In this issue of Blood, Sanchorawala and colleagues provide 10-year follow-up of their efforts to treat immunoglobulin light chain (AL) amyloidosis with high-dose melphalan in the 1990s. They report median survival durations approaching 5 years for all patients and exceeding 10 years for those attaining hematologic complete remission.
The remarkable results reported by Sanchorawala and colleagues in this issue of Blood support the continued use of myeloablative doses of melphalan in selected amyloidosis patients, despite some controversy that still dogs autologous hematopoietic stem-cell transplantation in this disease.1,2 The major criticism of transplantation studies is their bias toward selection of good-risk patients.3 This criticism is unjustifiable because, although transplantation is applicable to a limited number of patients, it has the potential of improving their outcome dramatically, as shown by the Boston University experience. A quest for therapies that are widely applicable and well-tolerated by all patients cannot supplant the exploration of riskier options that may be applicable to, and highly beneficial for, a small subgroup of patients.
The combination of prolonged oral low-dose melphalan and dexamethasone has been found to result in excellent outcomes in patients ineligible for transplantation, albeit with a shorter follow-up of 5 years.4 However, a multicenter French study showing apparent equivalence of melphalan-dexamethasone and autotransplantation5 must be interpreted with caution because the follow-up is short (patient enrollment from 2001 to 2005), 26% of the patients randomized to transplantation did not undergo the procedure, 27% of autografted patients received a dose of melphalan (140 mg/m2) associated with inferior long-term survival,6 and 24% of autografted patients died early from toxicity as a number of participating centers did not have sufficient experience to perform transplantation safely in amyloidosis. As pointed out previously,2 a meritocratic rather than a democratic approach in selecting a transplantation center is essential for minimizing the risk and maximizing the benefit associated with high-dose chemotherapy in amyloidosis.
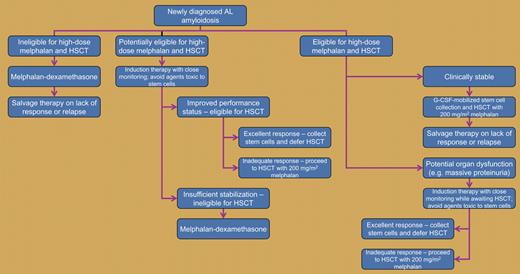
Approach to AL amyloidosis (an appropriate clinical trial is reasonable at every step). HSCT indicates autologous hematopoietic stem-cell transplantation. Enough stem cells are collected for 2 cycles of high-dose chemotherapy and transplantation.
Approach to AL amyloidosis (an appropriate clinical trial is reasonable at every step). HSCT indicates autologous hematopoietic stem-cell transplantation. Enough stem cells are collected for 2 cycles of high-dose chemotherapy and transplantation.
How, then, should one approach a patient with AL amyloidosis? Unless it is clear that the patient is totally unsuitable for high-dose chemotherapy, it is important to choose options that will not prevent future stem-cell collection and transplantation. Thus, treatment sequence is important: while transplantation does not preclude future melphalan-dexamethasone salvage therapy, induction with melphalan-dexamethasone is likely to prevent successful stem-cell collection for subsequent salvage transplantation. Newer agents such as thalidomide, bortezomib, and lenalidomide are used to treat recurrent amyloidosis. Unlike oral melphalan, combinations of these agents with corticosteroids do not cause permanent stem-cell damage. If used as initial therapy, they may permit disease control with subsequent stem-cell collection for possible transplantation.
But is induction therapy always required if transplantation is to be performed? The use of conventional cytoreductive therapy (melphalan-prednisone) prior to transplantation has been shown to be of no value,7 and patients eligible for high-dose chemotherapy usually undergo transplantation as their first therapy. However, patients who are at risk of life-threatening disease progression if left untreated for a few weeks while awaiting transplantation can be treated with agents that do not damage stem cells as a temporizing measure. If induction therapy is administered, measures to avoid opportunistic infections, gastrointestinal hemorrhage, and fluid overload during induction therapy and stem-cell mobilization are critical.
The figure shows our treatment algorithm, which keeps in mind long-term disease control as well as options for managing relapse—and what Nabarzanes said to Alexander in the context of the conquest of Persepolis: “Sed medici quoque graviores morbos asperis remediis curant.” (Indeed doctors too cure serious diseases with harsh remedies.)8
Conflict-of-interest disclosure: The author declares no competing financial interests. ■