Abstract
Background. Intracellular one-carbon transfer reactions are essential for nucleotide synthesis and methylation of biologic compounds including DNA. Previous studies have linked genetic variants in one-carbon metabolism genes with risk of developing NHL, but little is known regarding the impact of these variants on disease outcome. We evaluated the hypothesis that inherited genetic variation in genes involved in one-carbon metabolism is associated with overall survival in DLBCL.
Methods. We genotyped 30 single nucleotide polymorphisms (SNPs) from 18 candidate one-carbon metabolism genes in 215 DLBCL patients who participated in a population-based case-control study conducted from 1998–2000 using the SEER (Surveillance, Epidemiology and End Results) cancer registries in the Detroit, Iowa, Los Angeles and Seattle. Stage, B-symptoms, first course of therapy, date of last follow-up and vital status through early 2005 were obtained from cancer registry files. Cox proportional hazards analysis was used to estimate hazard ratios (HR) and corresponding 95% confidence intervals for the association between individual SNPs and overall survival, adjusting for age, demographic and clinical factors. We also used parallel modeling strategies to identify the best summary multi-SNP risk score to predict survival.
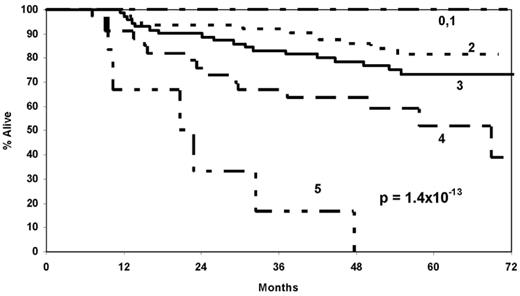
Results. The median age at diagnosis was 57 years (range, 20–74), and 50 (23%) of the patients died during follow-up, with a median follow-up of 57 months (range, 31–78 months) for surviving patients. After adjusting for demographic and clinical variables, SNPs in SHMT1 (rs1979276; HRCT/TT=2.47, 1.31–4.67), BHMT (rs585800; HRAT/TT=2.02, 1.16–3.54), and TCN1 (rs526934; HRTT=1.86, 1.04–3.33) were the strongest and most robust predictors of survival. A summary score of the number of deleterious genotypes (0–3) from these three genes was strongly associated with survival (p=7.9 x 10−5) after accounting for demographic and clinical variables (HR=2.58 per deleterious genotype, 95% CI 1.75–3.80). A risk score combining the three SNPs with clinical and demographic variables (score of 0 to 5) was even more strongly associated with survival (p=1.4 x 10−13); the Kaplan Meier survival curves are shown in the Figure. In a time-dependent ROC analysis, the combined risk score had a concordance index of 0.75 at 5 years of follow-up (95% CI 0.69–0.81).
Conclusion: Host genetic variation in the one-carbon metabolism genes SHMT1, BHMT, and TCN1, individually and particularly in combination, was associated with overall DLBCL survival after accounting for clinical and demographic factors, supporting a role for this pathway in disease progression. Future work should evaluate interactions of genes from this pathway with dietary nutrients and therapeutic agents in DLBCL prognosis.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal