Abstract
Background. SAA patients were transplanted from unrelated donors on two regimes. Regimen A, is a combination of fludarabine (FLU) 30 mg/m^2 /day ×4, cyclophopshamide (CY) 300 mg/m^2/day ×4 and anti-thymocyte globulin (ATG) (Genzyme, USA) 3.75 mg/kg ×4. Regimen B includes FLU -CY at the same dose as Regimen A, with the addition of total body irradiation (TBI) 2 Gy, on day 0,and a reduced does of ATG (7.5 mg/kg). Graft-versus-host disease (GvHD) prophylaxis was cyclosporin and short methotrexate.
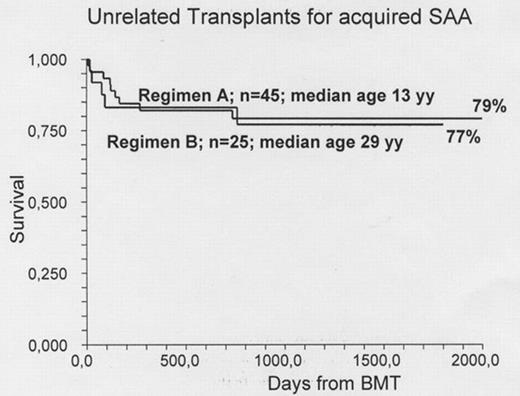
Patients. We are now reporting 70 consecutive patients. Clinical data and major outcome measures are detailed in the Table. The major difference is that Regimen A included mainly children, the median age being 13 years, with only 3 patients over the age of 30, whereas Regimen B was designed for adults, with the addition of low dose TBI becauses of increase rejection: the median age for Regimen B is 29 years, with only one patient under 16. All patients had failed at least 1 course of immunosuppressive treatment. Graft Rejection was seen in 20% of patients receiving Regimen A: 6/22 for patients >13 years of age (27%) and 3/23 for patients aged <=13 years (12%). Rejection was seen in 3 patients receiving Regimen B (12%). Acute GvHD grade II-IV was scored in 11% and 12% in the two regimens respectively. Chronic GvHD was scored as limited/ extensive in 14%/2% of patients in Regimen A ; these percentages were 27% and 9% for Regimen B.. Survival: The overall actuarial survival is 78% and 77% respectively. In Regimen A survival was 91% vs 67% for patients aged </=>13 years. It was 87% for patients transplanted before the median interval from diagnosis (496 days), compared with 73% for patients grafted later. In Regimen B there was no effect of age on outcome; survival was 92% vs 70% for patients grafted within or beyond the median interval diagnosis-BMT. Causes of death in Regimen A were as follows: graft failure (n= 6), EBV-related lymphoprolipherative disease (n=2) and evolution to myelodysplasia (n=1). For Regimen B causes of death were: graft failure (n=2), infections (n=2) and GvHD (n=1).
Conclusion: The FLU-CY-ATG conditioning regimen seems suitable for young children with acquired SAA undergoing an UD transplant. In patients above the age of 13 years, graft rejection remains a problem. The addition of low dose TBI, reduces graft rejection and produces encouraging outcome in adults with acquired SAA. The current survival exceeding 75%, the low incidence of acute and chronic GvHD, and the negative effective of delayed transplantation, suggest the opportunity to consider UD BMT early in the treatment strategy of patients with acquired SAA.
Clinical data of patients prepared for transplant with Regimen A or Regimen B
| . | Regimen A: FLU CY ATG . | Regimen B: FLU CY ATG + TBI 2 Gy . |
|---|---|---|
| Patients | 45 | 25 |
| Median age (yy) | 13 (3–56) | 29 (7–49) |
| Median Interval Dx-Tx (dd) | 496 | 572 |
| Unrelated donor | 37 | 20 |
| Family mismatched donor | 8 | 5 |
| Stem cell source: BM | 41 | 25 |
| Median follow up (dd) | 1160 (6–3364) | 765 (151804) |
| Graft Rejection | 9 (20%) | 3 (12%) |
| Acute GvHD II-IV | 6 (13%) | 3 (12%) |
| Deceased | 9 (20%) | 5 (20%) |
| Actuarial survival | 79% | 77% |
| . | Regimen A: FLU CY ATG . | Regimen B: FLU CY ATG + TBI 2 Gy . |
|---|---|---|
| Patients | 45 | 25 |
| Median age (yy) | 13 (3–56) | 29 (7–49) |
| Median Interval Dx-Tx (dd) | 496 | 572 |
| Unrelated donor | 37 | 20 |
| Family mismatched donor | 8 | 5 |
| Stem cell source: BM | 41 | 25 |
| Median follow up (dd) | 1160 (6–3364) | 765 (151804) |
| Graft Rejection | 9 (20%) | 3 (12%) |
| Acute GvHD II-IV | 6 (13%) | 3 (12%) |
| Deceased | 9 (20%) | 5 (20%) |
| Actuarial survival | 79% | 77% |
Figure
Author notes
Disclosure:Research Funding: Research funding from Pfizer, Roche, Amgen. Honoraria Information: Speakers agreement. Membership Information: Speakers agreement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal