Abstract
Plasminogen (Plg) facilitates inflammatory cell recruitment, a function that depends upon its binding to Plg receptors (Plg-Rs). However, the Plg-Rs that are critical for cell migration are not well defined. Three previously characterized Plg-Rs (α-enolase, annexin 2, and p11) and a recently identified Plg-R (histone H2B [H2B]) were assessed for their contribution to Plg binding and function on macrophages. Two murine macrophage cell lines (RAW 264.7 and J774A.1) and mouse peritoneal macrophages induced by thioglycollate were analyzed. All 4 Plg-Rs were present on the surface of these cells and showed enhanced expression on the thioglycollate-induced macrophages compared with peripheral blood monocytes. Using blocking Fab fragments to each Plg-R, H2B supported approximately 50% of the Plg binding capacity, whereas the other Plg-Rs contributed less than 25%. Anti-H2B Fab also demonstrated a major role of this Plg-R in plasmin generation and matrix invasion. When mice were treated intravenously with anti-H2B Fab, peritoneal macrophage recruitment in response to thioglycollate was reduced by approximately 45% at 24, 48, and 72 hours, with no effect on blood monocyte levels. Taken together, these data suggest that multiple Plg-Rs do contribute to Plg binding to macrophages, and among these, H2B plays a very prominent and functionally important role.
Introduction
In addition to the essential role of plasminogen (Plg) in fibrinolysis, nonfibrinolytic functions have been ascribed to the zymogen and/or its active protease plasmin (Plm).1-4 Both in vitro and in vivo studies have implicated Plg in cell migration in a variety of physiologic and pathophysiologic settings, including inflammatory cell recruitment,5-7 tumor cell invasion,8-10 and endothelial cell and smooth muscle cell migration.11-14 Integral to these responses is the binding of Plg to cell surfaces, which is mediated by a heterogeneous set of Plg receptors (Plg-Rs). Interaction of Plg with these cell-surface binding sites accelerates conversion of Plg to Plm,15-18 enhances the catalytic activity of Plm itself,19 and protects bound Plm from inactivation by inhibitors.15,20 Thus, Plg-Rs endow the cell surface with the broad and sustained proteolytic activity of Plm
Plg-Rs are broadly distributed; they are present at high densities on platelets, monocytes, macrophages, neutrophils, endothelial cells, and many transformed cells.21 Multiple Plg-Rs have been identified; many contain C-terminal lysines, which interact with the lysine binding sites (LBSs) of Plg. As a consequence of this similar recognition mechanism, despite their heterogeneity, the affinity of Plg for its receptors is similar1 ; lysine analogues, ϵ-aminocaproic acid (EACA), and tranexamic acid (TXA) block interactions and treatment with basic carboxypeptidases reduces Plg binding and activation.22,23
Monocytes/macrophages play a central role in pathogenic inflammatory responses associated with atherosclerosis, restenosis,24,25 tumor surveillance,26 and arthritis.27 The importance of Plg in monocyte/macrophage recruitment has been demonstrated in distinct inflammatory models conducted in Plg-deficient mice and in a variety of in vitro studies using monocyte/macrophage cell lines.1,28 Such cell lines have been used frequently in studies of Plg-Rs, and several Plg-Rs have been identified. These include α-enolase (45 kDa), which has a C-terminal lysine22,29 and functions as a Plg-R on a variety of other cell types.30-33 Annexin 2 (36 kDa), a calcium-dependent phospholipid-binding protein, has been found on the surface of monocytoid cells and macrophages.28,34 Annexin 2 does not possess a C-terminal lysine, and proteolytic processing is required for it to acquire Plg binding capacity.34-36 The N-terminus of annexin 2 can associate with p11 (11 kDa) to form a heterotetramer.37 p11 does have a C-terminal lysine, and Waisman36 proposes that it is the p11 subunit that imparts Plg binding activity to the annexin 2 heterotetramer. Recently, we have identified histone H2B (H2B) as another Plg-R with a C-terminal lysine on the surface of leukocytes.38 Although histones are primarily nuclear proteins, we and others have found H2B on the surface of human blood monocytes39 and lymphocytes,40,41 and enhanced expression of H2B on stimulated monocytoid cells is associated with the marked up-regulation of Plg binding.38
In this study, we sought to further evaluate the role of H2B as a Plg-R. Our study focused primarily on murine macrophages using 2 cells lines, RAW 264.7 and J774A.1, as models and naturally occurring macrophages elicited in response to an inflammatory stimulus, but our results are also shown to be applicable to human cells, using THP-1 monocytoid cells. The focus on murine cells ultimately allowed us to examine the function of H2B as a Plg-R in macrophage recruitment in vivo. Our results establish that H2B is a functionally important Plg-R both in model systems and in vivo.
Materials and methods
Cell lines
Murine macrophage cell lines RAW 264.7 and J774A.1 were obtained from ATCC (Manassas, VA). Both lines were cultured in DMEM containing 10% fetal bovine serum, 4 mM l-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 1 mM sodium pyruvate.
Mouse blood leukocytes and peritoneal macrophages
All animal experiments were performed under institutionally approved protocols. Blood was collected from C57BL/6 mice from the inferior vena cava or by heart puncture into sodium citrate (3.2%). Leukocytes from blood were purified by lysing erythrocytes using ACK solution (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA). The monocyte population among leukocytes was identified by flow cytometry as described in “Flow cytometry.” To obtain macrophages, C57BL/6 mice were injected intraperitoneally with 0.5 mL of a 4% Brewer thioglycollate (TG) solution (DIFCO Laboratories, Detroit, MI). After 72 hours, when most recruited leukocytes are macrophages,5,42,43 mice were killed and peritoneal cells were collected by lavage.
Antibodies and Fab fragments
Antibodies to Plg-Rs were raised in rabbits using synthetic peptides corresponding to the C-terminal region of the target proteins as immunogens as previously detailed.38,44 Specific peptides and the purification of Fab are provided as Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Flow cytometry
RAW 264.7 and J774A.1 cells were dissociated from culture dishes with enzyme-free cell dissociation buffer (GIBCO, Grand Island, NY). To assess surface expression of the various Plg-Rs, cells were Fc blocked with mouse IgG and then stained with the Plg-R antibodies in HBSS (Hanks balanced salt solution)–HEPES buffer (Worthington Biochemical, Lakewood, NJ) containing 0.1% bovine serum albumin (HBSS-BSA). Cells were further stained with fluorescein isothiocyanate (FITC) goat anti–rabbit IgG (Zymed Laboratories, San Francisco, CA). Cell fluorescence was measured by fluorescence-activated cell sorter (FACS). Monocytes among blood leukocytes and macrophage populations among the TG-induced peritoneal lavage cells were identified by side scatter and αMβ2 expression45 using CellQuest Software, version 3.3 (BD Biosciences, Bedford, MA).
Biotin cell-surface labeling, Alexa labeling, and Western blotting
Details are provided in Document S1.
Confocal microscopy
Staining of individual Plg-Rs with antipeptide antibodies was performed as previously described.38 To assess colocalization of Plg and Plg-Rs, cells were preincubated with human Glu-Plg (Enzyme Research Laboratories, South Bend, IN), washed, and incubated with a combination of anti–Plg-R and rat monoclonal anti-Plg (R&D Systems, Minneapolis, MN) in HBSS-BSA. Nonimmune rabbit or rat IgG served as controls. Cells were stained with Alexa 488 antirabbit and Alexa 568 antirat IgG antibodies (Invitrogen, Carlsbad, CA). Images were captured by a Leica TCS-SP2 laser scanning confocal microscope at room temperature (RT; Leica Microsystems GmbH, Heidelberg, Germany). In separate experiments, J774A.1 cells were treated with 10 U/mL carboxypeptidase B from porcine pancreas (CpB; Worthington) in 0.7 mM ZnCl2 for 1 hour at 37°C, washed, incubated with Plg, washed, and then stained for bound Plg and anti–Plg-R. All images shown are representative of numerous areas examined on multiple slides.
Solid-phase Plg binding assays
The various Plg-Rs (H2B provided by Dr S. Sanker, Cleveland Clinic; α-enolase, Biogenesis, Oxford, United Kingdom; annexin 2, uncleaved or trypsin cleaved; or p11, Dr David M. Waisman, Dalhousie University) were immobilized onto 96-well (Costar, Cambridge, MA) microtiter plates. Plates were preincubated with Fab (0-16 μM) for 1 hour. Alexa 488–labeled Glu-Plg (1 μM) was added to wells in the presence of Fab and incubated for 2 hours at 37°C in HBSS-BSA. Nonspecific Plg binding to each Plg-R was defined as residual binding of Alexa 488 Glu-Plg in the presence of EACA (100 mM). Plates were washed and bound Plg was quantified by measuring fluorescence (excitation wavelength, 480 nm; emission wavelength, 530 nm).
Plg binding assays
Unless otherwise indicated, all murine and human cells used in Plg binding assays were washed and maintained in the absence of serum for 2 hours to reduce serum protein levels, including Plg, for subsequent binding. Such serum deprivation had no effect on expression of the Plg-Rs and did not induce apoptosis as verified by FACS. Cells were incubated with 200 nM Alexa 488 Glu-Plg, either with or without 100 mM EACA, for 1 hour at 4°C in HBSS-BSA. Plg binding was measured on viable cells by FACS, excluding apoptotic/necrotic cells using annexin V and propidium iodide staining (BD Biosciences). In inhibition experiments, cells were preincubated with 8 μM Fab for 30 minutes at 4°C followed by 200 nM Alexa 488 Glu-Plg for 1 hour at 4°C; fluorescence was measured by FACS.
Plasminogen activation
RAW 264.7 cells were pretreated with Fab to various Plg-Rs or nonimmune Fab (8 μM) and then Plg (200 nM) was added for 1 hour at 4°C in HBSS-BSA. Plm generation was measured at 22°C after simultaneous addition of the chromogenic substrate S-2251 (0.5 mM; Chromogenix Diapharma Group, Franklin, OH) and uPA (3 nM, low molecular weight from human urine; Calbiochem, San Diego, CA), monitoring the absorbance at 405 nm over 2.5 hours.
Matrigel invasion and cell migration
RAW 264.7 cells were pretreated with anti-H2B Fab or nonimmune rabbit Fab (8 μM) and loaded (5 × 105 cells/well) into the upper portion of transwell chambers, coated with Matrigel in the case of invasion assays or uncoated in the case of migration assays (BD Biosciences). Plg (200 nM) was added in the absence or presence of EACA (100 mM) or TXA (200 μM). As a chemoattractant, MCP-1 (50 ng/mL; R&D Systems) was added to the lower chamber for both the invasion and migration assays. After 16 hours, cells in the Matrigel or lower chamber were quantified using the Cyquant cell proliferation assay (Invitrogen) as described previously.46
Fab injection into mice
Selected Fab (500 μg protein in 200 μL phosphate-buffered saline) was injected intravenously into C57Bl/6 mice (8-10 weeks old of mixed sex). Fifteen minutes later, the mice were injected intraperitoneally with TG. When a second dose of Fab was injected, it was administered intravenously 24 hours after TG. Mice were killed at 24, 48, and 72 hours.
Results
Cell-surface expression of Plg-R on murine macrophage cell lines
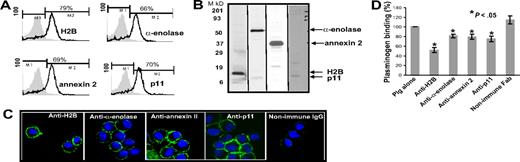
Based upon the literature, α-enolase and the p11 and annexin 2 subunits of the annexin 2 heterotetramer have been implicated in mediating Plg binding to a variety of cells, including cells of monocytic lineage. Recently, we identified H2B as a Plg-R on human monocytoid cells and neutrophils.38 To compare the contribution of H2B relative to these extensively characterized Plg-Rs, blocking antisera were prepared by immunizing rabbits with peptides corresponding to putative Plg binding sequences in the 4 Plg-Rs. Once high-titer antisera had been obtained, the immunoglobulin fractions were purified and Fab fragments were generated. The ability of these reagents to block Plg binding to their target receptors was evaluated using the purified proteins. These were immobilized on microtiter plates, and the capacity of various concentrations of each Fab to inhibit Alexa 488 Plg binding was tested. As shown in Figure 1A-D, each Fab fragment produced dose-dependent inhibition of Plg binding, and the extent of inhibition was 80% to 90% at the highest concentration. Consistent with the data of Hajjar,47 Plg binding to annexin 2 could only be demonstrated if the protein was first cleaved, and to observe Plg binding to the annexin 2 subunit, it was first cleaved with trypsin. The blocking effect of each Fab was specific; none of the Fab preparations inhibited Plg binding to any other candidate Plg-R (Figure S1).
Blocking plasminogen (Plg) binding to isolated plasminogen receptors (Plg-Rs) by Fab fragments. H2B-coated (A), α-enolase–coated (B), annexin 2–coated (trypsin-cleaved 15 μg annexin 2 was cleaved in 50 μL of 1 mg/mL trypsin immobilized onto sepharose beads suspended in 10 mM CaCl2, 50 mM Tris-Cl [pH 7.4] for 2.5 h at 37°C) (C), or p11-coated (D) plates were preincubated with Fab (0–16 μM) fragments of antipeptide antibodies raised to the Plg binding sites in each candidate Plg-R. Nonimmune rabbit Fab (0–16 μM) was used as a control with each Plg-R. Alexa 488 Glu-Plg (1 μM) was added to coated plates, with or without 100 mM EACA, and incubated for 2 hours at 37°C. The wells were washed, and bound Plg was quantified in a fluorescence plate reader using an excitation wavelength of 485 nm and emission wavelength of 530 nm. Values in the presence of EACA, which was 5% to 8% of total binding, were subtracted to obtain the specific binding values displayed. Means of duplicate determinations are plotted. Each Fab inhibited Plg binding by 80% to 90% at 16 μM. Error bar indicates SD.
Blocking plasminogen (Plg) binding to isolated plasminogen receptors (Plg-Rs) by Fab fragments. H2B-coated (A), α-enolase–coated (B), annexin 2–coated (trypsin-cleaved 15 μg annexin 2 was cleaved in 50 μL of 1 mg/mL trypsin immobilized onto sepharose beads suspended in 10 mM CaCl2, 50 mM Tris-Cl [pH 7.4] for 2.5 h at 37°C) (C), or p11-coated (D) plates were preincubated with Fab (0–16 μM) fragments of antipeptide antibodies raised to the Plg binding sites in each candidate Plg-R. Nonimmune rabbit Fab (0–16 μM) was used as a control with each Plg-R. Alexa 488 Glu-Plg (1 μM) was added to coated plates, with or without 100 mM EACA, and incubated for 2 hours at 37°C. The wells were washed, and bound Plg was quantified in a fluorescence plate reader using an excitation wavelength of 485 nm and emission wavelength of 530 nm. Values in the presence of EACA, which was 5% to 8% of total binding, were subtracted to obtain the specific binding values displayed. Means of duplicate determinations are plotted. Each Fab inhibited Plg binding by 80% to 90% at 16 μM. Error bar indicates SD.
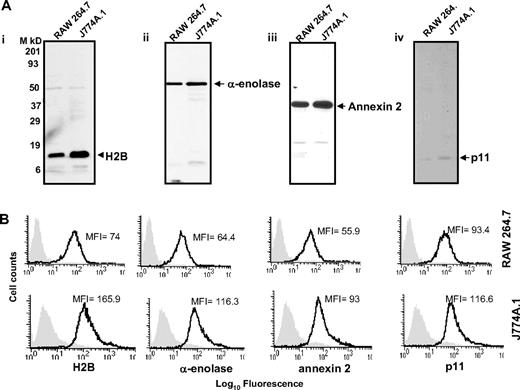
With these reagents in hand, we evaluated the presence of the Plg binding sequence of each Plg-R on the surface of murine macrophages, using J774A.1 and RAW 264.7 as models. Three independent approaches were used. First, cells were surface labeled with sulfo-NHS-biotin, lysed, then biotin-labeled proteins were precipitated with streptavidin conjugated to agarose followed by Western blotting with purified antibody against each Plg-R. As shown in Figure 2A, these antibodies reacted with a major band corresponding in estimated molecular weight to each target antigen. In each case, antibody reactivity with the major band accounted for greater than 98% of the staining as estimated by densitometric scanning. These data further document the specificity of the antibodies and suggest that all of the candidate Plg-Rs are present on the surface of RAW 264.7 and J774A.1 cells.
Cell-surface expression of H2B, α-enolase, annexin 2, and p11 on RAW 264.7 and J774A.1 cells. (A) Cell-surface biotinylation. RAW 264.7 and J774A.1 (i-iv) were dissociated from culture flasks, and cell-surface proteins were labeled with sulfo-NHS-biotin at 4°C for 30 minutes. Cells were lysed and equal amounts of surface-biotinylated proteins were precipitated with streptavidin-agarose, resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 12.5% gels) under reducing conditions, and immunoblotted with anti-H2B (i), anti–α-enolase (ii), anti–annexin 2 (iii), or anti-p11 (iv). Each antibody recognized a single major protein with a mobility appropriate for the target antigen. (B) FACS analysis. RAW 264.7 (top panels) and J774A.1 (bottom panels) cells were stained with antibodies to the indicated Plg-R (20 μg/mL) for 30 minutes at 4°C in HBSS-BSA buffer. Cells were then stained with FITC-conjugated goat anti–rabbit IgG. Staining with this antibody is indicated with the black line, and nonimmune IgG is shown by light gray areas. Mean fluorescence intensity (MFI) values, after subtracting the MFI values of the control antibody, are displayed in each histogram. Results are representative of 3 independent experiments and show that each Plg-R is present on the surfaces of these cells.
Cell-surface expression of H2B, α-enolase, annexin 2, and p11 on RAW 264.7 and J774A.1 cells. (A) Cell-surface biotinylation. RAW 264.7 and J774A.1 (i-iv) were dissociated from culture flasks, and cell-surface proteins were labeled with sulfo-NHS-biotin at 4°C for 30 minutes. Cells were lysed and equal amounts of surface-biotinylated proteins were precipitated with streptavidin-agarose, resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 12.5% gels) under reducing conditions, and immunoblotted with anti-H2B (i), anti–α-enolase (ii), anti–annexin 2 (iii), or anti-p11 (iv). Each antibody recognized a single major protein with a mobility appropriate for the target antigen. (B) FACS analysis. RAW 264.7 (top panels) and J774A.1 (bottom panels) cells were stained with antibodies to the indicated Plg-R (20 μg/mL) for 30 minutes at 4°C in HBSS-BSA buffer. Cells were then stained with FITC-conjugated goat anti–rabbit IgG. Staining with this antibody is indicated with the black line, and nonimmune IgG is shown by light gray areas. Mean fluorescence intensity (MFI) values, after subtracting the MFI values of the control antibody, are displayed in each histogram. Results are representative of 3 independent experiments and show that each Plg-R is present on the surfaces of these cells.
Second, the purified antibodies were used in FACS. As depicted in Figure 2B, J774A.1 and RAW 264.7 cells express H2B, α-enolase, annexin 2, and p11 on their surface. Mean fluorescence intensity (MFI) values obtained with each antibody and each cell type are summarized in Table 1. Reactivity of all the antibodies was higher with the J774A.1 cells, consistent with Plg binding to J774A.1 cells being 3.5-fold greater than Plg binding to RAW 264.7 cells as determined by FACS (Table 1). These data were corroborated using 125I-Plg in binding assays48,49 from Scatchard plots; J774.1 bound 3.2-fold more Plg than RAW 264.7 cells. In the above analyses, Plg binding to apoptotic/necrotic cells (< 15% of the cells) as assessed by annexin V/PI staining was excluded (not shown).
Plasminogen binding and Plg-R expression on RAW 264.7 and J774A.1 macrophage lines
| Molecules detected . | Expression levels, MFI . | |
|---|---|---|
| RAW 264.7 . | J774A.1 . | |
| Plasminogen | 71.5 | 280.5 |
| H2B | 74.0 | 165.9 |
| α-Enolase | 64.4 | 116.3 |
| Annexin 2 | 55.9 | 93.0 |
| p11 | 93.4 | 116.6 |
| Molecules detected . | Expression levels, MFI . | |
|---|---|---|
| RAW 264.7 . | J774A.1 . | |
| Plasminogen | 71.5 | 280.5 |
| H2B | 74.0 | 165.9 |
| α-Enolase | 64.4 | 116.3 |
| Annexin 2 | 55.9 | 93.0 |
| p11 | 93.4 | 116.6 |
Plasminogen binding was measured by incubating both RAW 264.7 and J774A.1 cells with Alexa 488–labeled Plg (200 nM) for 60 minutes at 4°C. The cells were washed and FACS analyses were used to measure Plg binding and Plg-R expression. Plg-R expression patterns were measured by reacting the cells with each anti–Plg-R, followed by secondary staining with FITC-conjugated goat anti–rabbit IgG. Mean fluorescence intensities (MFIs) have background MFI values in the presence of EACA for Plg or nonimmune IgG for Plg-R subtracted.
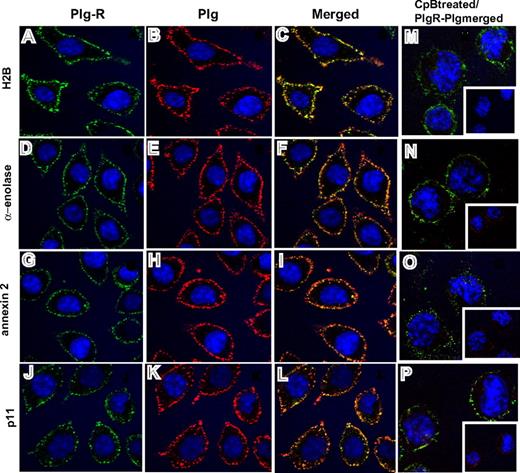
Third, confocal microscopy was performed using the antibodies to the various Plg-Rs. J774A.1 cells were preincubated with Plg and stained with a combination of the rabbit anti–Plg-R antibodies (green) and a rat monoclonal anti-Plg antibody (red). Representative confocal images are shown in Figure 3 and reveal that H2B, α-enolase, annexin 2, and p11 (Figure 3A,D,G,J; green) all localize to the cell surface. Bound Plg (Figure 3B,E,H,K; red) was also detected on the cell surface and colocalized with each Plg-R as revealed by merged images (Figure 3C,F,I,L; yellow). To further determine whether the observed colocalization of Plg and the Plg-Rs was LBS dependent, cells were pretreated with CpB. Colocalization of bound Plg and each candidate Plg-R was markedly diminished; only diffuse staining and little colocalization with the various Plg-Rs in the merged images was observed (Figure 3M-P). Similar results were obtained with RAW 264.1 cells, although staining intensities were less prominent. Collectively, these data indicate that all of the candidate Plg-Rs (H2B, α-enolase, annexin 2, and p11) are present on the surface of murine macrophage cell lines.
Colocalization of Plg-R with Plg on the surface of J774.1 cells. Localization of Plg with either H2B, α-enolase, annexin 2, or p11 on the cell surface of J774A.1 cells assessed by confocal microscopy. The cells were grown on coverslips, serum starved, and incubated with Plg (200 nM). After washing, the cells were incubated with a combination of rabbit anti–Plg-R (20 μg/mL) and rat monoclonal anti-Plg (20 μg/mL) for 30 minutes at 4°C in 1.5% BSA-HBSS (A-L). Nonimmune rabbit IgG and rat IgG served as controls. Cells were washed and then stained with Alexa 488 antirabbit and Alexa 568 antirat antibodies. Cells were fixed in 2% paraformaldehyde and mounted in Vectashield DAPl (4′,6-diamidine-2′-phenylindole dihydrochloride) mounting medium (Vector Laboratories, Burlingame, CA). Images were captured by confocal microscopy (Leica TCS-SP2 laser scanning confocal microscope) with 40×/1.25 NA oil-objective lens at RT and photographed using Leica confocal software v.2.61 (Leica Microsystems, Heidelburg, Germany). All images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). In separate experiments (M-P), J774A.1 cells were treated with CpB (10 U/mL), washed, and then incubated with Plg (200 nM). Cells were further washed and stained for bound Plg and Plg-R as described for panels A-L. Colocalization of Plg (red) with the cell-surface Plg-Rs (green) is detected as yellow in the merged images (C,F,I,L). Upon CpB treatment, the predominant staining seen in merged images (M-P) was only green, showing that Plg bound to cell-surface H2B, α-enolase, annexin 2, or p11 via their C-terminal lysines. Insets of panels M-P show the anti-Plg staining upon CpB treatment. The images shown are representative of numerous areas on the slides.
Colocalization of Plg-R with Plg on the surface of J774.1 cells. Localization of Plg with either H2B, α-enolase, annexin 2, or p11 on the cell surface of J774A.1 cells assessed by confocal microscopy. The cells were grown on coverslips, serum starved, and incubated with Plg (200 nM). After washing, the cells were incubated with a combination of rabbit anti–Plg-R (20 μg/mL) and rat monoclonal anti-Plg (20 μg/mL) for 30 minutes at 4°C in 1.5% BSA-HBSS (A-L). Nonimmune rabbit IgG and rat IgG served as controls. Cells were washed and then stained with Alexa 488 antirabbit and Alexa 568 antirat antibodies. Cells were fixed in 2% paraformaldehyde and mounted in Vectashield DAPl (4′,6-diamidine-2′-phenylindole dihydrochloride) mounting medium (Vector Laboratories, Burlingame, CA). Images were captured by confocal microscopy (Leica TCS-SP2 laser scanning confocal microscope) with 40×/1.25 NA oil-objective lens at RT and photographed using Leica confocal software v.2.61 (Leica Microsystems, Heidelburg, Germany). All images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). In separate experiments (M-P), J774A.1 cells were treated with CpB (10 U/mL), washed, and then incubated with Plg (200 nM). Cells were further washed and stained for bound Plg and Plg-R as described for panels A-L. Colocalization of Plg (red) with the cell-surface Plg-Rs (green) is detected as yellow in the merged images (C,F,I,L). Upon CpB treatment, the predominant staining seen in merged images (M-P) was only green, showing that Plg bound to cell-surface H2B, α-enolase, annexin 2, or p11 via their C-terminal lysines. Insets of panels M-P show the anti-Plg staining upon CpB treatment. The images shown are representative of numerous areas on the slides.
Contribution of Plg-Rs to plasminogen binding to mouse and human macrophage cell lines
While H2B, α-enolase, annexin 2, and p11 have all been implicated in Plg binding to cells, their relative contributions to binding have not been compared. Accordingly, the Fab for each Plg-R was tested as an inhibitor of Plg binding to RAW 264.7 and J774A.1 cells, which were preincubated for 30 minutes with Fab (8 μM), incubated an additional 60 minutes with Alexa 488 Plg, then analyzed by FACS. The effects of the various Fab fragments on specific Plg binding (inhibitable by EACA) are summarized in Table 2. Nonimmune Fab had no effect on Plg binding. Of the various Fabs, anti-H2B had the greatest inhibitory effect, decreasing Plg binding to RAW 264.7 cells by 50% and to J774A.1 cells by 42%. Anti-enolase, anti–annexin 2, and p11 Fab inhibited Plg binding by 20% to 23% to RAW 264.7 cells and had negligible effects on its binding to J774A.1, even though these cells had a higher Plg binding capacity. In view of the potency of the anti-H2B, we considered whether this Fab might sterically block the function of other candidate Plg-Rs. Mixing experiments were performed in which the H2B Fab was added together with an Fab to another Plg-R. In each case, the second Fab produced an additive inhibition of Plg binding on RAW 264.7 cells (Figure S2). These data suggested that the various Plg-Rs function independently, and H2B is the major contributor to recognition of Plg by these cells. To assess whether the various Plg-Rs contributed to Plg binding to human cells in a similar manner, nonstimulated or PMA-stimulated (20 nM) THP-1 cells were used. The THP-1 is an extensively characterized human monocytoid cell line that acquires macrophage-like characteristics upon PMA treatment for 72 hours.50 As assessed by FACS, Plg binding increased 3-fold when the THP-1 cells were treated with PMA. As shown in Table 2, the Fab to the Plg-Rs had little effect on the low level of Plg binding to the nonstimulated THP-1 cells. With the macrophage-like cells, Plg binding was inhibited by the various Plg-R Fab. Similar to the RAW 264.7 cells, anti-H2B was the most effective inhibitor (58%), whereas Fabs to the other 3 Plg-Rs produced 20% to 30% inhibition.
Inhibition of Plg binding to murine and human macrophage-related cells by Plg-R Fab
| Plg-R . | Inhibition of Plg binding, % . | ||||
|---|---|---|---|---|---|
| RAW 264.7 . | J774A.1 . | Mouse mФs . | THP-1 . | PMA+THP-1 . | |
| H2B | 50.7 ± 4 | 42 ± 3 | 48 ± 5 | ND | 58 ± 9 |
| α-Enolase | 23 ± 3.5 | ND | 18 ± 2 | 12 ± 3 | 17 ± 7 |
| Annexin 2 | 23 ± 3 | ND | 20 ± 3 | ND | 25 ± 3 |
| p11 | 16 ± 2 | ND | 24 ± 4 | ND | 29 ± 5 |
| Plg-R . | Inhibition of Plg binding, % . | ||||
|---|---|---|---|---|---|
| RAW 264.7 . | J774A.1 . | Mouse mФs . | THP-1 . | PMA+THP-1 . | |
| H2B | 50.7 ± 4 | 42 ± 3 | 48 ± 5 | ND | 58 ± 9 |
| α-Enolase | 23 ± 3.5 | ND | 18 ± 2 | 12 ± 3 | 17 ± 7 |
| Annexin 2 | 23 ± 3 | ND | 20 ± 3 | ND | 25 ± 3 |
| p11 | 16 ± 2 | ND | 24 ± 4 | ND | 29 ± 5 |
RAW 264.7 cells, J774A.1 cells, TG-induced peritoneal mouse macrophages (mФs), THP-1 (human monocytoid), or PMA-stimulated THP-1 (macrophage-like) cells were preincubated with Fab to H2B (C-terminal), α-enolase, annexin 2, or anti-p11 for 30 minutes at 4°C, followed by incubation with Alexa 488–labeled Plg (200 nM) for 60 minutes. The cells were washed and bound Plg was measured by FACS. Calculations are based on inhibition of specific Plg binding, inhibitable by EACA. The data are means plus or minus SD from 3 independent experiments.
ND indicates no detectable inhibition.
Functional roles of Plg binding to H2B
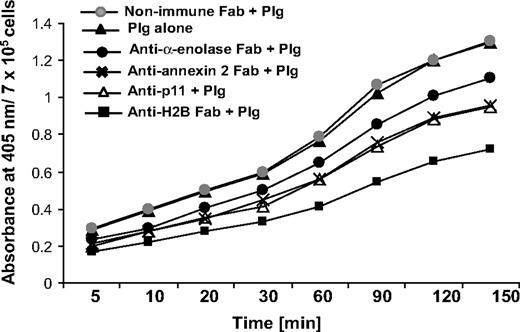
To address the functional role of H2B in Plg activation, we used RAW 264.7 cells. The cells were preincubated with Fab to H2B or nonimmune Fab (8 μM), followed by incubation with 200 nM Plg for 1 hour to allow its interaction with the cell surface. Plm generation was measured as a function of time upon addition of uPA and a chromogenic substrate for Plm, S-2251. As shown in Figure 4, the H2B Fab suppressed uPA-mediated Plm generation by 45% after 60 minutes, whereas nonimmune rabbit Fab had no inhibitory effect. As previously reported,15-18 Plg activation was 3.5- to 4-fold less in solution than in the presence of cells. The contributions of other Plg-Rs to plasmin generation were also analyzed. uPA-mediated Plm generation was suppressed by 19%, 29%, and 26% with the Fabs to α-enolase, annexin 2, and p11, respectively. Thus, the inhibitory activities of the various Fabs approximated their effects on Plg binding to these cells.
Role of H2B in Plg activation. RAW 264.7 cells were dissociated and washed. The cells were either pretreated with anti-H2B, anti–α-enolase, anti–annexin 2, anti-p11, or nonimmune rabbit Fab or left untreated, and then 200 nM Plg was added. After 1 hour, uPA (3 nM) was added with the chromogenic substrate S-2251, and plasmin generation was measured at 405 nm over 2.5 hours.
Role of H2B in Plg activation. RAW 264.7 cells were dissociated and washed. The cells were either pretreated with anti-H2B, anti–α-enolase, anti–annexin 2, anti-p11, or nonimmune rabbit Fab or left untreated, and then 200 nM Plg was added. After 1 hour, uPA (3 nM) was added with the chromogenic substrate S-2251, and plasmin generation was measured at 405 nm over 2.5 hours.
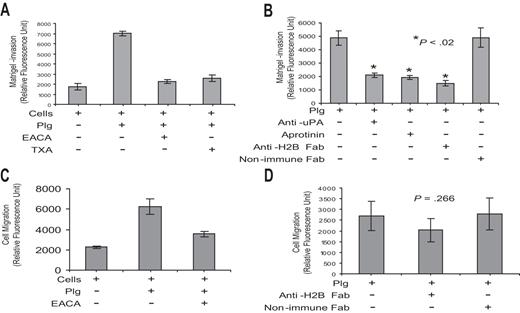
As a second functional assay, we determined whether Plg-H2B interaction is involved in macrophage migration. These experiments used Boyden chambers in which cells were placed in the upper chamber, the membrane between chambers was coated with Matrigel, and a chemoattractant stimulus, MCP-1, was in the lower chamber. Plg exerted a marked influence on the migration of the RAW 264.7 cells across the Matrigel, enhancing migration by approximately 4-fold, and this effect was suppressed by greater than 90% by EACA and 84% by TXA (Figure 5A). Plg-mediated migration was uPA and Plm dependent, since pretreatment of cells with anti-uPA and aprotinin inhibited Plg-mediated cell migration (Figure 5B). Preincubation of cells with anti-H2B Fab, but not with nonimmune Fab, diminished Plg-mediated Matrigel invasion by 70% (Figure 5B). In the absence of Matrigel, Plg has been shown to enhance the motility of cells,56 and this observation was reproduced with the RAW 264.7 cells (Figure 5C). However, anti-H2B had no significant effect (P = .266) on this migration (Figure 5D).
Role of H2B in Matrigel invasion and cell migration. (A,B) RAW 264.7 cells (5 × 104) were either pretreated with anti-uPA (0.5 μg/mL; American Diagnostica, Greenwich, CT), anti-H2B Fab, or nonimmune rabbit Fab or left untreated for 30 minutes. Cells were loaded into the upper portion of Matrigel-coated chambers, together with Plg, and in the absence or presence of aprotinin (50 U/mL; Calbiochem, San Diego, CA), EACA, or TXA, and were allowed to migrate toward MCP-1 in the lower chamber. The invading cells were quantified as described in “Cell invasion and cell migration.” The data are expressed as the means plus or minus SD of 2 independent experiments, and statistical significance was determined by a Student t test. Note that EACA and TXA inhibited 90% and 84% of Plg-dependent RAW 264.7 cell transmigration, respectively, and that anti-H2B Fab inhibited the response by 70% (B). (C,D) RAW 264.7 cells were either pretreated with anti-H2B Fab or nonimmune Fab or left untreated. Cells were loaded into the upper chambers with Plg, in the absence or presence of EACA or TXA, and with MCP-1 in the lower chambers. The membrane separating the upper and lower chamber was uncoated. Migrated cells were quantified as in panels A and B. The data are expressed as the means plus or minus SD of 2 independent experiments and statistical significance was determined by a Student t test.
Role of H2B in Matrigel invasion and cell migration. (A,B) RAW 264.7 cells (5 × 104) were either pretreated with anti-uPA (0.5 μg/mL; American Diagnostica, Greenwich, CT), anti-H2B Fab, or nonimmune rabbit Fab or left untreated for 30 minutes. Cells were loaded into the upper portion of Matrigel-coated chambers, together with Plg, and in the absence or presence of aprotinin (50 U/mL; Calbiochem, San Diego, CA), EACA, or TXA, and were allowed to migrate toward MCP-1 in the lower chamber. The invading cells were quantified as described in “Cell invasion and cell migration.” The data are expressed as the means plus or minus SD of 2 independent experiments, and statistical significance was determined by a Student t test. Note that EACA and TXA inhibited 90% and 84% of Plg-dependent RAW 264.7 cell transmigration, respectively, and that anti-H2B Fab inhibited the response by 70% (B). (C,D) RAW 264.7 cells were either pretreated with anti-H2B Fab or nonimmune Fab or left untreated. Cells were loaded into the upper chambers with Plg, in the absence or presence of EACA or TXA, and with MCP-1 in the lower chambers. The membrane separating the upper and lower chamber was uncoated. Migrated cells were quantified as in panels A and B. The data are expressed as the means plus or minus SD of 2 independent experiments and statistical significance was determined by a Student t test.
Plg-R expression on mouse blood monocytes and TG-recruited peritoneal macrophages
We sought to extend our observations to naturally occurring cells and assessed Plg binding to mouse blood monocytes and peritoneal macrophages harvested 72 hours after administration of the inflammatory stimulus TG. By FACS, we demonstrated LBS-dependent Plg binding to both blood monocytes and peritoneal macrophages using Alexa 488 Plg. As shown in Table 3, Plg binding to macrophages was higher than to blood monocytes: the MFI for Alexa 488 Plg was 31.5 for monocytes and 99.6 for macrophages.
Comparison of Plg-R expression and Plg binding to peripheral blood mouse monocytes (PBMs) and TG-induced mouse peritoneal macrophages (TGMΦs)
| Molecules detected . | PBMs . | TGMΦs . | ||
|---|---|---|---|---|
| % Positive cells . | MFI . | % Positive cells . | MFI . | |
| H2B | 2.5 | 19.5 | 72.8 | 43.8 |
| α-Enolase | 4.3 | 17.7 | 66.1 | 45.8 |
| Annexin 2 | 31.1 | 20.6 | 69.4 | 42.2 |
| p11 | 26.5 | 22.4 | 70 | 48.3 |
| Plasminogen | — | 31.6 | — | 99.6 |
| Molecules detected . | PBMs . | TGMΦs . | ||
|---|---|---|---|---|
| % Positive cells . | MFI . | % Positive cells . | MFI . | |
| H2B | 2.5 | 19.5 | 72.8 | 43.8 |
| α-Enolase | 4.3 | 17.7 | 66.1 | 45.8 |
| Annexin 2 | 31.1 | 20.6 | 69.4 | 42.2 |
| p11 | 26.5 | 22.4 | 70 | 48.3 |
| Plasminogen | — | 31.6 | — | 99.6 |
Next we evaluated H2B, α-enolase, annexin 2, and p11 expression on the monocytes and macrophages using the specific Fab for each. As assessed by FACS, expression of the Plg-Rs on the peripheral blood monocytes was detected but was low, ranging from 2.5% of cells being positive for H2B to 31.1% for annexin 2 (Table 3). Expression of each Plg-R was greater on the peritoneal macrophages (Table 3; Figure 6A); approximately 70% of the macrophages were positive for H2B. To confirm cell-surface localization of the Plg-Rs on the TG-induced macrophages, the cells were surface biotinylated and analyzed by Western blot or immunostained with antibodies against various Plg-Rs and analyzed by confocal microscopy. Data obtained from cell-surface biotinylation confirmed surface expression of H2B, α-enolase, annexin 2, and p11 on these cells (Figure 6B). Confocal microscopy also revealed that these Plg-Rs are localized to the surface of peritoneal macrophages (Figure 6C).
Expression patterns of Plg-Rs on TG-recruited peritoneal macrophages. (A) FACS. Thioglycollate-induced mouse peritoneal cells were incubated with the antibodies to H2B, α-enolase, annexin 2, and p11 (20 μg/mL) for 30 minutes at 4°C and were then stained with FITC-labeled goat anti–rabbit IgG for 30 minutes. Viable macrophages (low scatter, high integrin αMβ2 expression) were analyzed by FACS. Staining with each Plg-R antibody is highlighted with the black line and the light gray area is the reaction with nonimmune rabbit IgG. (B) Cell-surface biotinylation. TG-induced mouse peritoneal macrophages were harvested, subjected to surface biotinylation, captured on streptavidin-agarose, and analyzed by SDS-PAGE, followed by Western blotting with each of the anti–Plg-Rs. (C) Immunofluorescence. Macrophages were reacted for antibodies to each Plg-R or nonimmune rabbit IgG. After washing, the cells were then stained with Alexa 488 antirabbit IgG, fixed in 2% paraformaldehyde, and mounted in mounting medium containing DAPI. Images were captured as in Figure 3. The images shown are representative of numerous areas of the slides. (D) Inhibition of Plg binding to macrophages by Fab to Plg-Rs. Mouse peritoneal macrophages were collected after 72 hours from TG-treated mice. Cells were preincubated with Fab fragments (8 μM) of anti-H2B, anti–α-enolase, anti–annexin 2, anti-p11, or control nonimmune Fab for 30 minutes at 4°C followed by incubation with Alexa 488–labeled Plg (200 nM) at 4°C for 1 hour. The cells were washed and bound Plg was measured by FACS. Specific binding was determined by subtracting the binding values in the presence of EACA. Percentages of specific binding are plotted. Data are means plus or minus SD of 3 independent experiments.
Expression patterns of Plg-Rs on TG-recruited peritoneal macrophages. (A) FACS. Thioglycollate-induced mouse peritoneal cells were incubated with the antibodies to H2B, α-enolase, annexin 2, and p11 (20 μg/mL) for 30 minutes at 4°C and were then stained with FITC-labeled goat anti–rabbit IgG for 30 minutes. Viable macrophages (low scatter, high integrin αMβ2 expression) were analyzed by FACS. Staining with each Plg-R antibody is highlighted with the black line and the light gray area is the reaction with nonimmune rabbit IgG. (B) Cell-surface biotinylation. TG-induced mouse peritoneal macrophages were harvested, subjected to surface biotinylation, captured on streptavidin-agarose, and analyzed by SDS-PAGE, followed by Western blotting with each of the anti–Plg-Rs. (C) Immunofluorescence. Macrophages were reacted for antibodies to each Plg-R or nonimmune rabbit IgG. After washing, the cells were then stained with Alexa 488 antirabbit IgG, fixed in 2% paraformaldehyde, and mounted in mounting medium containing DAPI. Images were captured as in Figure 3. The images shown are representative of numerous areas of the slides. (D) Inhibition of Plg binding to macrophages by Fab to Plg-Rs. Mouse peritoneal macrophages were collected after 72 hours from TG-treated mice. Cells were preincubated with Fab fragments (8 μM) of anti-H2B, anti–α-enolase, anti–annexin 2, anti-p11, or control nonimmune Fab for 30 minutes at 4°C followed by incubation with Alexa 488–labeled Plg (200 nM) at 4°C for 1 hour. The cells were washed and bound Plg was measured by FACS. Specific binding was determined by subtracting the binding values in the presence of EACA. Percentages of specific binding are plotted. Data are means plus or minus SD of 3 independent experiments.
Involvement of the various Plg-Rs in Plg binding to mouse macrophages
The contribution of each Plg-R to peritoneal macrophage Plg binding was evaluated by FACS using each Fab as an inhibitor of Alexa 488 Plg binding. Nonimmune Fab has no effect on Plg binding, whereas anti-H2B Fab decreased Plg binding significantly (Figure 6D). The other Fabs were less inhibitory. For quantitation, specific binding was determined by subtracting the MFI values of Plg binding in the presence of EACA. On this basis, the inhibition by anti-H2B Fab was 48% (Table 2); enolase, annexin 2, and p11 Fab inhibited Plg to the macrophages by 20% to 24%. Thus, the contributions of the various Plg-Rs to Plg binding to macrophages closely recapitulated the results observed with the RAW 264.7 cells.
Involvement of H2B in the inflammatory response
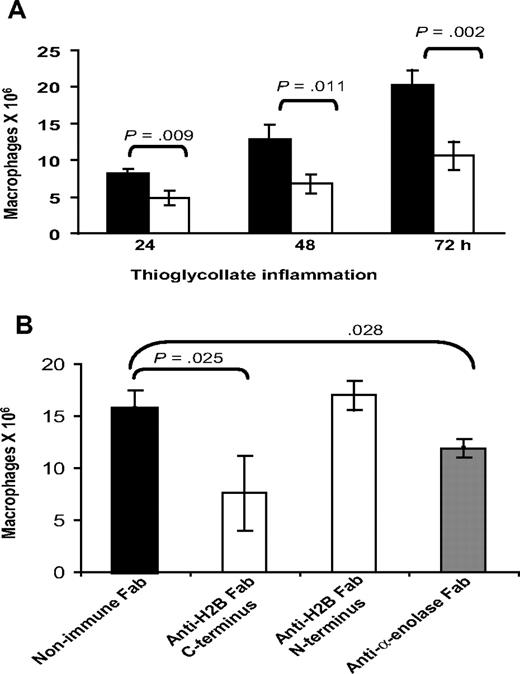
With our evidence implicating H2B as a major Plg-R on macrophages, experiments were undertaken to see if its role could be demonstrated in vivo. Wild-type C57BL/6 mice were given intravenous injections of 500 μg anti-H2B Fab or nonimmune Fab, each in 200 μL PBS, 15 minutes prior to intraperitoneal injection of TG. Another dose of each type of Fab was administered intravenously 24 hours after TG. Peritoneal exudates were collected at 24, 48, and 72 hours. At these time points, the lavage cells were collected by centrifugation, washed, and lysed in 1% Triton. Macrophages within the lavages were quantified by nonspecific esterase activity.51 As shown in Figure 7A, 24 hours after TG injection, macrophage recruitment was reduced significantly (41.5%, P = .009) by H2B Fab compared with nonimmune Fab. At 48 hours, macrophage recruitment remained reduced (47.2%) in the H2B Fab–treated animals, and this suppression remained the same at 72 hours (47.7%, P = .004). Based on FACS analysis, Fab against the N-terminal peptide of H2B binds to mouse peritoneal macrophages in a very similar fashion as Fab against the C-terminal peptide (MFI = 45.3 ± 5 for N-terminal Fab vs 47.2 ± 2.5 for C-terminal Fab). However, the N-terminal peptide Fab has no effect on Plg binding on the cells. MFI values for Alexa 488 Plg binding were 88.5 (± 3) for Plg alone and 90.9 (± 5) for Plg plus N-terminal Fab. Based on this difference in the effects of Fab to the N- and C-terminal H2B peptides on Plg binding, we compared their effects on macrophage recruitment in vivo. Mice were injected with a single dose of anti–C-terminal H2B, anti–N-terminal H2B, anti–α-enolase, or nonimmune Fab 15 minutes prior to TG, then at 72 hours peritoneal macrophages were enumerated (Figure 7B). Macrophage recruitment was significantly impaired (51.6%, P = .003) by the single dose of the C-terminal H2B Fab (7.6 ± 3.6 × 106 macrophages) compared with the mice injected with nonimmune Fab (15.7 ± 1.73 × 106 macrophages). In contrast, the N-terminal H2B Fab was without effect (17 ± 1.3 × 106 macrophages). Mice injected with anti-enolase Fab fragments also had suppressed macrophage recruitment (24.2% reduction, P = .028) compared with nonimmune Fab-injected mice, although the reduction was substantially less than that observed with the C-terminal H2B Fab.
Effect of anti-H2B and antienolase Fab on macrophage recruitment in response to TG. (A) Fab (500 μg in 200 μL PBS) was injected intravenously into C57BL/6 mice 15 minutes prior to intraperitoneal injection of TG. Another dose of each Fab was injected intravenously at 24 hours. Peritoneal lavage was collected into cold sterile HBSS, and the cells were collected by centrifugation, washed with HBSS-HEPES, and lysed in 1% TritonX. The number of macrophages in the lavage was determined using nonspecific esterase activity (n = 3). ■ indicate mice treated with nonimmune Fab; ▧, mice treated with anti-H2B Fab. Significance was calculated by a Student t test. (B) C57BL/6 mice were injected intravenously with Fab to C-terminus and N-terminus H2B, α-enolase, or nonimmune Fab, 15 minutes prior to TG administration. Lavage cells (from 2-mL lavage) were collected at 72 hours and macrophages were quantified as in panel A.
Effect of anti-H2B and antienolase Fab on macrophage recruitment in response to TG. (A) Fab (500 μg in 200 μL PBS) was injected intravenously into C57BL/6 mice 15 minutes prior to intraperitoneal injection of TG. Another dose of each Fab was injected intravenously at 24 hours. Peritoneal lavage was collected into cold sterile HBSS, and the cells were collected by centrifugation, washed with HBSS-HEPES, and lysed in 1% TritonX. The number of macrophages in the lavage was determined using nonspecific esterase activity (n = 3). ■ indicate mice treated with nonimmune Fab; ▧, mice treated with anti-H2B Fab. Significance was calculated by a Student t test. (B) C57BL/6 mice were injected intravenously with Fab to C-terminus and N-terminus H2B, α-enolase, or nonimmune Fab, 15 minutes prior to TG administration. Lavage cells (from 2-mL lavage) were collected at 72 hours and macrophages were quantified as in panel A.
Discussion
The importance of components of the Plg system in cell migration in vivo has been extensively documented using genetically engineered mice52 and uPAR,53 and a particularly prominent role of Plg per se in leukocyte migration has been demonstrated in inflammatory models conducted in Plg−/− mice.5,7 Based on the inhibition of inflammatory cell recruitment by TXA7 and enhanced migration in TAFI (which removes C-terminal lysines)–deficient mice,54 recognition of Plg-Rs by the LBSs of Plg has been implicated in leukocyte responses. We have recently added H2B to the list of Plg-Rs on leukocytes, which includes α-enolase and the 2 subunits of the annexin 2 heterotetramer, and have sought to assess the relative contribution of H2B to Plg binding and monocyte/macrophage migration.
In this assessment, Fabs that blocked Plg binding to these candidate Plg-Rs were used. Specificity of the antibodies for their target proteins and their selective inhibition was established in the solid-phase assays. Consistent with data in the literature, we could not detect Plg binding to annexin 2 unless it was cleaved and only then was the antibody we raised effective in inhibiting Plg. We confirmed that each candidate Plg-R was expressed on the surface of 2 widely used murine macrophage cell lines by 3 independent approaches: precipitation from surface-labeled cells, FACS, and confocal microscopy. Each Plg-R colocalized with Plg on the surfaces of these cells by confocal microscopy, and treatment of the cells with CpB disrupted this colocalization. Nevertheless, after removal of C-terminal lysines with CpB, our antibodies still reacted with the various Plg-Rs, indicating that the recognized epitopes were not entirely dependent on the C-terminal lysine. Upon treatment with CpB, we noted some rounding of the cells. This was due to the ZnCl2, which is necessary for CpB activity; however, the cells were not apoptotic as assessed by PI and annexin V staining. Based upon FACS and the intensity of the confocal images (not shown), we found that each Plg-R showed greater surface expression on the surface of J774A.1 than on RAW 264.7 cells, and Plg binding to the J774A.1 cells was also greater.
In inhibition experiments, Fab to each Plg-R showed some capacity to inhibit Plg binding to RAW 264.7 cells. The inhibition produced by the Fab to α-enolase, annexin 2, and p11 was 20% to 23%, but these reagents had no significant effect on Plg binding to J774A.1 cells. For both cell types, anti-H2B Fab inhibited Plg binding by 40% to 50%. These results indicate a major role of H2B in Plg recognition by cells with relatively high and low Plg binding capacities. The contribution of the other Plg-Rs was low and Plg-Rs other than these targets must contribute to Plg binding to the J774A.1 cells (eg, integrin αMβ255 ). The potency of the H2B Fab was not due to its blockade of other Plg-Rs. Addition of Fab to α-enolase, p11, and annexin 2 produced additive inhibition of Plg binding in combination with the H2B Fab. We also looked for colocalization of the various Plg-Rs by confocal microscopy. Annexin 2 and p11 showed extensive colocalization, as would be expected from the complex formation. Some colocalization of H2B with α-enolase, annexin 2, and p11 was observed but was much less extensive. To further test for direct interaction between H2B and the other Plg-Rs, we performed coimmunoprecipitation experiments from membranes isolated from RAW 264.7 cells. When H2B was precipitated, none of the other candidate Plg-Rs were detected by Western blots, although α-enolase, annexin 2, and p11 were all detected in blots of the membrane preparations. These data, coupled with the additive effects on the inhibition of Plg binding of Fab to α-enolase, annexin 2, or p11 when combined with anti-H2B (Figure S2), suggested that the potency of anti-H2B was not due to its blockade of Plg binding to other Plg-Rs. Fab to H2B was found to be very effective (58% inhibition) in inhibiting Plg binding to PMA-stimulated THP-1 cells, which display human macrophage-like characteristics.50 Altogether, these data support the contention that multiple receptors contribute to Plg binding to cells, and H2B is prominent among these.
In view of the importance of H2B in Plg binding, we assessed its function in 2 widely used assays. Cell-surface H2B on RAW 264.7 cells was found to reduce uPA- induced Plm generation by approximately 50%. Of note, this level of inhibition is in line with the contribution of H2B to Plg binding. The second function analyzed was Matrigel invasion. RAW 264.7 cell invasion depended upon endogenous uPA produced by the cells and Plm generation, as cell invasion was blocked by an anti-uPA monoclonal antibody (mAb) and by aprotinin. The H2B Fab inhibited this response by 70%. Although Plg also enhanced the migration/motility of these cells in the absence of a matrix, consistent with a previously reported activity of Plg,56 only the invasion of Matrigel and not the migration response was H2B dependent.
Armed with information showing that H2B is a major and functionally important Plg-R on murine macrophage cell lines, we evaluated its role in Plg binding to naturally occurring cells during an inflammatory response in vivo. Macrophages that accumulated in the peritoneum in response to TG bound 3-fold more Plg than peripheral blood monocytes. Numerous studies have demonstrated that the vast majority of recruited macrophages in the TG model are derived from peripheral blood. Hence, it appears that Plg binding and, presumably, the Plg-Rs that mediate binding are up-regulated during the recruitment and/or differentiation of blood monocytes into macrophages. It is noted that Plg binding to mouse macrophages was intermediate between the levels observed with the RAW 264.7 and J774A.1 cells but closer to that observed with RAW 264.7 cells in capacity. On blood monocytes, α-enolase and H2B were expressed at very low levels as assessed by FACS, whereas the subunits of the annexin heterotetramer were more prominent. On peritoneal macrophages, all 4 Plg-Rs were prominent. Hence, up-regulation of α-enolase and H2B is particularly extensive relative to monocytes, but all 4 Plg-Rs showed increased expression with approximately 70% of cells expressing the 4 Plg-Rs, as assessed by FACS. Inhibition experiments with the various Fabs showed a pattern very similar to that observed with RAW 264.7 cells. Namely, anti-H2B inhibited Plg binding by approximately 50% and the other 3 Fab fragments produced approximately 20% inhibition.
In view of the major role of H2B in Plg binding to macrophages, as well as in Plm generation and cell migration, we investigated whether this receptor facilitated macrophage recruitment in vivo. Upon intravenous injection of Fab to the C-terminus peptide from H2B, macrophage recruitment to TG was suppressed. At each time point tested (24, 48, and 72 h), this Fab reduced macrophage recruitment by 42% to 48%. As a control, the Fab to H2B and nonimmune Fab were injected into the peritoneal cavity 1 hour prior to harvesting the recruited cells. The H2B Fab had no effect on recovery of cells from the peritoneal cavity per se (not shown). In contrast to the profound inhibitory effect of the Fab to the C-terminal peptide of H2B, Fab reactive with the N-terminal peptide of H2B had minimal effect on macrophage recruitment even though it reacted with the macrophages to a similar extent as the C-terminal Fab. The reduction by the Fab to the C-terminal H2B peptide was observed with a 2-injection regimen of anti-H2B (time 0 and 24 hours) with no change in blood monocyte numbers. The particular regimen used was arbitrary and not optimized, and the inhibition produced by a single intravenous injection of anti-H2B was almost as effective. It is noteworthy that macrophage recruitment is suppressed by approximately 50% in Plg−/− mice in this same model, consistent with a major contribution of H2B and its Plg receptor function to the response. In this same setting, Fab to α-enolase produced 21.8% inhibition. Thus, there is remarkable concordance between the contribution of these Plg-Rs to Plg binding in vitro and their effect on macrophage recruitment in vivo. Recent preliminary studies suggest that the decrease in macrophage recruitment in the Plg−/− mice is not due to failure of monocytes to pass through the endothelium but rather to the accumulation of cells in the peritoneal tissue, probably due to an inability to degrade matrix constituents.57 This observation appears consistent with the inhibition of cell invasion through Matrigel by H2B Fab. Taken together, our data directly demonstrate that H2B is a functionally critical Plg-R that influences macrophage migration during inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge Dr Subramanian Sanker (Department of Genomic Medicine, Cleveland Clinic, Cleveland, OH) for purified H2B and Dr David M. Waisman (Department of Biochemistry and Molecular Biology, Dalhousie University, Halifax, NS) for the kind gift of purified annexin 2 and p11 subunits. We thank Dr Judy Drazba of the Imaging Core facility at Lerner Research Institute for help with confocal microscopy.
This work was supported by National Institutes of Health grant HL17964.
National Institutes of Health
Authorship
Contribution: R.D. designed and performed research, analyzed data, and wrote the manuscript; T.B. performed research; E.F.P designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edward F. Plow, Department of Molecular Cardiology, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue/NB50, Cleveland, OH 44195; e-mail:plowe@ccf.org.

![Figure 1. Blocking plasminogen (Plg) binding to isolated plasminogen receptors (Plg-Rs) by Fab fragments. H2B-coated (A), α-enolase–coated (B), annexin 2–coated (trypsin-cleaved 15 μg annexin 2 was cleaved in 50 μL of 1 mg/mL trypsin immobilized onto sepharose beads suspended in 10 mM CaCl2, 50 mM Tris-Cl [pH 7.4] for 2.5 h at 37°C) (C), or p11-coated (D) plates were preincubated with Fab (0–16 μM) fragments of antipeptide antibodies raised to the Plg binding sites in each candidate Plg-R. Nonimmune rabbit Fab (0–16 μM) was used as a control with each Plg-R. Alexa 488 Glu-Plg (1 μM) was added to coated plates, with or without 100 mM EACA, and incubated for 2 hours at 37°C. The wells were washed, and bound Plg was quantified in a fluorescence plate reader using an excitation wavelength of 485 nm and emission wavelength of 530 nm. Values in the presence of EACA, which was 5% to 8% of total binding, were subtracted to obtain the specific binding values displayed. Means of duplicate determinations are plotted. Each Fab inhibited Plg binding by 80% to 90% at 16 μM. Error bar indicates SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/10/10.1182_blood-2007-03-079392/6/m_zh80230709650001.jpeg?Expires=1769129860&Signature=FdPi5-fkFXlmcCE5EzMiw2O0oAdg9PQRGlJM~F4LTjHDJaNvsdiD79nC2hkr9JEGwAuf2zT-BKxELzR8jbM8qFnB7CYifWp8pe6AP5ukqhCyEvxPnJ4g8IC6fhy0nkWH4tdE~wLFcDzMzFeG2nY7buG5Vw0lkwXEJVYGiu7PXeAIJfvkOfyMYvlfttekVVlhkDsLWe-upZeUc4G10C3VWQsq-sOpxRMunjkXC1Us25-Q4pANtr5UNXJewXmhXvVNQ0QiDjEbzjryN7A~-pVu6cHwseCzntRfEjpLVMj~~b3W-yNZXfVoF1g45j-Z3gKSNLQP7WLrnD3xb~vR73ubig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal