Abstract
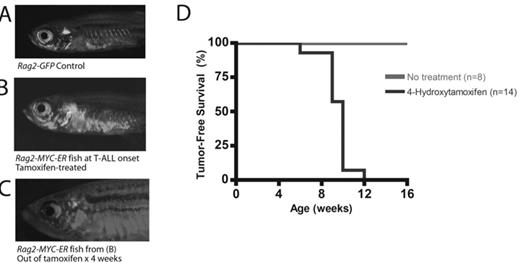
The MYC oncogene plays a central role in the pathogenesis of human T cell acute lymphoblastic leukemia (T-ALL), and our laboratory has previously developed a zebrafish model of Myc-induced T-ALL. The primary strength of the zebrafish as a model system for human disease lies in is its suitability for unbiased forward genetic and small molecule screens. Our central hypothesis is that forward screens performed using our zebrafish model of MYC-induced T-ALL will lead to the identification of entirely novel genes and pathways that play critical roles in MYC-induced leukemogenesis. However, zebrafish from our original line develop rapidly progressive T-ALL prior to achieving reproductive maturity, making this line poorly suited for the performance of large-scale screens. Therefore, a conditional model was required. We have now generated a transgenic zebrafish line that expresses a human MYC-estrogen receptor fusion construct under the control of the zebrafish recombination activating gene 2 (Rag2) promoter, which is lymphocyte-specific. When mated against fish transgenic for a Rag2-GFP transgene, the development and progression of T-ALL can be readily tracked in live fish by fluorescent microscopy. Upon treatment with 4-hydroxytamoxifen (4HT), zebrafish from this line develop fully penetrant T-ALL, with a mean time to tumor onset of 8 weeks. Additionally, removal from 4HT invariably led to complete morphologic remission in leukemic zebrafish from this line, and all of these fish remained alive and were able to mate successfully for greater than 6 months after removal from 4HT. This conditional zebrafish model of MYC-induced T-ALL will now allow the successful performance of forward genetic and small molecule screens to identify known and novel genes and pathways that play critical roles in T-ALL leukemogenesis and MYC-induced transformation.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal