Wang and colleagues present the novel candidate gene lysocardiolipin acyltransferase (Lycat) driving the blast colony-forming cell (BL-CFC) toward an endothelial and hematopoietic cell differentiation. Because this cell type is believed to be equivalent to the hemangioblast, this finding implies important mechanisms for in vivo natal and postnatal differentiation processes.
It has been known for some time that BL-CFCs are able to generate both endothelial and hematopoietic cells during mice ES cell differentiation, especially when stimulated by VEGF. But the exact mechanisms driving this development have remained unclear. An important finding in this context was the zebrafish mutant cloche (clo), which results in a defect of the endocard and substantial anemia.1 Within this genetic cloche interval Wang et al were able to clone the Lycat gene. Lycat, a protein involved in the remodeling of cardiolipin, a mitochondrial structure and function protein, has never been discussed in stem-cell differentiation before.2 Interestingly, even the mouse Lycat gene was able to partially rescue the cloche mutant phenotype (Jing-Wei Xiong, Qingming Yu, Jiaojiao Zhang, and John D. Mably, “An acyltransferase controls the generation of hematopoietic and endothelial lineages in zebrafish,” manuscript submitted July 2007).
In the present study, the authors demonstrate a homology among zebrafish, mouse, and human Lycat genes that is highly conserved in its acyltransferase domain. They also demonstrate that its expression pattern is restricted to Flk1+ embryoid bodies (EBs) of ES cell cultures as well as Lin−Sca-1+C-Kit+ and CD31+CD45− bone marrow cells, all of which are markers of a hematopoietic or endothelial cell fate. Interestingly, when the Lycat gene is overexpressed within ES cell clones (Lycat transgene), both the expression of hematopoietic (Runx1, CD41, β-major hemoglobin) and endothelial (Flk1, CD31, VE-Cadherin, Tie1) genes in day-4 EBs is substantially increased using semiquantitative and quantitative RT-PCR. This is confirmed on the protein level, with FACS analysis revealing compatible results. The functional data derived from colony-forming assays are also impressive. In these, the authors demonstrate that, when driven with the Lycat transgene, BL-CFCs increase 3-fold and lead to higher differentiation rates of hematopoietic lineages and higher endothelial cell sprouting. On the other side, Lycat siRNA experiments leading to a significant knockdown of this gene result in a marked inhibition of endothelial and hematopoietic gene and protein expression within EBs. Further evaluation using microarray experiments reveals that, in the Lycat transgene, 65 of 200 (32.5%) affected genes with a significant increase (2-fold) of signal are related to endothelial or hematopoietic signaling or differentiation.
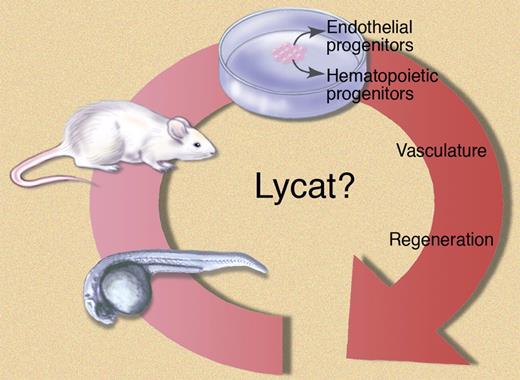
Role of Lycat in cell differentiation: a cardiovascular continuum? The cloche mutant zebrafish characterized by severe anemia and defective endocardium. Out of the cloche genetic interval the Lycat gene was cloned. Mouse derived ES cells transfected with the Lycat transgene are driven to an endothelial and hematopoietic phenotype. Although the results are very promising, many questions remain to be answered before potential translation to clinical applications. Illustration by Marie Dauenheimer.
Role of Lycat in cell differentiation: a cardiovascular continuum? The cloche mutant zebrafish characterized by severe anemia and defective endocardium. Out of the cloche genetic interval the Lycat gene was cloned. Mouse derived ES cells transfected with the Lycat transgene are driven to an endothelial and hematopoietic phenotype. Although the results are very promising, many questions remain to be answered before potential translation to clinical applications. Illustration by Marie Dauenheimer.
The novelty of this study is based on the fact that the acyltransferase Lycat is involved in differentiation processes of EBs toward an endothelial and hematopoietic phenotype under in vitro conditions and that it is enriched in endothelial and hematopoietic progenitors of the bone marrow. We do not know which signaling pathway is utilized to achieve this goal. It remains for future studies to perform engrafting experiments with Lycat modified ES cells in mice to elucidate whether Lycat stays true to its attributed character under in vivo conditions. This would serve as a prerequisite to further exploration of this new pathway and eventual translation to clinical science.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal