Abstract
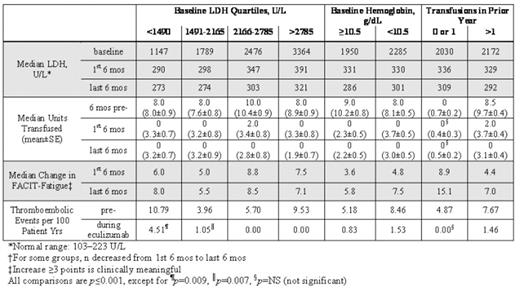
Paroxysmal nocturnal hemoglobinuria (PNH) is a debilitating and life-threatening disease. However, disease burden in patients with lower lactate dehydrogenase (LDH), higher hemoglobin and minimal transfusion support has not been determined. The terminal complement inhibitor, eculizumab (Soliris™), was shown to significantly reduce hemolysis, improve anemia and fatigue, and reduce thrombosis in a broad PNH population. We examined the long-term efficacy of eculizumab in multiple patient subgroups. Efficacy data included hemolysis (as assessed by LDH), transfusion requirements, fatigue (measured by Functional Assessment of Chronic Illness Therapy-Fatigue instrument, FACIT-Fatigue), and thrombosis. Data were analyzed by lower baseline hemolysis (LDH <1490 U/L, n=46), mild anemia (Hgb ≥10.5 g/L, n=54), and minimal transfusion (0 or 1 episodes in prior yr, n=21) among 187 PNH patients who had been enrolled in eculizumab trials (Phase 2 Pilot, Phase 3 TRIUMPH and SHEPHERD studies) and who continued to receive eculizumab in a long-term extension trial (median duration, 22 months). Eculizumab provided significant clinical benefit to patients regardless of baseline degree of hemolysis, anemia, or transfusion requirement (Table). Fatigue improved with eculizumab across all baseline LDH quartiles. Of interest, reduced hemolysis with eculizumab improved fatigue even in patients with only mild anemia and little or no transfusion requirement, suggesting that lowering hemolysis improves fatigue, independent of anemia. Fatigue scores increased during the first 6 mos of treatment and continued to improve throughout the last six months of treatment. Transfusion requirements also maintained during long-term treatment; patients requiring >1 transfusion in the year prior received a median of 8.5 units in the 6 months prior to eculizumab, 2.0 units in the first 6 months of treatment, and 0 units in the last 6 months of treatment. In patients with evidence of marrow failure (n=25; platelets <100,000/mm3 and no prior thrombosis) compared with pre-treatment, hemolysis (LDH) was reduced by 1217±211 U/L, median units transfused were reduced from 8 (mean 9.2±1.2) to 0 (4.4±1.4) and fatigue improved by 8.1±2.1 points (p≤0.001 for each) during the last six mos of eculizumab therapy. Thromboembolism (TE) was examined in all eculizumab trial patients (N=195). The TE rate was elevated in the pre-treatment period for patients with lower baseline hemolysis, mild anemia and minimal transfusion; eculizumab reduced TE in these patient subgroups by 58% (p=0.009, n=48), 84% (p<0.001, n=55) and 100% (p=0.063, n=22), respectively. In conclusion, patients who may be expected to have less severe disease suffer from significant disease burden, and long-term treatment with eculizumab provides substantial clinical improvement.
Author notes
Disclosure:Employment: Dr Rollins is an employee of Alexion Pharmaceuticals, Inc. Consultancy: Drs. Bessler, Schrezenmeier, and Hill have served as consultants for Alexion Pharmaceuticals, Inc. Ownership Interests:; Dr Rollins owns stock in Alexion Pharmaceuticals, Inc. Honoraria Information: Drs. Schrezenmeier, Maciejewski, Hill, and Luzzatto have received lecture fees from Alexion Pharmaceuticals, Inc. Membership Information: Drs. Bessler, Maciejewski, Hill, and Luzzatto have served on advisory boards for Alexion Pharmaceuticals, Inc.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal