Abstract
Iron overload at the time of bone marrow transplantation has recently been associated with increased mortality. The mechanism by which iron overload negatively impacts survival is unclear. In an effort to better define iron overload and its potential role in the transplant setting, we examined 78 consecutive patients at risk for transfusion-related iron overload (AML, ALL, MDS, and aplastic anemia) undergoing either autologous or allogeneic stem cell transplant at our institution between 1999 and 2004. To estimate transfusion-related iron overload, we devised a novel scoring system based on routinely available laboratory variables obtained prior to transplant. In our scoring system, a single point was awarded for each of the following:
number of red cell units transfused prior to transplantation > 25;
serum ferritin > 1000 ng/ml; and
a semi-quantitative bone marrow iron stain of 6+.
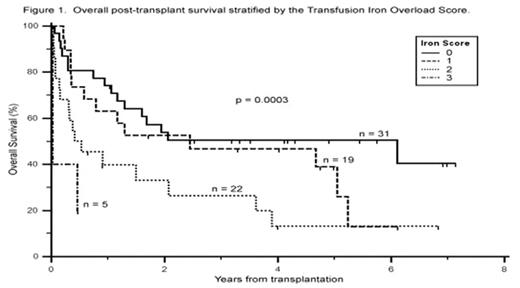
The Transfusion Iron Overload Score (range of 0 to 3) predicted for overall survival in our patient sample (Fig. 1, p = 0.0003 by Kaplan-Meier log rank trend) and was more powerful than using serum ferritin alone (p = 0.02). The calculated score retained its significant association with overall survival while controlling for traditional risk factors (age, sex, diagnosis, transplant type, donor relation, and remission status), inflammation (c-reactive protein), and measures of organ damage (transaminases, total bilirubin, INR, and cardiac ejection fraction) (p = 0.04 by Cox proportional-hazards regression). The 27 patients (35%) with a high score (≥ 2) had a dramatically lower median survival of 5.0 months compared to 29.3 months in those with an iron score of less than two (unadjusted hazard ratio of 2.5; 95% CI of 1.6 to 6.3). The observed mortality seen in those with a high score was primarily attributable to treatment-related mortality (p = 0.018) rather than disease-related mortality (p = 0.48). This finding likely accounts for the significant survival impact of iron overload seen in the allogeneic (p = 0.006) but not the autologous (p = 0.48) subgroup. In conclusion, the Transfusion Iron Overload Score served as a strong independent predictor of survival in our stem cell transplant population, identifying patients at significant risk for early treatment-related mortality. Our results lend further support to the notion that iron overload is detrimental in the transplant setting, and for the first time suggest that transfusion-related iron overload increases mortality via a mechanism independent of pre-existing inflammation and end-organ dysfunction.
Overall post-transplant survival by the Transfusion Iron Overload Score.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal