Abstract
Signaling mediated by various Notch receptors and their ligands regulates diverse biological processes, including lymphoid cell fate decisions. Notch1 is required during T-cell development, while Notch2 and the Notch ligand Delta-like1 control marginal zone B (MZB) cell development. We previously determined that Mastermind-like (MAML) transcriptional coactivators are required for Notchinduced transcription by forming ternary nuclear complexes with Notch and the transcription factor CSL. The 3 MAML family members (MAML1-MAML3) are collectively essential for Notch activity in vivo, but whether individual MAMLs contribute to the specificity of Notch functions is unknown. Here, we addressed this question by studying lymphopoiesis in the absence of the Maml1 gene. Since Maml1−/− mice suffered perinatal lethality, hematopoietic chimeras were generated with Maml1−/−, Maml1+/−, or wild-type fetal liver progenitors. Maml1 deficiency minimally affected T-cell development, but was required for the development of MZB cells, similar to the phenotype of Notch2 deficiency. Moreover, the number of MZB cells correlated with Maml1 gene dosage. Since all 3 Maml genes were expressed in MZB cells and their precursors, these results suggest that Maml1 is specifically required for Notch2 signaling in MZB cells.
Introduction
The evolutionarily conserved Notch signaling pathway regulates multiple cell fate decisions during hematopoietic and lymphoid development (reviewed in Maillard et al,1 Radtke et al,2 and Tanigaki and Honjo3 ). Notch is essential for the emergence of definitive hematopoietic stem cells4,5 and at multiple steps of T-cell development.6-15 Together with other molecular pathways, Notch signaling also plays an essential role in the generation of marginal zone B (MZB) cells in the spleen.16-19 MZB cells are a distinct subset of peripheral B cells that reside in the splenic marginal zone and are capable of mounting rapid antibody responses, providing vital early protection against bloodborne infections, particularly encapsulated bacteria (reviewed in Martin and Kearney18 )
Mammalian cells express 4 Notch receptors (Notch1-Notch4) and 5 ligands from the Jagged (Jagged1-Jagged2) or Delta-like families (Dll1, Dll3, and Dll4). Signaling mediated by specific combinations of Notch receptors and ligands have unique roles in lymphoid lineage commitment. For instance, loss of the Notch1 receptor perturbs T-cell development, yet the B-cell compartment seems unaffected.15 Conversely, the targeted inactivation of the Notch2 receptor or Dll1 ligand results in a loss of MZB cells, but T-cell development appears normal.16,17 These effects are consistent with phenotypes caused by inactivation of canonical Notch signaling in these compartments, because loss of the transcription factor CSL blocks both T-cell development and MZB cell generation.13,19 Overall, these studies indicate that specific Notch ligands and receptors control distinct cell fate decisions in the lymphoid system. However, such specificity is not completely explained by differential expression patterns or preferential molecular interactions of Notch receptors or ligands, suggesting that other genes can contribute to specifying Notch receptor functions.
Our group and others have previously identified a family of Mastermind-like (MAML) coactivators as essential regulators of Notch-induced transcriptional events.20-25 This MAML family currently consists of 3 members, MAML1-MAML3. When activated by ligand binding, the Notch receptor is cleaved, and its intracellular domain (ICN) translocates to the nucleus.26 ICN then interacts with the transcription factor CSL and recruits the MAML coactivator proteins to form an ICN/MAML/CSL transcriptional complex, which regulates the transcription of specific target genes.27,28 Structurally, MAML proteins contain a basic domain that is responsible for ICN binding, and a domain required for the transcriptional activation of Notch target genes. Biochemically, all MAML proteins appear to form stable DNA-binding complexes with all 4 ICNs and the transcription factor CSL. Functionally, the 3 MAML proteins have overlapping and differential activities based on reporter assays in cultured cells.23 MAML1 and MAML2 are similarly able to coactivate all 4 Notch receptors, whereas MAML3 seems only to effectively coactivate Notch4. Importantly, interference with endogenous MAML function through expression of a dominant-negative MAML1 mutant (DNMAML1) in bone marrow cells caused early inhibition of T-cell development and MZB cell generation, indicating that Maml genes are collectively essential for physiologic Notch functions.8,9,29 However, the precise roles of individual MAML coactivators in vivo, and whether they contribute to the diverse biological effects of Notch signaling, have not been addressed.
Because individual Notch receptors have specific effects, the hematolymphoid system is an interesting context to evaluate the individual contribution of MAML1-MAML3 to Notch-mediated transcriptional activation in vivo. To this end, we examined the effect of Maml1 deficiency on lymphopoiesis using Maml1-deficient mice.30 Since Maml1 deficiency induces perinatal lethality, we generated hematopoietic chimeras from Maml1−/−, Maml1+/−, or wild-type fetal liver progenitors, in order to comprehensively assess all adult hematopoietic lineages in the absence of the Maml1 gene. The targeted deletion of Maml1 resulted in a specific B-cell phenotype with an absence of the MZB cell lineage. Moreover, the number of MZB cells correlated with Maml1 gene dosage. Conversely, T-cell development was largely unaffected, with only a modestly but significantly increased number of γδ T cells. Taken together, these results identified a unique signaling cascade in which the ligand Dll1 activates the Notch2 receptor, resulting in a putative Notch2/MAML1/CSL transcriptional complex that is essential for MZB cell development. Furthermore, these findings demonstrate that individual MAML coactivators provide molecular specificity and are dose limiting in the regulation of Notch signaling in vivo.
Materials and methods
Mice
Maml1−/− mice were generated as described.30 Briefly, Maml1 exon 1 was replaced with a neo cassette through homologous recombination in J129 embryonic stem (ES) cells. After germ-line transmission, the null Maml1 allele was maintained through heterozygous breeding due to the neonatal lethality of Maml1−/− mice. Genotyping was performed by polymerase chain reaction (PCR), as described.30 C57BL/6.Ly5.2 mice (B6-SJL, CD45.1+) were purchased from Taconic (Germantown, NY). Experiments were performed according to protocols approved by the Institutional Animal Care and Use Committees (IACUC) of the Dana-Farber Cancer Institute and the University of Pennsylvania.
Fetal liver hematopoietic chimeras
Timed pregnant mice were generated through intercrossing of Maml1+/− mice. Mice were killed at embryonic day (E) 15.5. A cell suspension was prepared from individual fetal livers. After purification on Ficoll, cells were washed and kept on ice until genotyping had been completed. Recipient female B6-SJL (CD45.1+) mice were irradiated (9 Gy [900 rad]) and reconstituted through tail-vein injection with 1 to 2 × 106 fetal liver cells (CD45.2+). Recipient mice were analyzed 12 weeks after reconstitution.
Antibodies
Antibodies were from Pharmingen (San Diego, CA), or eBioscience (San Diego, CA). The following antibodies were used: anti-CD4, anti-CD8α, anti-CD3, anti–T-cell receptor (TCR) β, anti-TCRγ, anti– c-Kit, anti-CD25, anti-B220, anti-CD19, anti-sIgM, anti-CD43, anti-AA4.1, anti-CD21/35, anti-CD23, anti-CD11b, anti-Gr1, anti-NK1.1, anti-CD11c, anti-CD45.1, anti-Sca1, anti-IL-7Rα, and anti-F1t3. Biotinylated antibodies were revealed with streptavidin–Pacific Blue (Molecular Probes, Eugene, OR) or PE–Texas Red (Caltag, Burlingame, CA). Lineage+ cells in the thymus were defined with anti-CD11b, anti-Gr1, anti-B220, anti-CD19, anti-CD11c, anti-CD8α, anti-CD3ϵ, anti-TCRβ, anti-TCRγ, and anti-NK1.1.
Flow cytometry and cell sorting
Cells were stained on ice after blocking with rat/mouse IgG (Sigma, St Louis, MO). Cells were sorted on FACSAria (Becton Dickinson, San Jose, CA) or MoFlo (Cytomation, Ft Collins, CO). Analysis was performed on LSR II (Becton Dickinson). Doublets were excluded with forward scatter (FSC)–W and side scatter (SSC)–W characteristics. DAPI was used to exclude dead cells. Data were analyzed in FlowJo (Tree Star, San Carlos, CA).
Quantitative RT-PCR
RNA from sorted cell populations was isolated using the RNEasy Micro Kit (QIAGEN, Valencia, CA). cDNA was prepared with the Superscript II kit (Invitrogen, Carlsbad, CA). Real-time PCR was performed with SYBRGreen PCR Master Mix (Applied Biosystems, Foster City, CA) and analyzed on an ABI Prism 7900HT (Applied Biosystems). A standard curve was generated from 10-fold dilutions of a positive control with a known amount of target DNA, so that the abundance of Maml1, Maml2, and Maml3 transcripts could be compared. The following primers were used: Maml1, 5′-ACAAGTCCCAAGGGTGTCAG-3 and 5′-TCACACAGCTGTTCCCAGAC-3′; Maml2, 5′-TTTCCCCTCAGGATCAGATG-3′ and 5′-AGAGGAGCCACCCGAATACT-3′; Maml3, 5′-CTGGTGAACTCGGCTCTCTC-3′ and 5′-GACGGTTCCCATGACACTCT-3′; and Hprt, 5′-CTCCTCAGACCGCTTTTTGC-3′ and 5′-TAACCTGGTTCATCA-TCGCTAATC-3′.
Results
The 3 Maml genes are differentially expressed during lymphoid development
The 3 members of the Maml gene family (Maml1-Maml3) have a nonoverlapping pattern of expression in nonhematopoietic tissues.23 To assess the effects of individual Maml genes during Notch-mediated cell fate decisions in the hematolymphoid system, we first examined the relative expression levels of Maml1-Maml3 during hematopoietic and lymphoid development, focusing on developmental steps with known involvement of Notch signaling. We purified various cell populations including hematopoietic precursors as well as cells at different stages of B and T lineage development from the bone marrow (BM), spleen, and thymus. The expression of Maml1, Maml2, and Maml3 genes was assessed by quantitative reverse transcription (RT)–PCR in such a way that the relative expression level of Maml family members could be directly compared.
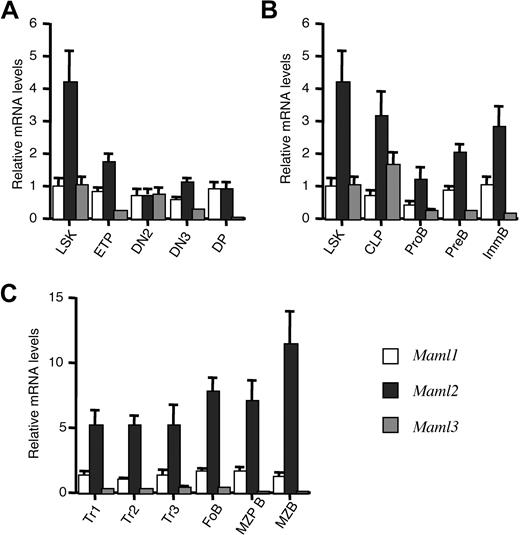
As shown in Figure 1, the 3 Maml genes had different patterns of expression. Transcripts from all 3 Maml genes (Maml1-Maml3) were present in multipotent hematopoietic progenitors with a Lin−Sca-1+c-kit+ (LSK) phenotype, containing hematopoietic stem cells, as well as in early progenitors of the T-cell and B-cell lineages in the thymus and bone marrow (ETP, early T lineage progenitors; CLP, common lymphoid progenitors; Figure 1A,B). Maml1 expression was generally stable during T-cell development in the thymus (Figure 1A) and B-cell development in the BM (Figure 1B), as well as in splenic B-cell subsets (Figure 1C). In contrast, Maml2 was expressed at 3- to 4-fold higher levels than Maml1 in LSK progenitors, before decreasing during T- and B-cell development (Figure 1A,B). Maml2 expression appeared to increase again in all splenic B-cell subsets, including MZB precursors (MZP B) and MZB cells, which undergo Notch2-mediated signaling. Finally, Maml3 transcripts were detected in LSK progenitors and during early lymphoid development, but decreased significantly to very low levels at late stages of T- and B-cell development.
Maml genes are differentially expressed in hematopoietic and lymphoid progenitor subsets. Quantitative RT-PCR was performed on cDNA isolated from multipotent BM progenitors, thymocyte subsets, BM B lineage progenitors, and splenic B-cell subsets from C57BL/6 mice fractioned by flow cytometry. Maml1-Maml3 transcript levels were normalized using Hprt expression. The Maml1/Hprt ratio in LSK progenitors was normalized to 1. The measurements were performed in triplicates. Results are shown as means plus or minus SD. (A) Relative expression of Maml1-Maml3 in multipotent BM progenitors (LSK subset) and thymocyte subsets. LSK indicates Lin−Sca-1+c-kit+; ETP, early T lineage progenitors (Lin−c-Kit+CD25−); DN2, CD4−CD8− double-negative 2 (Lin−c-Kit+CD25+); DN3, double-negative 3 (Lin−c-Kit+CD25−); and DP, CD4+CD8+ double-positive thymocytes. (B) Maml expression in BM B-cell progenitor subsets. CLP indicates common lymphoid progenitors (Lin−Sca-1loc-kitloIL-7Rα+Flt3+); ProB, pro-B cells (B220+CD43+AA4.1+CD19+sIgM−); PreB, pre-B cells (B220+CD43−AA4.1+sIgM−); and ImmB, immature B cells (B220+CD43−AA4.1+sIgM+). (C) Maml expression in splenic B-cell subsets. Tr1-Tr3 indicates successive stages of immature transitional B cells (Tr1, B220+AA4.1+sIgMhiCD23−; Tr2, B220+AA4.1+sIgMhiCD23+; Tr3, B220+AA4.1+sIgMintCD23+); FoB, follicular B cells (most abundant mature B-cell type in the spleen; B220+AA4−sIgMintCD23+); MZP B cells, marginal zone B cell precursors (B220+AA4.1−sIgMhiCD21hiCD23+); MZB, marginal zone B cells (B220+AA4.1−sIgMhiCD21hiCD23lo).
Maml genes are differentially expressed in hematopoietic and lymphoid progenitor subsets. Quantitative RT-PCR was performed on cDNA isolated from multipotent BM progenitors, thymocyte subsets, BM B lineage progenitors, and splenic B-cell subsets from C57BL/6 mice fractioned by flow cytometry. Maml1-Maml3 transcript levels were normalized using Hprt expression. The Maml1/Hprt ratio in LSK progenitors was normalized to 1. The measurements were performed in triplicates. Results are shown as means plus or minus SD. (A) Relative expression of Maml1-Maml3 in multipotent BM progenitors (LSK subset) and thymocyte subsets. LSK indicates Lin−Sca-1+c-kit+; ETP, early T lineage progenitors (Lin−c-Kit+CD25−); DN2, CD4−CD8− double-negative 2 (Lin−c-Kit+CD25+); DN3, double-negative 3 (Lin−c-Kit+CD25−); and DP, CD4+CD8+ double-positive thymocytes. (B) Maml expression in BM B-cell progenitor subsets. CLP indicates common lymphoid progenitors (Lin−Sca-1loc-kitloIL-7Rα+Flt3+); ProB, pro-B cells (B220+CD43+AA4.1+CD19+sIgM−); PreB, pre-B cells (B220+CD43−AA4.1+sIgM−); and ImmB, immature B cells (B220+CD43−AA4.1+sIgM+). (C) Maml expression in splenic B-cell subsets. Tr1-Tr3 indicates successive stages of immature transitional B cells (Tr1, B220+AA4.1+sIgMhiCD23−; Tr2, B220+AA4.1+sIgMhiCD23+; Tr3, B220+AA4.1+sIgMintCD23+); FoB, follicular B cells (most abundant mature B-cell type in the spleen; B220+AA4−sIgMintCD23+); MZP B cells, marginal zone B cell precursors (B220+AA4.1−sIgMhiCD21hiCD23+); MZB, marginal zone B cells (B220+AA4.1−sIgMhiCD21hiCD23lo).
These results indicated that Maml1-Maml3 were differentially expressed in hematopoietic and lymphoid progenitor compartments, but with more than 1 Maml family member expressed at all stages of development. We therefore asked if this pattern of expression was associated with functional redundancy of Maml1-Maml3, or if individual Maml family members could contribute to the specificity of Notch functions in vivo.
Maml1 haploinsufficiency leads to a reduction of MZB cells
To assess specifically the role of Maml1 in Notch-mediated cell fate decisions, we generated a null Maml1 allele through homologous recombination in ES cells. We previously described Maml1-deficient mice, which fail to thrive, exhibit a severe muscular dystrophy, and die within 10 days of birth.30
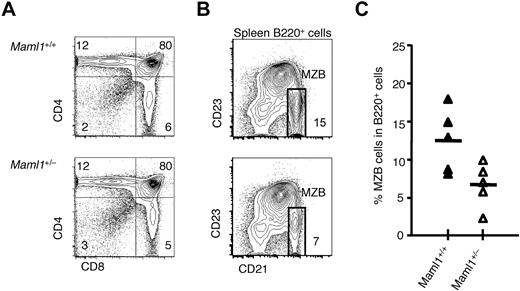
In contrast to Maml1− mice, Maml1+/− littermates were viable and fertile and had no overt phenotypic abnormality. Therefore, we first assessed hematopoietic and lymphoid development in adult Maml1+/− mice compared with control littermates (Figure 2). The cellularity of the BM, thymus, and spleen were similar in Maml1+/− mice and their Maml1+/+ littermates (data not shown). T-cell development in the thymus was not affected by the loss of 1 Maml1 allele, as shown by the normal generation of αβ and γδ T lineage cells in Maml1+/− thymi (Figure 2A; data not shown). Given the importance of Notch1 at multiple steps of T-cell development, this indicated that Notch1 functions were well preserved in Maml1 heterozygous mice. We next assessed splenic MZB cells through their characteristic B220+CD21hiCD23lo/neg immunophenotype (Figure 2B). We observed a statistically significant decrease in the percentage of CD21hiCD23lo/neg among B220+ cells in the spleen of Maml1+/− mice compared with control littermates (Figure 2C). On average, the number of MZB cells was approximately 2-fold lower in the Maml1 heterozygous spleens. Such reduction of the MZB cell number in Maml1 heterozygous mice are correlated with the reduced level of Maml1 expression at the mRNA and protein levels as compared with the wild-type littermates, based on the assessment of mouse embryonic fibroblasts prepared from mouse embryos from our previous study.30
Normal T-cell development and decreased numbers of MZB cells in Maml1+/− mice. (A) Flow cytometric analysis showed a similar percentage of CD4+CD8+ double-positive and CD4+ or CD8+ single-positive cells in the thymus of Maml1+/− mice compared with wild-type littermates. Numbers indicate the percentage of cells in each quadrant. (B) Flow cytometric analysis of splenic B-cell subsets showing a reduced percentage of B220+CD21hiCD23lo MZB cells in Maml1+/− compared with wild-type spleens. Numbers indicate the percentage of cells in the rectangular box identifying MZB cells. (C) Data from multiple mice were compiled, indicating significant Maml1 dosage-dependent decrease in MZB cells. Each triangle represents a data point for a single mouse (Maml1+/+, ▴, n = 5; Maml1+/−, ▵, n = 5).
Normal T-cell development and decreased numbers of MZB cells in Maml1+/− mice. (A) Flow cytometric analysis showed a similar percentage of CD4+CD8+ double-positive and CD4+ or CD8+ single-positive cells in the thymus of Maml1+/− mice compared with wild-type littermates. Numbers indicate the percentage of cells in each quadrant. (B) Flow cytometric analysis of splenic B-cell subsets showing a reduced percentage of B220+CD21hiCD23lo MZB cells in Maml1+/− compared with wild-type spleens. Numbers indicate the percentage of cells in the rectangular box identifying MZB cells. (C) Data from multiple mice were compiled, indicating significant Maml1 dosage-dependent decrease in MZB cells. Each triangle represents a data point for a single mouse (Maml1+/+, ▴, n = 5; Maml1+/−, ▵, n = 5).
The significant reduction of MZB cell numbers in the Maml1 heterozygous background suggested that Maml1 haploinsufficiency could disrupt Notch2-mediated MZB cell development. Interestingly, MZB cell development is influenced by the gene dosage of other individual components of the Notch pathway, since both the Notch ligand gene Dll1 and the Notch receptor gene Notch2 are essential and haploinsufficient for MZB cell development.16,17
Maml1 deletion results in a total loss of MZB cells in fetal liver hematopoietic chimeras
To study hematopoiesis in the complete absence of Maml1, it was necessary to bypass the early postnatal lethality of Maml1−/− mice. To accomplish this, we generated fetal liver hematopoietic chimeras through fetal liver cell reconstitution of irradiated recipients. Specifically, fetal liver cells were isolated from E15.5 wild-type, heterozygous, and Maml1−/− embryos and transplanted into lethally irradiated wild-type recipient mice (B6-SJL, CD45.1+). The recipient mice expressed a different CD45 allele from the donor cells, thus allowing us to specifically track donor-derived cells (CD45.2+). The donor-derived lymphoid cells were analyzed at 12 weeks after transplantation by flow cytometry.
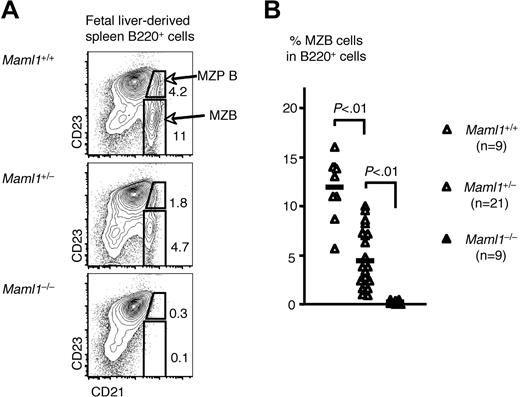
We found that BM B-cell development was preserved in recipients of Maml1−/− progenitors (data not shown). The same was true for immature transitional B cells in the spleen of these recipients, indicating that B-cell production was normal in the absence of Maml1. In contrast, there was a virtual absence of both donor-derived MZB precursors (MZP B, B220+CD21hiCD23+) and MZB cells in Maml1−/− fetal liver cell chimeras (Figure 3). Moreover, the number of MZB cells was reduced to approximately 50% in Maml1+/− fetal liver chimeras compared with recipients of wild-type progenitors, consistent with observations in Maml1+/− adult mice (Figure 2). These data indicate that Maml1 is absolutely required for MZB cell development and that in vivo Maml1 levels are limiting downstream of Notch2-mediated signaling.
Characterization of splenic B-cell subsets in fetal liver hematopoietic chimeras reveals an absolute dosage-dependent requirement for Maml1 in MZB development. (A) Representative contour plots showing that MZB precursors (MZP B) and MZB populations were absent in Maml1−/− fetal liver chimera, while reduced to about half of normal in Maml1+/− fetal liver chimera compared with the wild-type controls. Host-derived CD45.1+ cells were excluded from the analysis. (B) Compilation of data collected from all the fetal liver chimeras that were analyzed (Maml1+/+, n = 9; Maml1+/−, n = 21; Maml1−/−, n = 9). MZB cells were essentially absent in Maml1−/− chimeras and decreased in numbers in Maml1+/− heterozygous chimeras. Differences were highly statistically significant (P < .01; Student t test).
Characterization of splenic B-cell subsets in fetal liver hematopoietic chimeras reveals an absolute dosage-dependent requirement for Maml1 in MZB development. (A) Representative contour plots showing that MZB precursors (MZP B) and MZB populations were absent in Maml1−/− fetal liver chimera, while reduced to about half of normal in Maml1+/− fetal liver chimera compared with the wild-type controls. Host-derived CD45.1+ cells were excluded from the analysis. (B) Compilation of data collected from all the fetal liver chimeras that were analyzed (Maml1+/+, n = 9; Maml1+/−, n = 21; Maml1−/−, n = 9). MZB cells were essentially absent in Maml1−/− chimeras and decreased in numbers in Maml1+/− heterozygous chimeras. Differences were highly statistically significant (P < .01; Student t test).
Modest abnormalities of T-cell development in the absence of Maml1
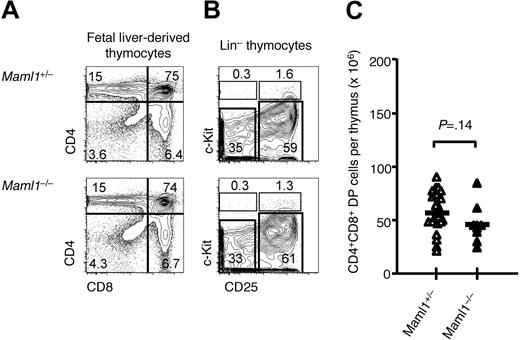
We then studied T-cell development in recipients of Maml1−/− fetal liver progenitors. Because no significant impairment in T-cell development had been detected in adult Maml1+/− mice compared with wild-type mice (Figure 2), we used recipients of Maml1+/− progenitors as controls. The percentages of CD4−CD8− double-negative, CD4+CD8+ double-positive (DP), and CD4+ or CD8+ single-positive cells were similar in the thymi of Maml1−/− and Maml1+/− recipients (Figure 4A). When Lin− thymocytes were examined, we found no significant difference in the relative or absolute numbers of early T lineage progenitors (c-Kit+CD25−) and DN2 (c-Kit+CD25+), DN3 (c-Kit+CD25−), or DN4 (c-Kit−CD25−) thymocytes (Figure 4B). There was also no difference in the absolute number of CD4+CD8+ DP thymocytes, although a nonsignificant trend for a modestly decreased number of Maml1−/− DP thymocytes was apparent (Figure 4C). These findings indicated that αβ T-cell development was largely preserved in the absence of Maml1 and suggested that Notch1 signaling can function efficiently through the coactivators Maml2 and/or Maml3.
Preserved development of αβ lineage T cells in the absence of Maml1. (A) Flow cytometric analysis showed a similar percentage of CD4+CD8+ double-positive and CD4+ or CD8+ single-positive thymocytes in recipients of Maml1−/− compared with Maml1+/− progenitors. Numbers indicate the percentage of cells in each quadrant. Host-derived CD45.1+ cells were excluded from the analysis. (B) Normal distribution of Lin− thymocyte progenitor subsets, as defined using c-Kit and CD25 expression, in recipients of Maml1−/− compared with Maml1+/− progenitors. Numbers indicate the percentage of cells in each quadrant. Host-derived CD45.1+ cells were excluded from the analysis. (C) Absolute number of donor-derived double-positive thymocytes in all the fetal liver chimeras that were analyzed (Maml1+/−, ▵, n = 21; Maml1−/−, ▴, n = 9). The trend for decreased numbers of double-positive thymocytes in Maml1−/− compared with Maml1+/− progenitors was not statistically significant (P = .14; Student t test).
Preserved development of αβ lineage T cells in the absence of Maml1. (A) Flow cytometric analysis showed a similar percentage of CD4+CD8+ double-positive and CD4+ or CD8+ single-positive thymocytes in recipients of Maml1−/− compared with Maml1+/− progenitors. Numbers indicate the percentage of cells in each quadrant. Host-derived CD45.1+ cells were excluded from the analysis. (B) Normal distribution of Lin− thymocyte progenitor subsets, as defined using c-Kit and CD25 expression, in recipients of Maml1−/− compared with Maml1+/− progenitors. Numbers indicate the percentage of cells in each quadrant. Host-derived CD45.1+ cells were excluded from the analysis. (C) Absolute number of donor-derived double-positive thymocytes in all the fetal liver chimeras that were analyzed (Maml1+/−, ▵, n = 21; Maml1−/−, ▴, n = 9). The trend for decreased numbers of double-positive thymocytes in Maml1−/− compared with Maml1+/− progenitors was not statistically significant (P = .14; Student t test).
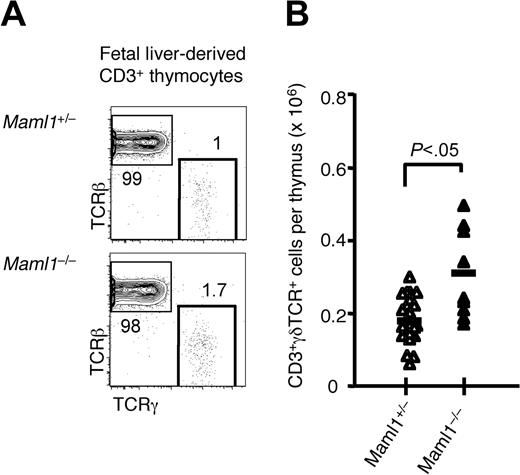
Next, we examined γδ T-cell development in the absence of Maml1 (Figure 5). We found a modest but significant increase in the percentage of TCRγ+ cells among CD3+ thymocytes in recipients of Maml1−/− cells compared with Maml1+/− controls (Figure 5A). This translated into a significant increase in the absolute number of donor-derived γδ T cells in the thymus (Figure 5B) and spleen (data not shown) of Maml1−/− recipients.
Increased numbers of γδ lineage T cells in the absence of Maml1. (A) Flow cytometric analysis showed an increased percentage of TCRγ+ cells among CD3+ thymocytes in recipients of Maml1−/− compared with Maml1+/− progenitors (representative examples). Numbers indicate the percentage of cells in each box. Host-derived CD45.1+ cells were excluded from the analysis. (B) Absolute number of donor-derived CD3+ γδ T cells in the thymus of all the fetal liver chimeras that were analyzed (Maml1+/−, ▵, n = 21; Maml1−/−, ▴, n = 9). The increased number of γδ T cells in Maml1−/− compared with Maml1+/− recipients was statistically significant (P < .05; Student t test).
Increased numbers of γδ lineage T cells in the absence of Maml1. (A) Flow cytometric analysis showed an increased percentage of TCRγ+ cells among CD3+ thymocytes in recipients of Maml1−/− compared with Maml1+/− progenitors (representative examples). Numbers indicate the percentage of cells in each box. Host-derived CD45.1+ cells were excluded from the analysis. (B) Absolute number of donor-derived CD3+ γδ T cells in the thymus of all the fetal liver chimeras that were analyzed (Maml1+/−, ▵, n = 21; Maml1−/−, ▴, n = 9). The increased number of γδ T cells in Maml1−/− compared with Maml1+/− recipients was statistically significant (P < .05; Student t test).
Discussion
Maml1 is 1 of 3 members of the MAML family of transcriptional coactivators, which are essential for mediating Notch signaling via coactivation of target gene transcription. Importantly, however, the Notch molecules display unique and important functions. This functional specificity is best exemplified by the genetic mouse models in which Notch1 deficiency results in impaired T-cell development, while Notch2 deficiency causes a dramatic absence of MZB cells.15,17 Strikingly, it remains a critical question how various mammalian Notch receptors and ligands exert their unique functional activities. In this study, we discovered an unexpected, in vivo role for Maml1 in mediating the signaling of a specific Notch receptor, Notch2. Using the knockout mice we established recently, we observed that the targeted deletion of 1 member of the MAML transcriptional coactivators, the Maml1 gene, led to the absence of MZB cells, similar to the phenotype of Notch2 or Dll1 ligand deficiency. Therefore, this work strongly implicates individual Maml coactivators in regulating molecular specificity of Notch receptor functions in vivo. Thus, our present study provides evidence for at least 1 mechanism by which this occurs: specifically, by unique transcriptional coactivators of the Notch pathway, the MAML family.
Our studies show only modest abnormalities in T-cell development from Maml1−/− progenitors, indicating that Maml1 is largely dispensable to mediate Notch1 function in the thymus, although it has mild effects on γδ T-cell development. Previous studies have provided different conclusions as to the role of Notch signaling during γδ T-cell development. Inactivation of Notch signaling through Lck-Cre–mediated inactivation of Notch1 or Lck-Cre–mediated expression of the Notch inhibitor DNMAML1 have not revealed significant effects on γδ T-cell development.8,14 In contrast, inactivation of the CSL/RBP-J gene through the same Lck-Cre transgene was reported to cause an increase in the number of thymic and splenic γδ T cells associated with increased export of these cells into the periphery.12 In addition, recent data using the OP9-DL1 culture system to drive T-cell development in vitro have revealed that γδ T-cell development is less sensitive to Notch inhibition than αβ T-cell development, due to a cooperative effect of Notch and pre-TCR signals in the αβ lineage.6,7 Our observations in the absence of Maml1 reveal an increase in the γδ/αβ T-cell ratio, but also a true increase in the absolute numbers of γδ T cells in the thymus and in peripheral lymphoid organs. These findings are most reminiscent of the effects observed after conditional deletion of CSL/RBP-J,12 and cannot be completely explained by cooperativity between Notch and pre-TCR signaling, since disruption of this effect might give rise to an increased γδ/αβ ratio but not to an increase in the absolute number of γδ T cells. Therefore, the effect of Notch signaling on γδ T-cell development is more complex in vivo than it is in culture systems.
With respect to the B-cell phenotype, Maml1-deficient mice displayed identical MZB cell phenotype as those mouse models that lack Notch ligand Dll1, the receptor Notch2, or the transcription factor CSL.16,17,19 Our data revealed that the targeted deletion of Maml1 in hematopoietic cells led to the specific loss of MZB cells, indicating an absolute requirement for Maml1 in MZB cell development. Moreover, the Maml1 haploinsufficiency leads to a reduction in MZB cells. Though the possible functional compensation by other Maml members and the effect of the Maml1 deficiency on Notch receptor functions in the Maml1 knockout mice remain to be further characterized, we recently found no significant differences in the expression levels of Maml2, Maml3, Notch2, or CSL in the Maml1-deficient embryonic fibroblasts (L.W., July 2007, unpublished data). Therefore, combined with previous studies showing that there is an absence of MZB cells in the absence of the Notch ligand Dll1, the receptor Notch2, or the transcription factor CSL, our data indicate that a unique signaling cascade controls MZB cell development: (1) the Notch ligand Dll1 activates the Notch2 receptor, resulting in ICN2 translocation to the nucleus; and (2) this complex interacts with the CSL transcription factor and recruits MAML1 as a coactivator to drive the transcription of genes involved in MZB cell formation. Thus, the Notch2/MAML1/CSL complex is essential for MZB development, and there is a functional haploinsufficiency for each of its components.
Currently, the underlying basis for the specific interaction and function of the Notch2/MAML1/CSL complex in the nucleus remains unclear, and warrant further investigations because such studies will provide mechanistic explanations for specific modulation of various Notch receptor signaling in different cellular contexts. Because both Maml1 and Maml2 transcripts were present in splenic B cells (Figure 1), the specific requirement for Maml1 could not be explained by the absence of other Maml family members in cells exposed to Notch2 signals. Instead, the data suggested that MAML1 provided specific molecular features to the Notch2 transcriptional activation complex that could not be substituted by Maml2. In addition, the preservation of nearly normal T-cell development in the absence of Maml1 suggested that Notch1 functions are either mediated by Maml2 and/or Maml3, or that both Maml1 and other Mamls can interact well with Notch1 in vivo. Another possibility to consider is that Maml1 might associate with other, distinct cellular factors that are required for the expression of genes necessary for MZB cell development. Finally, it is possible (although unlikely) that only a small subset of cells in the pre-MZB population represents true MZB precursors, and that these cells might express only MAML1.
In summary, our study provides in vivo evidence for the functional specificity of Maml family members in a well-defined Notch function and indicates that Maml expression levels can be limiting in vivo. It should be noted that our work suggests the existence of Notch2/MAML1/CSL complex based on the strong mouse genetic data from our and other groups, and currently we do not have direct biochemical data supporting that such unique complex regulating MZB cell development due to the inherent low level of endogenous Notch activity and the lack of specific Notch antibodies. Moreover, the specific interaction of Dll1/Notch2/MAML1 could not be clearly predicted from in vitro biochemical data showing that overexpressed MAML proteins appear to form stable complexes with all 4 ICNs in immunoprecipitation assays.23 Therefore, more sensitive assays will be required to test if a higher binding affinity between MAML1 and Notch2 can be detected at physiologic levels in vivo. In addition, detailed structural information is now available regarding the trimolecular complex between CSL, Notch1, and the N-terminal Notch-binding domain of MAML1.27 Such emerging structural insights will provide a basis to investigate whether structural differences underlie the in vivo specificity of various transcriptional complexes involving combinations of individual Notch and MAML family members. In the future, this information might lead to novel strategies to inhibit some but not all Notch functions in cancer and other diseases where Notch signaling plays a pathogenic role.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO1CA097148 to L.W., RO1AI047833 to W.S.P., and RO1CA036167 to J.D.G.). I.M. was supported by a grant from the Damon Runyon Cancer Research Foundation (DRG-102–05). Additional support was provided by a Specialized Center of Research (SCOR) grant from the Leukemia and Lymphoma Society (to W.S.P.).
National Institutes of Health
Authorship
Contribution: L.W. and I.M. participated in the design of the study, performed research, and wrote the manuscript. M.N. performed part of the research. W.S.P. and J.D.G. were responsible for study conception, design, and oversight as well as help with manuscript preparation. All authors read and approved the final manuscript. L.W. and I.M. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lizi Wu, Cancer and Genetics Research Complex, Rm 362, Department of Molecular Genetics and Microbiology, Shands Cancer Center University of Florida, 1376 Mowry Rd, Gainesville, FL 32610-3633; e-mail:lzwu@ufl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal