole patients cohort.
Materials and methods
Study samples
The phenotype-genotype association study was conducted by analyzing JAK2 V617F mutation on DNA isolated from peripheral blood granulocytes of 304 consecutive patients with primary myelofibrosis. The patients attended a routine examination of the Italian Registry for Myelofibrosis in different Italian centers between 1980 and 2007. Subjects were excluded from the study if they had postpolycythemia vera or postessential thrombocythemia myelofibrosis. Patients with a diagnosis of “prefibrotic” myelofibrosis were also excluded for disease homogeneity. The diagnosis of primary myelofibrosis was established according to the Italian Consensus Conference criteria.20 All patients met also the World Health Organization (WHO) criteria for the diagnosis of fibrotic myelofibrosis.21 The Registry policies for collection and use of blood samples had been approved by the institutional review board of the Fondazione IRCCS Policlinico S. Matteo, and informed consent was obtained in accordance with the Declaration of Helsinki for the donation of samples to the tissue bank. A complete list of the members of the GIMEMA-Italian Registry of Myelofibrosis is provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
The outcome study was conducted in a subset of the whole cohort, constituted by 174 patients who had the DNA sample for JAK2 V617F mutation taken at diagnosis or no longer than 4 months from diagnosis, and who were prospectively followed up according to the Registry protocol.
Qualitative and quantitative JAK2 V617F genotyping
The mutational status for JAK2 V617F was determined using the allele-specific polymerase chain reaction (PCR) assay,12 on DNA purified from granulocytes. Digestion of PCR products with BsaXI restriction enzyme was performed, and samples were scored as homozygous if the proportion of the mutant allele was greater than 50%. In selected patients, measurement of JAK2 V617F allele burden was also performed using a quantitative real-time PCR assay that provides a sensitivity of 1% for the detection of mutant alleles.22
Study design and statistical analysis
The phenotype-genotype association analysis was conducted using patient characteristics recorded at the time of sampling. We examined the following variables that could be influenced by the JAK2 V617F mutation: requirement of cytostatic treatment (hydroxyurea, busulfan, or interferon); aquagenic pruritus (nonoccasional itching exacerbated by water contact); level of hemoglobin; white blood cell number; percentage of immature myeloid cells, erythroblasts, and blasts in peripheral blood; platelet count; spleen size; and serum lactate dehydrogenase level. Moreover, immunofluorescence CD34+cell count in peripheral blood was selected as a biomarker because of biologic plausibility.23 Two indicators of spleen size were recorded: the distance from the splenic tip to the costal margin, and the spleen index (ie, the product of the longitudinal by the transverse spleen axis), the latter defined as the maximal width of the organ. Because the quantitative variables were not normally distributed (Shapiro-Wilk test), they were summarized as median and interquartile range. Subject characteristics were compared by nonparametric analysis of variance (Kruskal-Wallis test) or by Mann-Whitney U test. Chi-square test or Fisher exact test was used to compare qualitative variables. Multiple regression models were used to account for the confounding effects of potential covariates. Results were expressed as coefficient or odds ratios. Confounding factors considered were age, sex, disease duration, disease-modifying therapies (cytostatic drugs, thalidomide, danazol, erythropoietin). To deal with missing data, we applied a multiple imputation by chained estimation model.24 To study the timing of the mutation prevalence, data were grouped into 7 time intervals from the diagnosis up to the last follow-up arbitrarily selected to have a quasi-homogeneous number of patients in each interval, and JAK2 mutation frequency trends were analyzed with Wilcoxon rank-sum test.
In the study of risk of progression to the clinically relevant outcomes of the disease, the primary outcome measures considered were death from any cause, death from leukemic transformation, major thrombosis (ie, nonfatal myocardial infarction, stroke, deep vein thrombosis, pulmonary embolism), development of severe anemia (hemoglobin < 100 g/L), large splenomegaly (spleen tip extending > 10 cm from left costal margin), thrombocytopenia (platelet count < 150 × 109/L), and leukopenia (white blood cell count < 4 × 109/L), need of splenectomy, and leukemic transformation. A diagnosis of leukemic transformation required the peripheral blood blasts to be greater than 20% of the white blood cell count or blasts in the bone marrow greater than 30% or both.1 French-American-British (FAB) classification was used to categorize the leukemic cells.25 To avoid therapy confounding, the censoring for the development of the hematologic outcomes and of large splenomegaly was considered at the beginning of any disease-modifying treatment. The distribution for overall and progression-free survival was estimated by using the method of Kaplan and Meier. The log-rank test was used to test for differences in survival between groups. Cox proportional-hazards analysis, adjusted for conventional risk factors measured at diagnosis, including age, sex, hemoglobin level, white blood cell count, percentage of blasts in peripheral blood, and Dupriez risk categories, was used to assess whether JAK2 V617F status independently predicted the outcomes. Term for splenectomy, stem cell transplantation, or use of a potentially mutagenic drug (hydroxyurea, busulfan) for at least 6 months, and their interaction, was fitted to the data. Having severe anemia, large splenomegaly, thrombocytopenia, or leukopenia at diagnosis were exclusion factors in models for hematologic outcomes and for development of large splenomegaly.
Results were considered statistically significant when P values were less than .05. All computations were performed with STATA Statistics/Data analysis software (version 9.0; (Stata, College Station, TX).
Results
Prevalence of JAK2 V617F mutation
Three hundred four patients having primary myelofibrosis were genotyped for JAK2 V617F mutation. One hundred ninety-three harbored a JAK2 V617F mutation, giving a frequency of the mutation of 63.5% (95% CI, 58.0%-68.9%). One hundred nine had a heterozygous mutation (35.8%; 95% CI, 30.4%-41.3%), and 84 (27.6%; 95% CI, 22.6%-32.7%) had a homozygous mutation (Table 1).
Laboratory and clinical features of the patients with primary myelofibrosis at the time of sample collection for JAK2 V617F assay
| . | Total population . | JAK2 V617F wild-type . | P* . | JAK2 V617F heterozygous . | P† . | JAK2 V617F homozygous . | P‡ . |
|---|---|---|---|---|---|---|---|
| No. of patients (%) | 304 | 111 (36.5) | — | 109 (35.8) | — | 84 (27.6) | — |
| Males, n (%) | 201 (66.1) | 74 (66.7) | NS | 70 (64.2) | NS | 57 (67.9) | NS |
| Age, y (IQR) | 63 (52-71.5) | 63 (54-73) | NS | 64 (53-72) | NS | 61.5 (49.5-70) | NS |
| Duration of disease, mo (IQR) | 16 (1-50) | 15 (1-60) | NS | 9 (1-30) | <.001 | 28 (3-67) | .001 |
| Hemoglobin level, g/L (IQR) | 108 (90-133) | 100 (84-118) | <.001 | 119.5 (93.5-138.5) | NS | 120 (96-143) | <.001 |
| White blood cell count, × 109/L (IQR)§ | 9.3 (5.7-14.8) | 7.5 (4.8-12.55) | NS | 9.01 (5.55-13.4) | .03 | 13.1 (7.74-18.76) | .004 |
| Immature myeloid cells in peripheral blood, % (IQR) | 5 (2-12) | 5 (2-12) | NS | 5 (0-11) | NS | 5 (1-14) | NS |
| Blasts in peripheral blood, % (IQR) | 0 (0-2) | 0.5 (0-2) | NS | 0 (0-1) | NS | 0 (0-2.5) | NS |
| Erythroblasts in peripheral blood, % (IQR) | 2 (0-3) | 2 (0-3) | NS | 1 (0-4) | NS | 1 (0-4) | NS |
| Platelet count, × 109/L (range) | 246 (126-446) | 204 (111-382) | .005 | 319 (153-594) | .009 | 224 (98-446) | NS |
| Spleen index, cm2 (IQR)‖ | 210 (130-400) | 202 (130-380) | NS | 185 (120-300) | <.001 | 287 (187-540) | <.001 |
| Serum lactate dehydrogenase, U/L (IQR) | 730 (456-1018) | 744 (438-1003) | NS | 718 (438-1045) | NS | 840 (532-1343) | NS |
| CD34+ cell no. in peripheral blood, × 106/L (IQR) | 40 (8-106) | 31 (7-118) | NS | 40 (7-106) | NS | 42 (14-106) | NS |
| Abnormal cytogenetics, no. of patients (%) | 210 (10) | 70 (15) | NS | 80 (12) | NS | 60 (7.5) | NS |
| Aquagenic pruritus, no. of patients (%) | 202 (44.9) | 40 (26.3) | .001 | 81 (48.2) | NS | 81 (62.1) | <.001 |
| Patients taking disease-modifying therapies, n (%) | 174 (25.2) | 66 (23.9) | NS | 51 (21.5) | NS | 57 (32.2) | NS |
| Patients taking cytostatic treatment, n (%) | 158 (48.2) | 42 (33.9) | NS | 54 (47.4) | .02 | 62 (68.9) | .02 |
| . | Total population . | JAK2 V617F wild-type . | P* . | JAK2 V617F heterozygous . | P† . | JAK2 V617F homozygous . | P‡ . |
|---|---|---|---|---|---|---|---|
| No. of patients (%) | 304 | 111 (36.5) | — | 109 (35.8) | — | 84 (27.6) | — |
| Males, n (%) | 201 (66.1) | 74 (66.7) | NS | 70 (64.2) | NS | 57 (67.9) | NS |
| Age, y (IQR) | 63 (52-71.5) | 63 (54-73) | NS | 64 (53-72) | NS | 61.5 (49.5-70) | NS |
| Duration of disease, mo (IQR) | 16 (1-50) | 15 (1-60) | NS | 9 (1-30) | <.001 | 28 (3-67) | .001 |
| Hemoglobin level, g/L (IQR) | 108 (90-133) | 100 (84-118) | <.001 | 119.5 (93.5-138.5) | NS | 120 (96-143) | <.001 |
| White blood cell count, × 109/L (IQR)§ | 9.3 (5.7-14.8) | 7.5 (4.8-12.55) | NS | 9.01 (5.55-13.4) | .03 | 13.1 (7.74-18.76) | .004 |
| Immature myeloid cells in peripheral blood, % (IQR) | 5 (2-12) | 5 (2-12) | NS | 5 (0-11) | NS | 5 (1-14) | NS |
| Blasts in peripheral blood, % (IQR) | 0 (0-2) | 0.5 (0-2) | NS | 0 (0-1) | NS | 0 (0-2.5) | NS |
| Erythroblasts in peripheral blood, % (IQR) | 2 (0-3) | 2 (0-3) | NS | 1 (0-4) | NS | 1 (0-4) | NS |
| Platelet count, × 109/L (range) | 246 (126-446) | 204 (111-382) | .005 | 319 (153-594) | .009 | 224 (98-446) | NS |
| Spleen index, cm2 (IQR)‖ | 210 (130-400) | 202 (130-380) | NS | 185 (120-300) | <.001 | 287 (187-540) | <.001 |
| Serum lactate dehydrogenase, U/L (IQR) | 730 (456-1018) | 744 (438-1003) | NS | 718 (438-1045) | NS | 840 (532-1343) | NS |
| CD34+ cell no. in peripheral blood, × 106/L (IQR) | 40 (8-106) | 31 (7-118) | NS | 40 (7-106) | NS | 42 (14-106) | NS |
| Abnormal cytogenetics, no. of patients (%) | 210 (10) | 70 (15) | NS | 80 (12) | NS | 60 (7.5) | NS |
| Aquagenic pruritus, no. of patients (%) | 202 (44.9) | 40 (26.3) | .001 | 81 (48.2) | NS | 81 (62.1) | <.001 |
| Patients taking disease-modifying therapies, n (%) | 174 (25.2) | 66 (23.9) | NS | 51 (21.5) | NS | 57 (32.2) | NS |
| Patients taking cytostatic treatment, n (%) | 158 (48.2) | 42 (33.9) | NS | 54 (47.4) | .02 | 62 (68.9) | .02 |
Aggregate data are medians with ranges or interquartile ranges (IQRs).
NS indicates not significant; —, not applicable.
Difference between wild-type and heterozygous patients.
Difference between heterozygous and homozygous patients.
Difference between homozygous and wild-type patients.
Corrected for the number of circulating erythroblasts.
At the time of sampling, 16 patients had undergone splenectomy. The values of spleen size refer to 288 patients.
JAK2 V617F association analysis
The phenotypic characteristics of the patients classified according to the genotype are shown in Table 1. The hemoglobin level was higher in mutated (either heterozygous or homozygous) than in nonmutated subjects, whereas platelet count was higher in heterozygous than in wild-type or homozygous subjects. Patients who carried a homozygous mutation had significantly higher spleen index and white blood cell count than did those who carried wild-type or heterozygous mutation. The frequency of patients with aquagenic pruritus increased significantly from heterozygous to homozygous genotype. Patients with a homozygous mutation had longer disease duration and more frequent requirement of cytostatic therapies than did heterozygous or wild-type patients.
In a multivariate analysis, the genotype was adjusted for other possible phenotype-modifying characteristics. This analysis confirmed the association of a mutated JAK2 V617F with hemoglobin level (average increase of hemoglobin for mutated genotype versus wild-type genotype, 16.4 g/L; 95% CI, 10.2-22.6 /L; P < .001), aquagenic pruritus (odds ratio for mutated genotype compared with wild-type genotype, 3.7; 95% CI, 1.9-6.9; P < .001), and the association of heterozygous mutation with platelet count (average increase of platelet count for heterozygous genotype compared with wild-type or homozygous genotype, 104.1 × 109/L; 95% CI, 17.8-190.5 × 109/L; P = .018). The analysis also showed a significant association of a homozygous V617F genotype with spleen size (average increase of spleen size for homozygous genotype compared with wild-type or heterozygous genotype, 95.0 cm2; 95% CI, 32.6-157.3 cm2; P = .003), and white blood cell count (average increase of white blood cell count for homozygous genotype compared with wild-type or heterozygous genotype, 2.2 × 109/L; 95% CI, 1.3-3.6 × 109/L; P = .003).
Time trend of V617F mutation rates and analysis of mutation progression
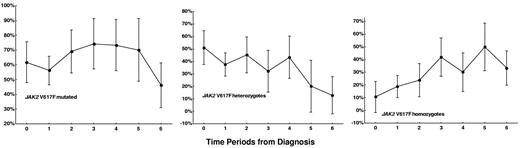
The prevalence rate and 95% CIs of the JAK2 V617F mutation for the analyzed 7 time periods from the diagnosis are reported in Figure 1. The crude prevalence rate of the mutation had no significant trend from the time of diagnosis to the time period of 8 years or more of disease duration (P for trend = .35). In contrast, the crude prevalence rate of homozygous mutation rose steadily from 19.6% to 50% (P = .003), and that of heterozygous mutation dropped from 51% to 12.8% (P < .001) from diagnosis to 8 years or more of disease duration. This result suggests a consistent rate of transformation from heterozygous to homozygous mutation.
The trend of the crude prevalence rates of JAK2 V617F mutation in samples taken during the 7 time periods from the diagnosis to the last follow-up. Time periods: 0 = at diagnosis (n = 70), 1 = from diagnosis to 1 year of follow-up (n = 72), 2 = from more than 1 year to 2 years of follow-up (n = 42), 3 = from more than 2 years to 3 years of follow-up (n = 31), 4 = from more than 3 years to 5 years of follow-up (n = 30), 5 = from more than 5 years to 8 years of follow-up (n = 20), 6 = from more than 8 years to the last follow-up (n = 39). Bars indicate 95% confidence interval.
The trend of the crude prevalence rates of JAK2 V617F mutation in samples taken during the 7 time periods from the diagnosis to the last follow-up. Time periods: 0 = at diagnosis (n = 70), 1 = from diagnosis to 1 year of follow-up (n = 72), 2 = from more than 1 year to 2 years of follow-up (n = 42), 3 = from more than 2 years to 3 years of follow-up (n = 31), 4 = from more than 3 years to 5 years of follow-up (n = 30), 5 = from more than 5 years to 8 years of follow-up (n = 20), 6 = from more than 8 years to the last follow-up (n = 39). Bars indicate 95% confidence interval.
We prospectively collected multiple DNA samples from 64 patients. Different time points of the disease were analyzed for a total follow-up of 809 patient-months (447 patient-months in patients initially wild-type and 363 patient-months in those initially heterozygous). By using allele-specific PCR, 4 of the 36 patients who initially were JAK2 V617F wild-type became V617F heterozygous, whereas 3 of the 28 who initially were V617F heterozygous became V617F homozygous. When the same samples was reanalyzed by the more sensitive quantitative PCR method, all the 4 patients primarily interpreted as JAK2 V617F negative were identified as JAK2 V617F positive, with a very low V617F alleles burden (median, 2%; range, 1%-5%), which is below the detection limit of the allele-specific PCR used originally. Thus, no patient converted from a wild-type to heterozygous V617F genotype. On the contrary, all the heterozygous to homozygous conversions were confirmed, providing an incidence of progression of 10 per 100 patient-years (95% CI, 2.1-26.5 per 100 patient-years).
Outcome studies
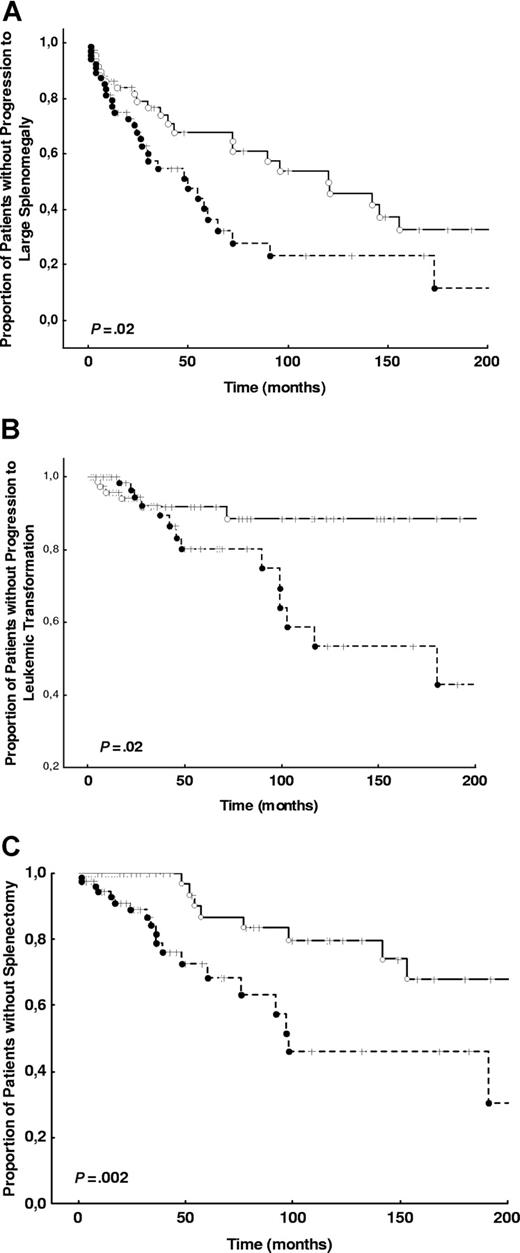
The characteristics and the frequency of outcomes of the 174 consecutive patients prospectively examined for outcome analysis are shown in Table 2. The results of univariate and multivariate outcome analysis are shown in Table 3. The risk of developing severe anemia, thrombocytopenia, and leukopenia were not influenced by JAK2 V617F mutation. There was, however, an increase in the risk to die of any cause, to develop large splenomegaly, to undergo splenectomy, to incur leukemic transformation, and to die of leukemic transformation in patients who were positive for the JAK2 V617F mutation (either heterozygous or homozygous) at diagnosis (Figure 2). When the hazard ratios of these outcomes were adjusted for the conventional risk factors, progression to large splenomegaly, undergoing splenectomy, and developing leukemic transformation were independently predicted by the JAK2 V617F mutation (Table 3). No significant interaction for leukemic transformation was noted between potentially transforming treatments, or splenectomy, and mutation status.
Clinical laboratory features and outcomes of the patients enrolled in the perspective outcome analysis
| . | . | JAK2 V617F nonmutated . | JAK2 V617F mutated . | P* . |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Patients, no. | 174 | 88 | 86 | — |
| Male sex, n (%) | 109 (62.2) | 54 (61.4) | 55 (63.9) | NS |
| Age, y (IQR) | 54 (43-63) | 50 (39-57) | 56 (45-64) | NS |
| Follow-up, mo (IQR) | 26 (7-66) | 24 (7-84) | 28 (7-58) | NS |
| Surviving at 5 y, % (95% CI) | 81.7 (71.4-88.8) | 86.5 (73.2-91.1) | 75 (60-81.2) | .04 |
| Laboratory and clinical features at diagnosis | ||||
| Hemoglobin level, g/L (IQR) | 130 (110-140) | 117 (95-132) | 130 (110-147) | .002 |
| White cell count, × 109/L (IQR) | 8 (6-13) | 7.6 (5.4-10.7) | 9.38 (6.8-13.8) | .02 |
| Immature myeloid cells in peripheral blood, % (IQR) | 2 (0-46) | 3 (1-12) | 2 (0-7) | NS |
| Blasts in peripheral blood, % (IQR) | 0 (0-40) | 0 (1-1.5) | 4 (0-1) | NS |
| Erythroblasts in peripheral blood, % (IQR) | 1 (0-24) | 1 (0-2) | 0 (0-2) | NS |
| Platelet count, × 109/L (IQR) | 347 (197-654) | 341 (187-661) | 354 (203-653) | NS |
| Spleen index, cm2 (IQR) | 150 (100-240) | 140 (100-250) | 160 (106-230) | NS |
| Serum lactate dehydrogenase, U/L (IQR) | 730 (456-1018) | 744 (438-1003) | 730 (469-1045) | NS |
| Dupriez score at diagnosis, no. of patients (%) | ||||
| Grade 0 | 126 (72.4) | 59 (67.1) | 67 (77.9) | NS |
| Grade 1 | 37 (21.3) | 24 (27.2) | 13 (15.1) | NS |
| Grade 2 | 11 (6.31) | 5 (5.7) | 6 (7.0) | NS |
| Events during follow-up | ||||
| Patients treated with a potentially transforming drug for <6 mo at the last follow-up, no. of patients (%) | 66 (47.1) | 31 (35.2) | 35 (40.7) | NS |
| Patients submitted to splenectomy, no. of patients (%) | 29 (16.6) | 9 (10.2) | 20 (23.3) | NS |
| Major thrombotic events, no. of patients (%) | 22 (12.7) | 10 (11.4) | 12 (13.9) | NS |
| Development of large splenomegaly, no. of patients (%)† | 55 (31.6) | 24 (27.3) | 31 (36.0) | NS |
| Development of severe anemia, no. of patients (%)† | 71 (40.8) | 41 (46.6) | 30 (35.3) | NS |
| Development of thrombocytopenia, no. of patients (%)† | 57 (32.8) | 30 (34.1) | 27 (31.4) | NS |
| Development of leukopenia, no. of patients (%)† | 36 (20.9) | 23 (26.1) | 13 (15.1) | NS |
| Leukemic transformation, no. of patients (%) | 21 (12.1) | 6 (6.8) | 15 (17.4) | NS |
| Time from diagnosis to leukemic transformation, mo; median (range) | 42 (22-99) | 9 (6-17) | 47 (28-101) | NS |
| FAB classification of leukemic transformation, no. of patients (%) | ||||
| M0 | 7 (33.3) | 5 (5.7) | 2 (2.3) | NS |
| M1 | 2 (9.5) | 2 (2.3) | 0 | NS |
| M2 | 0 (0) | 0 (0) | 0 (0) | — |
| M3 | 0 (0) | 0 (0) | 0 (0) | — |
| M4 | 0 (0) | 0 (0) | 0 (0) | — |
| M5 | 3 (14.3) | 1 (1.1) | 2 (2.3) | NS |
| M6 | 3 (14.3) | 0 (0) | 3 (3.5) | NS |
| M7 | 6 (28.6) | 4 (4.5) | 2 (2.3) | NS |
| Deaths from leukemic transformation, n (%) | 12 (7) | 4 (4.5) | 8 (9.3) | NS |
| Deaths from all causes, n (%) | 21 (12.1) | 7 (7.9) | 14 (16.2) | NS |
| . | . | JAK2 V617F nonmutated . | JAK2 V617F mutated . | P* . |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Patients, no. | 174 | 88 | 86 | — |
| Male sex, n (%) | 109 (62.2) | 54 (61.4) | 55 (63.9) | NS |
| Age, y (IQR) | 54 (43-63) | 50 (39-57) | 56 (45-64) | NS |
| Follow-up, mo (IQR) | 26 (7-66) | 24 (7-84) | 28 (7-58) | NS |
| Surviving at 5 y, % (95% CI) | 81.7 (71.4-88.8) | 86.5 (73.2-91.1) | 75 (60-81.2) | .04 |
| Laboratory and clinical features at diagnosis | ||||
| Hemoglobin level, g/L (IQR) | 130 (110-140) | 117 (95-132) | 130 (110-147) | .002 |
| White cell count, × 109/L (IQR) | 8 (6-13) | 7.6 (5.4-10.7) | 9.38 (6.8-13.8) | .02 |
| Immature myeloid cells in peripheral blood, % (IQR) | 2 (0-46) | 3 (1-12) | 2 (0-7) | NS |
| Blasts in peripheral blood, % (IQR) | 0 (0-40) | 0 (1-1.5) | 4 (0-1) | NS |
| Erythroblasts in peripheral blood, % (IQR) | 1 (0-24) | 1 (0-2) | 0 (0-2) | NS |
| Platelet count, × 109/L (IQR) | 347 (197-654) | 341 (187-661) | 354 (203-653) | NS |
| Spleen index, cm2 (IQR) | 150 (100-240) | 140 (100-250) | 160 (106-230) | NS |
| Serum lactate dehydrogenase, U/L (IQR) | 730 (456-1018) | 744 (438-1003) | 730 (469-1045) | NS |
| Dupriez score at diagnosis, no. of patients (%) | ||||
| Grade 0 | 126 (72.4) | 59 (67.1) | 67 (77.9) | NS |
| Grade 1 | 37 (21.3) | 24 (27.2) | 13 (15.1) | NS |
| Grade 2 | 11 (6.31) | 5 (5.7) | 6 (7.0) | NS |
| Events during follow-up | ||||
| Patients treated with a potentially transforming drug for <6 mo at the last follow-up, no. of patients (%) | 66 (47.1) | 31 (35.2) | 35 (40.7) | NS |
| Patients submitted to splenectomy, no. of patients (%) | 29 (16.6) | 9 (10.2) | 20 (23.3) | NS |
| Major thrombotic events, no. of patients (%) | 22 (12.7) | 10 (11.4) | 12 (13.9) | NS |
| Development of large splenomegaly, no. of patients (%)† | 55 (31.6) | 24 (27.3) | 31 (36.0) | NS |
| Development of severe anemia, no. of patients (%)† | 71 (40.8) | 41 (46.6) | 30 (35.3) | NS |
| Development of thrombocytopenia, no. of patients (%)† | 57 (32.8) | 30 (34.1) | 27 (31.4) | NS |
| Development of leukopenia, no. of patients (%)† | 36 (20.9) | 23 (26.1) | 13 (15.1) | NS |
| Leukemic transformation, no. of patients (%) | 21 (12.1) | 6 (6.8) | 15 (17.4) | NS |
| Time from diagnosis to leukemic transformation, mo; median (range) | 42 (22-99) | 9 (6-17) | 47 (28-101) | NS |
| FAB classification of leukemic transformation, no. of patients (%) | ||||
| M0 | 7 (33.3) | 5 (5.7) | 2 (2.3) | NS |
| M1 | 2 (9.5) | 2 (2.3) | 0 | NS |
| M2 | 0 (0) | 0 (0) | 0 (0) | — |
| M3 | 0 (0) | 0 (0) | 0 (0) | — |
| M4 | 0 (0) | 0 (0) | 0 (0) | — |
| M5 | 3 (14.3) | 1 (1.1) | 2 (2.3) | NS |
| M6 | 3 (14.3) | 0 (0) | 3 (3.5) | NS |
| M7 | 6 (28.6) | 4 (4.5) | 2 (2.3) | NS |
| Deaths from leukemic transformation, n (%) | 12 (7) | 4 (4.5) | 8 (9.3) | NS |
| Deaths from all causes, n (%) | 21 (12.1) | 7 (7.9) | 14 (16.2) | NS |
Aggregate laboratory and clinical features data are medians.
NS indicates not significant; IQR, interquartile range; and —, not applicable.
Statistical significance of the difference between JAK2 V617F mutated and nonmutated patients.
Development of large splenomegaly was intended as spleen tip extending greater than 10 cm from left costal margin, development of severe anemia was intended as hemoglobin level less than 100 g/L, development of thrombocytopenia was intended as platelet count lower than 150 x109/L, development of leukopenia was intended as white blood cell count lower than 4 × 109 /L.
The genotype at the time of leukemic transformation was measured on the DNA purified from granulocytes. In 12 of the patients, CD34+ cells from peripheral blood were separated, and V617F mutation was detected on the separated cells. In all the cases, CD34+ cells had the same genotype of granulocytes.
Cox regression analysis: hazard ratio of JAK2 V617F mutation for different outcomes
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Univariate | ||
| Death from leukemic transformation | 4.0 (1.1-14.8) | .04 |
| Leukemic transformation | 3.7 (1.4-10.3) | .01 |
| Need of splenectomy | 3.5 (1.5-8.0) | <.05 |
| Death from all causes | 2.5 (1.0-6.2) | <.05 |
| Development of large splenomegaly | 2.0 (1.1-3.5) | .01 |
| Development of thrombocytopenia | 1.9 (0.8-4.5) | .14 |
| Major thrombosis | 1.7 (0.5-5.5) | .34 |
| Development of severe anemia | 1.3 (0.7-2.7) | .38 |
| Development of leukopenia | 0.7 (0.3-1.6) | .41 |
| Adjusted | ||
| Death from leukemic transformation | 3.2 (0.8-12.1) | .09 |
| Leukemic transformation | 5.2 (1.6-16.8) | <.01 |
| Need of splenectomy | 2.9 (1.2-6.8) | .01 |
| Death from all causes | 2.3 (0.8-6.1) | .10 |
| Development of large splenomegaly | 1.7 (1.0-3.0) | .05 |
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Univariate | ||
| Death from leukemic transformation | 4.0 (1.1-14.8) | .04 |
| Leukemic transformation | 3.7 (1.4-10.3) | .01 |
| Need of splenectomy | 3.5 (1.5-8.0) | <.05 |
| Death from all causes | 2.5 (1.0-6.2) | <.05 |
| Development of large splenomegaly | 2.0 (1.1-3.5) | .01 |
| Development of thrombocytopenia | 1.9 (0.8-4.5) | .14 |
| Major thrombosis | 1.7 (0.5-5.5) | .34 |
| Development of severe anemia | 1.3 (0.7-2.7) | .38 |
| Development of leukopenia | 0.7 (0.3-1.6) | .41 |
| Adjusted | ||
| Death from leukemic transformation | 3.2 (0.8-12.1) | .09 |
| Leukemic transformation | 5.2 (1.6-16.8) | <.01 |
| Need of splenectomy | 2.9 (1.2-6.8) | .01 |
| Death from all causes | 2.3 (0.8-6.1) | .10 |
| Development of large splenomegaly | 1.7 (1.0-3.0) | .05 |
*Hazard ratios were adjusted for age, sex, hemoglobin level, white blood cell count, percentage of blasts in peripheral blood, and Dupriez risk score.
Kaplan-Meier plots of the time to large splenomegaly development, time to splenectomy, and time to leukemic transformation in JAK2 V617F-negative and JAK2 V617F-positive groups. (A) The time to large splenomegaly development is shown in the population selected for outcome analysis (a total of 174 patients) for patients who were JAK2 V617F negative or JAK2 V617F positive at the diagnosis. (B) The time to splenectomy in both groups is shown. (C) The time to leukemic transformation in both groups is shown. P values for all analyses are from log-rank tests. ---- indicates JAK2 V617F-positive; —, JAK2 V617F-negative.
Kaplan-Meier plots of the time to large splenomegaly development, time to splenectomy, and time to leukemic transformation in JAK2 V617F-negative and JAK2 V617F-positive groups. (A) The time to large splenomegaly development is shown in the population selected for outcome analysis (a total of 174 patients) for patients who were JAK2 V617F negative or JAK2 V617F positive at the diagnosis. (B) The time to splenectomy in both groups is shown. (C) The time to leukemic transformation in both groups is shown. P values for all analyses are from log-rank tests. ---- indicates JAK2 V617F-positive; —, JAK2 V617F-negative.
Discussion
We have found that V617F mutation of the JAK2 gene is present in more than 63% of patients with primary myelofibrosis. We have documented that none of the V617F-negative patients who were sequentially genotyped acquired the mutation. This result is consistent with those reported in polycythemia vera26 and in essential thrombocythemia27 but contrasts with a previous report in primary myelofibrosis,28 in which 1 of 16 patients progressed from a wild-type to a heterozygous V617F genotype. However, the evidence of genetic progression was obtained by an allele-specific PCR method that has the chance to miss a very small amount of mutated V617F alleles, thus producing false-negative results at the baseline sample, as we documented in 4 of 36 patients.
We have documented that the JAK2 V617F mutation plays a significant and independent influence on the disease phenotype that is correlated with the expansion of clonal hematopoietic cells harboring the JAK2 mutant allele. Any level of mutant alleles favors higher concentration of hemoglobin and aquagenic pruritus. Low levels, as in the heterozygous state, favors higher platelet count, whereas high levels, as in the homozygous state, favor a hyperproliferative profile that was substantiated by higher white blood cell count, larger splenomegaly, and greater need of cytoreductive therapy. These results are generally in keeping with those reported in polycythemia vera29,30 and essential thrombocythemia,29,31,32 but they were only partially reported in primary myelofibrosis.15,16 What resulted originally in our results was the role of homozygous mutation on hyperproliferative characteristics of the disease (ie, high number of white blood cells and splenomegaly). These characteristics maintained their association with homozygous V617F mutation when submitted to multivariate analysis and corrected for confounding factors such as disease duration, age, sex, and therapy. These results confirm those in murine models mimicking the homozygous condition in which erythrocytosis and leukocytosis, but not thrombocytosis, develop after transplantation with JAK2V617F-transfected cells33-35 and shed light on the role JAK2 V617F plays in the phenotypic progression of the disease. In particular they point to a hyperproliferative disease profile as the clinical counterpart of the mitotic recombination that sustains the genetic conversion to a homozygous state.36
Insight on the phenotypic disease progression is further provided by the perspective outcome study. Development of severe anemia, thrombocytopenia, or leukopenia was not influenced by the JAK2 V617F mutational status at diagnosis. On the contrary, development of large splenomegaly, requirement of splenectomy, and leukemic transformation was more frequent in patients with a mutated genotype at diagnosis. The strongest influence of the mutated genotype was exerted on the risk of leukemic transformation that was 5.2 times as great as in nonmutated patients. The risk remained significant after adjustment for age, sex, Dupriez risk score, white blood cell count, and percentage of blasts in peripheral blood, the known main risk factors for leukemic transformation in primary myelofibrosis.5,7,37 We acknowledge that other predictors not tested might have provided additional information. We did not adjust for the other reported risk factor (ie, cytogenetic abnormalities), because we did not have statistical power to include this biomarker in the multivariate analysis.
Seventeen of 20 (85%) V617F-mutated patients who incurred splenectomy had a homozygous mutation at the time of splenectomy and 12 of 15 (80%) mutated patients who transformed into acute leukemia displayed a homozygous mutation at the time of leukemia. This corroborates the model in which the evolution from heterozygous to homozygous mutation (ie, the accumulation of a high load of mutated alleles) is a critical step in the progression of the disease as has been reported for the evolution toward secondary myelofibrosis among patients with polycythemia vera or essential thrombocythemia.29-32 Because this genetic conversion occurred at a rate of approximately 10 per 100 patient-years, we can speculate that it represents a major determinant for the disease evolution.
In our series, 6 of 21 leukemic transformations occurred in patients not bearing the JAK2 V617F mutation. This indicates that V617F mutation is not the sole mechanism of leukemogenesis in primary myelofibrosis, in accord with the known biology of leukemic transformation in which a number of genetic alterations contribute to the evolution to acute leukemia.
In conclusion, the JAK2 mutant represents the first biologic marker useful for stratify the risks in primary myelofibrosis, which is independent from conventional predictors. Such a stratification might be conveniently used for patient enrollment in clinical trials that use novel drugs, eventually including those targeting the constitutively active JAK2 kinase.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the European LeukemiaNet within the 6th European Community Framework Programme for Research and Technological Development; a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC); and a grant from Ministero dell'Istruzione, Università e Ricerca (MIUR #06488803).
Authorship
Contribution: G. Barosi designed the study, collected samples from patients in Pavia, and wrote the manuscript; G. Bergamaschi contributed to the JAK2 V617F mutation detection by allele-specific PCR; M. Marchetti contributed to study design, helped with collection of samples and clinical information, and contributed in writing the manuscript; A.M.V. helped with sample collection in Florence and contributed to JAK2 V617F mutation detection by quantitative PCR; P.G. and E.A. helped with sample collection in Florence; M. Massa helped to design the study; V.R. helped to organize the collection of samples in Pavia and to design of the study; R.C. helped with collection of clinical information; L.V. helped with the JAK2 V617F mutation detection by allele-specific PCR; G.V. helped with the assay of CD34+ cells; E.G. helped with collection of clinical information for patients from Pavia; G.G. helped with sample collection in Milan; G.S. helped with sample collection in Bari; C.T. performed statistical analysis; A.R. contributed to the design of the study and writing the manuscript; and T.B. contributed to the design of the study and writing the manuscript.
A complete list of members of the GIMEMA-Italian Registry of Myelofibrosis is provided in Document S1.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanni Barosi, Laboratorio di Epidemiologia Clinica, Fondazione IRCCS Policlinico S. Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail barosig@smatteo.pv.it.