Abstract
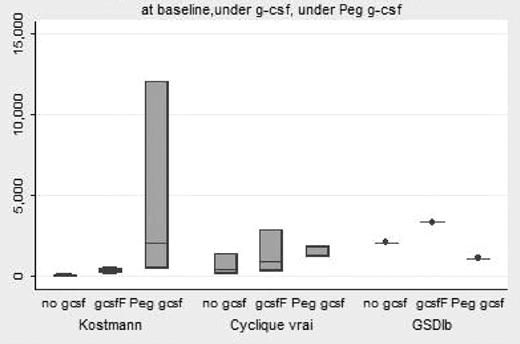
PegFilgrastim is registered in France since 2002. Its pharmacokinetic characteristic allows the possibility to limit the number of injections in patients with congenital neutropenia receiving long term G-CSF therapy. To date, no report has been issued about the outcome of patients with congenital neutropenia receiving this drug. At the cut-off date of July 31th, 2007, 339 patients with congenital neutropenia had been included in the French Severe chronic neutropenia (Severe congenital neutropenia n=142, WHIM n=16, Cyclic n=75; GSDIb n=16 SDS n=90). Eight patients (SCN n=4, Cyclic n=3 and GSDIb n=1) had been identified to receive PegFilgrastim. Median age at PegFilgrastim onset was 18.2 years (min 6.1-max 37.6). In 7 cases, PegFilgrastim was given in the continuation of Filgrastim (n=3) or Lenograstim(4), with a median delay of 13 years after G-CSF onset and in one case, PegFilgrastim was the initial prescription of cytokine. Dose of PegFilgrastim was usually a vial per injection, except in one child (1/2vials) resulting in a dose per injection between 90 and 286 μg/kg per injection. The rhythm of PegFilgrastim injection varied from 1 injection every 7 days until 1 injection every 14 days, with discontinuation period. The median duration (between onset of PegFilgrastim and the last dose) was 0.7 year (min 0.3–2.7). The efficiency measured by ANC criteria appears to better than G-CSF (fig 1: baseline Median ANC 156/mm3, Under G-CSF 501/mm3, Under PegFilgrastim 1901/mm3/ Kruskall Wallis test p=0.02). Severe infection was never reported under PegFilgrastim, but neither in the previous G-CSF therapeutic periods for the 7 patients. More frequent ENT infection was observed in one patient who consider the PegFilgrastim as less efficient than G-CSF. The side effects appeared to be more frequent compare to the G-CSF therapy. Bone pains were reported in 7 patients, anemia and thrombocytopenia (WHO gr II) in one, urticarian chronic skin rash in 3. Finally, at the last update, 7 patients had withdrawn PegFilgrastim, and had resumed G-CSF therapy. Only one patient is still receiving PegFilgrastim. In conclusion, PegFilgrastim appeared efficient in Congenital neutropenia, but more frequent short term side effects were observed compare to standard G-CSF therapy, resulting in a limited quality of life improvement, leading to drug withdrawn in most of the patients.
Median ANC by diagnosis
Author notes
Disclosure:Research Funding: The French SCN registry is an independant organisation. It had received grants from Amgen Inc.; Chugai Inc, the GIS maladies rares (publich fund), the association L Fugain and the association B Gonnot.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal