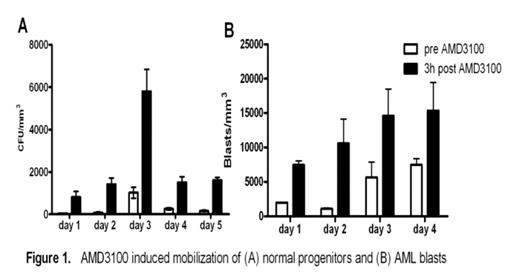
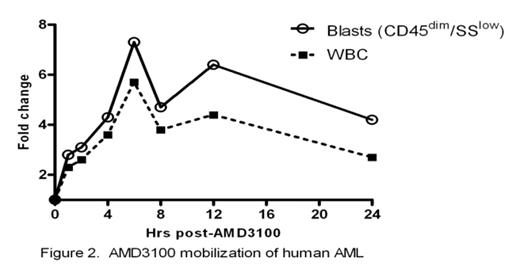
The CXCR4-SDF-1 axis possesses a central role in the trafficking and retention of both normal and malignant stem cells in the bone marrow. Previous work from our laboratory established that in a murine model, a single dose of the CXCR4 antagonist, AMD3100, sensitizes AML blasts to chemotherapy supporting the premise that the interaction between AML blasts and the marrow microenvironment confers resistance to genotoxic stress (Nervi et al., ASH 2006). Here we examine the effects of repetitive dosing of AMD3100 on the kinetics of normal and leukemic mobilization. Following SQ injection of AMD3100 5mg/kg into B6/129 F1 mice daily for 5 days (n=8), we observed a 2.4 fold increase in total leukocyte counts with a 12.4 increase in CFU-GM when compared to 3 hours post injection (Fig 1A). No differences were seen in the degree of mobilization between d1 and d5 with WBC and CFU-GM counts returning to baseline after 24 hours. We next tested repetitive doses of AMD3100 in our mouse model of AML in which 106 blasts derived from leukemic mice carrying the PML-RARα fusion gene in the murine cathepsin G locus are adoptively transferred into genetically compatible secondary recipients. AMD3100 at 5mg/kg was then administered to these AML mice for 4 consecutive days. At 3 hrs post AMD3100 injection, we observed a 1.8 fold increase in peripheral leukocyte counts with a 4.5 fold increase in circulating blasts compared to baseline (n=3). Again, no significant differences are seen in the degree of mobilization from d1 to d4 (Fig 1B). Based on these preclinical data, we have initiated a phase I/II trial of AMD3100 plus mitoxantrone, etoposide and cytarabine (MEC) in relapsed or refractory AML in which AMD3100 is administered 4 hours prior to MEC daily for 5 consecutive days. To study the kinetics of human AML mobilization, we administered AMD3100 by SQ injection followed by 24hr observation period prior to chemotherapy. Two patients have been treated at the first dose level of AMD3100, 80 μg/kg. In pt #1 following AMD3100 mobilization, total WBC increased from 3 × 103/mm3 to a peak of 17 × 103/mm3 at 6 hours post-AMD3100 representing a 5.7 fold increase in total white count (Fig 2). In addition, the blasts (CD45dim, SSlow) increased by 7.3 fold. Similarly in pt #2, we observed a 2 fold increase in the total WBC from 2.5 to 5.1 × 103/mm3 with a 2.3 fold increase in blasts (CD45dim, SSlow). Mobilization of AML was confirmed in both patients through informative FISH for 11q23 (MLL). No adverse events have been observed during mobilization. These data provide the preclinical rationale for repetitive dosing of AMD3100 and direct clinical evidence that AMD3100 mobilizes human AML blasts into the peripheral circulation. Our trial of AMD3100 plus MEC in relapsed or refractory AML is ongoing.
AMD3100 induced mobilization of (A) normal progenitors and (B) AML blasts
Disclosure:Consultancy: CNA has served as a consultant to Genzyme. Research Funding: Support for clinical trial provided by Genzyme. Honoraria Information: JFD has received honoraria from AnorMED and Genzyme. Off Label Use: Off-label use of AMD3100.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal