Abstract
Background: In both polycythemia vera (PV) and essential thrombocythemia (ET), aspirin and/or hydroxyurea therapy provide adequate protection from thrombosis but not from disease transformation into acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) or myelofibrosis (MF). In designing clinical trials with new drugs, including the use of small molecule JAK2 inhibitors, in either ET or PV, it is important to identify patients who are at a higher risk of early transformation into AML/MDS/MF as well as those in whom the long-term risk of these complications is too low to justify exposure to experimental drugs with unknown short-term and long-term toxicity profile.
Methods: Information on an institutional database of 1061 patients with either PV (n=458) or ET (n=603) was updated in July of 2007. The World Health Organization criteria were used for diagnosis of PV, ET, AML, MDS, and MF. Two different approaches were used to assess both early and overall risk of AML/MDS/MF development in both ET and PV. In the first approach, three distinct groups were delineated and their presenting features compared; group A was comprised of patients who have remained AML/MDS/MF free after a minimum follow-up of 20 years whereas groups B and C included patients who developed either AML/MDS or MF, respectively, in the first decade of their disease. In the second approach, prognostic factors for AML/MDS/MF-free survival were examined among the entire cohort of 1061 patients.
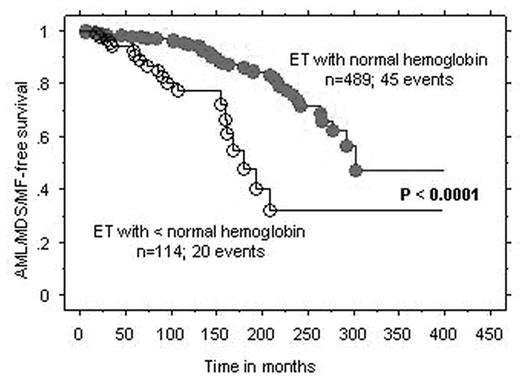
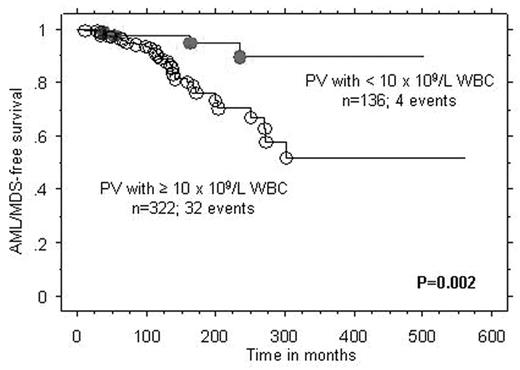
Results : In ET, the number of patients that fulfilled the above-mentioned criteria for inclusion in groups A, B, and C were 40, 12, and 8; the three groups displayed significant differences in the prevalence, at diagnosis, of lower than normal hemoglobin level (8% vs. 58% vs. 38%; p=0.0004), male sex (18% vs. 58% vs. 50%; p=0.01), and arterial thrombosis at diagnosis (p=0.03), respectively. However, only the former two remained significant during multivariable analysis that included age as a covariate. In PV, the number of patients in groups A, B, and C were 23, 18, and 12; the only difference between the three groups was the association between group B and leukocytosis (p=0.02). Cox regression analysis of the entire 1061 ET/PV patients cohort, including 65 AML/MDS/MF events in ET and 36 AML/MDS events in PV, confirmed the inferior AML/MDS/MF-free survival in ET associated with lower than normal hemoglobin and AML/MDS-free survival in PV associated with leukocytosis (Figure).
Conclusion : The current study identifies PV patients with leukocytosis as being the most appropriate and ET patients without anemia as the least appropriate for consideration of participation in experimental drug treatment trials.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal