Abstract
Introduction 1-Desamino-8-D-Arginine Vasopressin (DDAVP) is the mainstay of treatment for mild Type I von Willebrand Disease (vWD). The use of a DDAVP challenge is standard practice in the management of newly diagnosed patients. Pre- and Post- DDAVP administration laboratory studies for vWF: RCoF, vWF: Ag and FVIII:C are expected to rise 2–5 fold from baseline. PTT and Closure Time values would be expected to correct. In a subset of our patients, pre- and post- Platelet Function studies were also tested. We sought to retrospectively study responses in our vWD patient population in terms of time-related and intensity of incremental response to DDAVP.
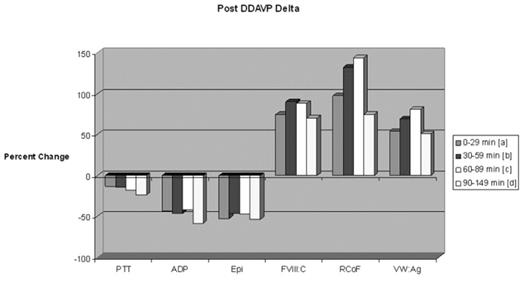
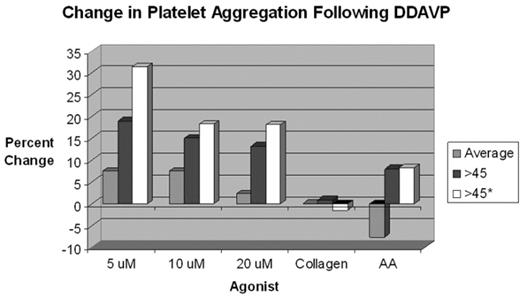
Results Closure Time prolongation was noted in over 55% of patients with vWD. The Collagen/Epi time appears to be more sensitive for the vWD defect (Collagen/Epi was more frequently prolonged than Collagen/ADP). In vWD patients with Closure Time defects at baseline, 86% were corrected following DDAVP administration, and this correction appears stable over all time points evaluated. Post-DDAVP laboratory responses for vWF: RCoF, vWF: Ag and FVIII:C, respectively: 9.1%, 31.2%, and 16.9% incremented by <50%; 24.7%, 33.8%, and 45.5% incremented by 50–99%; 36.4%, 33.8%, and 28.6% incremented by 100–199%; 18.2%, 0%, and 5.2% incremented by 200–299%; and 11.7%, 1.3% and 3.9% incremented by ≥300%. Peak responses occurred in the 60–89 minute post-DDAVP sampling period (see Figure 1). More than 92% of individuals tested achieved a post-DDAVP vWF: RCoF value of >0.80 U/mL. About 30% and 47.8% of individuals had a prolonged APTT and APTT Mix prior to DDAVP; only 7.8% and 16.7%, respectively, remained prolonged afterwards. There was a trend toward improvement in platelet aggregation variables over time (see Figure 2).
Conclusions Baseline Closure Time abnormalities occurred in over half of the patients and were reliably corrected in most following DDAVP administration. Consistent with established experience, DDAVP produces reliable vWF: RCoF, vWF: Ag and FVIII:C responses in patients with mild Type 1 vWD. Platelet function studies trended toward improvement at greater than 45 minutes post-DDAVP challenge, however, very few patients were studied pre- and post- for platelet function studies and therefore statistical significance was not achieved. Although all patients undergoing DDAVP challenge are asked to avoid antiplatelet drugs, inadvertent use may explain residual Closure Time and Platelet Function test abnormalities.
Post DDAVP Delta
Change in platelet aggregation Following DDAVP
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal