After allogeneic blood or bone marrow transplantation, donor T cells interact with a distorted antigen-presenting cell (APC) environment in which some, but not all, host APCs are replaced by APCs from the donor. Significantly, host APCs are required for the priming of acute graft-versus-host disease (GVHD). Donor APCs play a lesser role in the induction of acute GVHD despite their predicted capacity to cross-present host antigens. In contrast, donor APCs may play a role in perpetuating the tissue injury observed in chronic GVHD. Host APCs are also required for maximal graft-versus-leukemia responses. Recent studies have suggested potential strategies by which the continued presence of host APCs can be exploited to prime strong donor immunity to tumors without the induction of GVHD.
Introduction
Forty years ago, Richard Billingham proposed that graft-versus-host reactions could be explained by the response of donor immunocompetent cells to recipient antigens in recipients who are unable to reject donor cells.1 To this day, the central tenets of this hypothesis remain intact, but rapid advances in our understanding of innate and adaptive immunity have allowed refinements of the model. Thus, T cells were demonstrated to be the primary immunocompetent cells that induce graft-versus-host disease (GVHD)2 or graft-versus-leukemia (GVL) responses.3 Several groups4,,,,,,–11 demonstrated the importance of the initial inflammatory response to conditioning-induced injury. More recently, the role of additional cell populations such as natural killer (NK) cells,12 γδ T cells,13,14 and regulatory T cells15,,,–19 have been incorporated into a more complex conceptual framework that explains the pathogenesis and modulation of graft-versus-host responses.
Another major advance in our understanding is the demonstration of the critical role of professional antigen-presenting cells (APCs) in GVHD20,–22 and GVL.23,–25 In this review, we attempt to place these findings in context by exploring how APC functions integrate with those of other accessory or effector cell populations or cytokines that play a role in the graft-versus-host response. We then examine the potential implications for the design of novel strategies to boost GVL responses and reduce GVHD after transplantation.
Antigen-presenting cell populations
Many cell types, including both hematopoietic cells and nonhematopoietic cells (eg, endothelial cells), may participate in the process of antigen presentation with varying levels of efficiency.26,27 Professional APCs are hematopoietic cells that have a specialized role in processing and presenting antigens and do so with an effectiveness that is above the threshold required to promote an adaptive immune response. They include B cells, macrophages, and dendritic cells (DCs), although other hematopoietic cells such as CD34+ progenitor cells28 or γδ T cells29 and nonhematopoietic cells can develop antigen-presenting ability under specific conditions. Professional APCs are highly efficient at loading peptide antigens onto major histocompatibility complex (MHC) molecules and then delivering them to the cell surface as composite structures with costimulatory and leukocyte adhesion molecules that together aid formation of an immunological synapse with the T cell.30 The major APC populations occupy distinct strategic locations and recirculatory patterns. Naïve B cells are absent from the skin and most mucosal sites, but instead recirculate between blood and the secondary lymphoid organs. Here, they pick up antigens through specific B cell receptors for presentation on MHC class II and become competent APC on interaction with CD40L+ CD4+ T cells.31 Macrophages are resident at most sites, but are actively recruited to sites of inflammation, wherein a subset of cells develop a DC-like phenotype.32 Crucially, DCs possess the additional capacity to pick up antigens in peripheral tissues and traffic to secondary lymphoid organs.30 Accordingly, DCs are the most effective at priming naïve T cells, although the extent to which they do so is heavily dependent on their origin and activation history.
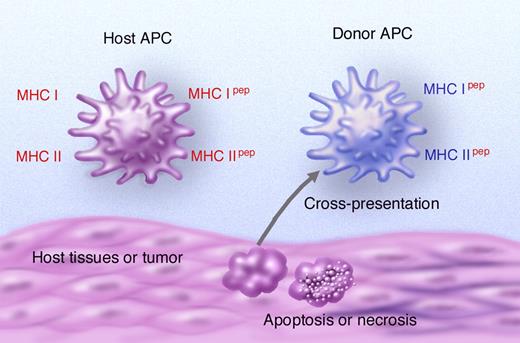
Separate endogenous and exogenous pathways exist for loading peptides onto MHC class I or MHC class II, respectively (see Ackerman and Cresswell33 for review). In the endogenous pathway, proteins or peptides within the cytosol are degraded by the proteasome before being transported into the endoplasmic reticulum where peptides are loaded onto MHC class I molecules. In the exogenous pathway, soluble or particulate antigens are taken up by phagocytosis or micropinocytosis and then hydrolyzed by peptidases before loading of the derived peptides onto MHC class II within the endolysosomal system.33 Certain APCs (eg, CD8α DCs in mice) have the additional capacity to divert proteins derived from particulate antigens (eg, phagocytosed necrotic or apoptotic cells) into the endosomal/cytosolic pathway, allowing the derived peptides to be presented in the context of MHC class I. This phenomenon is termed crosspresentation and is thought to be essential for the development of immunity to infectious agents (in which APCs are themselves not infected) or tumors. Antigen-laden DC populations or DC precursors34,–36 may act as “couriers” by trafficking from tissues to draining lymph nodes, delivering antigen to crosspresenting CD8α DCs.37 Recent studies have suggested that crosspresentation is a highly efficient process, although the extent to which priming occurs depends on whether presentation occurs within the context of an inflammatory or noninflammatory environment.38 After transplantation, host alloantigens may thus be presented directly by host APCs or be crosspresented by donor APCs (Figure 1).
Pathways of antigen presentation after allogeneic stem cell transplantation. Host alloantigens can be presented by host APC (direct presentation) or crosspresented by donor APC after uptake of particulate host material (indirect presentation). In direct presentation, donor T cells can potentially recognize allogeneic MHC antigens on host APC (MHC shown in red with or without a requirement for peptide) or may recognize host alloantigens (including allogeneic MHC or minor H antigens) that have been processed and presented by host APC. Alternatively, host allogeneic MHC or minor H antigens may be presented indirectly by donor APC.
Pathways of antigen presentation after allogeneic stem cell transplantation. Host alloantigens can be presented by host APC (direct presentation) or crosspresented by donor APC after uptake of particulate host material (indirect presentation). In direct presentation, donor T cells can potentially recognize allogeneic MHC antigens on host APC (MHC shown in red with or without a requirement for peptide) or may recognize host alloantigens (including allogeneic MHC or minor H antigens) that have been processed and presented by host APC. Alternatively, host allogeneic MHC or minor H antigens may be presented indirectly by donor APC.
The turnover of antigen-presenting cell populations after transplantation
After lethal conditioning and allogeneic hematopoietic stem cell transplantation (HSCT), the APC environment undergoes dramatic distortion. Radio- or chemosensitive APC and precursor populations derived from the host are lost within the first few days after transplantation. The void in host APC numbers is filled by differentiated APCs contained within the donor graft or by differentiation from donor progenitor cells. Numerical recovery of DCs and macrophages is more rapid than that of B cells,39 although the kinetics of full functional reconstitution are not known, especially in the context of posttransplantation immune suppression. The kinetics of the switch to donor APC populations is primarily dependent on the mode of conditioning, the site, and the presence or absence of donor T cells in the graft. After myeloablative conditioning, this process can be very rapid as indicated by data from animal models40,–42 and a number of recent clinical studies.43,44 In contrast and as expected, the extent and/or rate of switching are decreased after reduced intensity conditioning.44,45 However, even after myeloablative conditioning, the macrophage and skin DC (dermal DC and epithelial Langerhans cell [LC]) compartment appear fairly resistant to conversion to full donor chimerism, a fact that may reflect the capacity of these populations to be maintained by resident, radioresistant precursor cells.40,41,43,,–46 Thus, recipient DCs continue to be present for a considerable time posttransplantation within the skin epithelia, or draining lymph nodes.40,46 The presence of T cells in the donor graft is also critical, because residual host LCs are targeted to some extent by the developing graft-versus-host reaction.40,41 However, host LC can persist even in the presence of donor T cells40 (M. Sykes, W. Caughman, S. Katz, and D.H. Sachs, 1987, unpublished data).
Antigen-presenting cells and acute graft-versus-host disease
After their transfer to freshly irradiated allogeneic recipients, naïve donor T cells are initially retained within secondary lymphoid tissues.47 Within the lymph nodes, spleen, and Peyer patches, donor T cells undergo a rapid burst of proliferation. After an interval of 3 to 4 days, large numbers of lymphoblasts enter the peripheral circulation. Recruitment of effector T cells to peripheral tissues such as the skin and gut47,–49 leads to profound tissue injury that characterizes GVHD.
The initial “trapping” of donor T cells within secondary lymph nodes of recipients was originally described by Jonathan Sprent and colleagues in a series of elegant experiments in which they measured the number of graft-versus-host reactive T cells entering the thoracic duct in the first few days after allogeneic bone marrow transplantation (BMT). As graft-versus-host-reactive T cells are sequestered in the lymph nodes, thoracic duct output cells are initially depleted of cells capable of inducing GVHD on transfer to secondary recipients. Using this model in an MHC mismatched strain combination, they demonstrated that host bone marrow-derived cells (presumably host APCs expressing host class II alloantigen) were important but not absolutely critical in the initial retention of CD4+ T cells within the lymph nodes.50 In contrast, although host bone marrow-derived cells contributed to donor CD8+ T cell trapping, other cells also played a major role.51 Because MHC class I expression is ubiquitous (unlike class II), nonbone marrow-derived cells may contribute more to the initial retention of CD8+ than CD4+ T cells. An alternative explanation is that donor APC crosspresent peptides derived from the host to donor T cells.
The issue of whether such crosspresentation is sufficient to prime acute GVHD has been evaluated in experimental models of CD8+ T cell-dependent GVHD developing across MHC-matched, minor histocompatibility (H) antigen mismatched barriers.21,52 Early experiments demonstrating acute GVHD induction after transfer of strain donor b splenocytes to reirradiated strain b → strain a bone marrow chimeras may reflect the use of insufficient total body irradiation (TBI) doses to remove all host (strain a) APCs,52 because the opposite result was more recently obtained in similar experiments using higher TBI doses.21 In the latter study, marked resistance to GVHD was also evident after transfer of CD8+ T cells (from strain b) to irradiated strain a β2M-/-→ strain a chimeras (in which bone marrow-derived host APCs lacked MHC class I expression), indicating a requirement for the presence of host APCs expressing MHC class I.21 Thus, despite the capacity for crosspresentation by donor APCs in this model, no GVHD was observed in mice lacking class I on host APCs, indicating that crosspriming is not sufficient to initiate acute GVHD. A similar interpretation was suggested in the context of CD8+ or CD4+ T cell-dependent GVHD when donor T cells recognized single MHC class I or II alloantigens.53,54 Thus, resident host APCs that survive host conditioning are required and sufficient to initiate acute GVHD. Indeed, very small numbers of host CD11c+ DCs surviving for the first few days after conditioning are able to prime donor T cell activation and effector differentiation.42
Donor APCs transferred directly with the graft or developing from hematopoietic progenitor cells do not contribute to the initiation of the response, but they may drive further tissue injury by crosspresentation of host antigens. Thus, in experiments in which donor APCs are selectively prevented from crosspresenting host antigens, GVHD still occurs, but its incidence and severity are sharply diminished.55 Donor APCs that produce and present interleukin (IL)-15 through IL-15 receptor α on their cell surface may also be required for perpetuating tissue injury.56 The distinct roles of host and donor APCs that have emerged in these model systems are likely to reflect the fact that in the very early phase after transplantation, too few donor APCs have differentiated sufficiently to process and present host antigens effectively.
The identity or identities of the professional APCs required for inducing acute GVHD have not been established precisely. Depletion of phagocytic DCs and macrophages from the liver and spleen by exposure to liposomal clodronate leads to a specific reduction in donor T cell infiltration of these organs.57 Selective “add back” of individual APC populations to chimeras in which host APCs have been disabled (by failure to express host MHC alloantigen) suggest that host DCs rather than host B cells are important in the induction of both CD4+ and, to a lesser extent, CD8+ T cell-dependent acute GVHD.53 In fact, recent studies indicate that persistent host B cells negatively regulate acute GVHD induction through an IL-10-dependent mechanism.58 In a similar vein, although donor APCs contribute to the overall severity of GVHD, certain donor DC populations (e.g. CD11c+ CD11b+ CD4-CD8α-DCs)59 or reconstituting myeloid cells60 appear to impede its induction.
The role of antigen-presenting cells within graft-versus-host disease target tissues
The pattern of GVHD organ involvement (liver, skin, and gut) is striking. Three potential factors may contribute to this phenomenon. First, highly dense networks of DCs are organized into specific compartments within these tissues (eg, LC in the skin epithelia) and associated lymphoid structures (eg, Peyer patches of the gut). The functional capacities of these cells might also be relevant, as suggested by the increased potential immunogenicity of certain APC populations such as LC.61 Second, the distribution corresponds to the potential sites of exposure to microbes and microbial products through the skin, gut, and portal circulation. Loss of epithelial integrity after conditioning may increase exposure to pathogen-associated molecular patterns such as lipopolysaccharide62 that are recognized by pattern-recognition receptors expressed on APCs and lead to their activation (see subsequently). Third, priming DCs from these sites (eg, the Peyer patches or skin draining lymph nodes) are particularly adept at “imprinting” the expression of tissue-specific homing receptors on activated T cells that bias recirculation of effector T cells back to the tissue from which the DC were derived. For example, Peyer patch-derived DCs prime T cells to express the gut-homing receptors α4β7 and CCR9 and the subsequent recruitment of these cells to the gut.63 Similarly, DCs derived from skin-derived lymph nodes prime functional E/P-selectin ligand expression by activated T cells and selective trafficking to the skin.64 Imprinting could, for example, explain the development of skin GVHD (but not gut or liver GVHD) when naive donor T cells are transferred to allogeneic chimeras in which the only tissue containing host APCs is the skin 40,41 (Figure 2). To date, however, there is no formal proof that this tissue imprinting by APCs applies to the induction of GVHD. Indeed, Peyer patches are not required for the induction of GVHD in freshly irradiated allogeneic recipients, indicating that under these conditions, priming of T cells that home to the gut can occur elsewhere.65
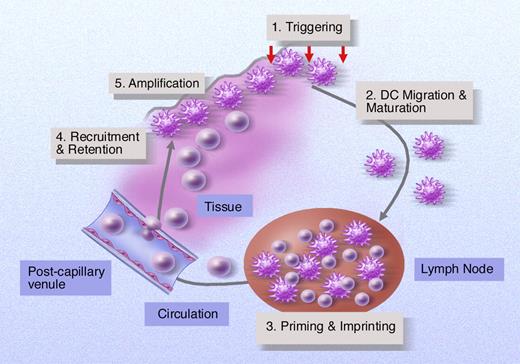
Antigen-presenting cells can influence development of GVHD at multiple levels. Initial tissue injury and innate immune activation may trigger APC within tissues and induce certain APC populations such as DCs to migrate to draining lymph nodes. Migrating DCs or other lymph node resident APCs “trap” graft-versus-host-reactive T cells at this site for 3 to 4 days where they induce T cell activation and may “imprint” a homing phenotype that permits selective trafficking of effector cells to GVHD target organs. APCs within inflamed tissues may act to amplify the developing GVH response by providing further priming signals to T cells in situ by producing proinflammatory cytokines or actively recruiting T cells and other cellular effectors to this site.
Antigen-presenting cells can influence development of GVHD at multiple levels. Initial tissue injury and innate immune activation may trigger APC within tissues and induce certain APC populations such as DCs to migrate to draining lymph nodes. Migrating DCs or other lymph node resident APCs “trap” graft-versus-host-reactive T cells at this site for 3 to 4 days where they induce T cell activation and may “imprint” a homing phenotype that permits selective trafficking of effector cells to GVHD target organs. APCs within inflamed tissues may act to amplify the developing GVH response by providing further priming signals to T cells in situ by producing proinflammatory cytokines or actively recruiting T cells and other cellular effectors to this site.
The relative importance of APCs versus host epithelial cells in generating the full manifestations of GVHD is controversial. By transferring donor CD4+ T cells to chimeric mice in which bone marrow-derived APCs expressed MHC class II alloantigen but in which other cells in peripheral tissues lacked MHC II expression, Teshima et al54 reported the surprising finding that expression of host alloantigen by epithelial cells was not required for the induction of GVHD. To a lesser extent, this was also true in CD8+ T cell-dependent GVHD directed against a single MHC class I alloantigen. The authors demonstrated that GVHD was mediated in this model by effector cytokines (tumor necrosis factor-α or IL-1β), and one possibility is that this process is amplified by host-derived bone marrow APCs resident within the target organs. In contrast to these findings, donor T cells could induce tissue damage only when alloantigens were expressed on epithelial cells in MHC-matched, minor H antigen mismatched murine models of CD8+ or CD4+ T cell-dependent acute GVHD.66,67 The discrepancy between these results might be explained by lower graft-versus-host reactive T cell precursor frequencies to mismatched minor H antigens versus MHC alloantigens and hence a reduced capacity to provoke an inflammatory cytokine cascade. In the relative absence of effector cytokines, tissue injury would then depend on direct cell-mediated cytotoxicity against epithelial targets expressing class I or class II MHC.68 The sparing of donor skin grafts but not recipient skin after the induction of GVHD in dogs undergoing minor H antigen-mismatched BMT and delayed transfer of donor T cells69 is consistent with this concept.
APCs within tissues may theoretically act at both the priming (including “imprinting”) and effector phase of the graft-versus-host response (Figure 2). In the former case, antigen-loaded tissue DCs could migrate to draining lymph nodes where they prime donor T cells directly or alternatively deliver antigens to specialized resident APC populations. In the latter scenario, tissue-resident APCs might actively recruit activated T cells to target organs through the secretion of inflammatory chemokines. Evidence in support of this latter hypothesis comes from models in which liposomal clodronate was used to selectively deplete host APCs (macrophages and most CD11c+ DCs) from the liver of BMT recipients before the transfer of primed, graft-versus-host reactive effector T cells.57 In these experiments, effector T cells were unable to access liver tissue if host APCs had been depleted just before transfer.
The effect of the posttransplantation environment
There are two distinct phases after myeloablative transplantation that influence the functional status of APCs. In the first, host conditioning leads to a loss of epithelial integrity, systemic exposure to microbial products, and activation of the innate immune system.62 Activation of the innate immune system as a result of direct tissue injury or exposure to microbial products is an important initiating event in the induction of GVHD. NK cells can, through activating receptors, detect altered self-ligands whose expression is increased on cellular stress.70 Mucosal γδ T cells detect microbial antigens (eg, pyrophosphate monoesters) as well as stress-induced self-molecules such as heat shock proteins.71 TBI-induced tissue injury causes translocation of bacterial products such as lipopolysaccharide across damaged epithelium, leading to macrophage activation and the release of proinflammatory cytokines.6,7,9,10 The importance of this pathway in the development of GVHD is emphasized by experiments in which blockade of lipopolysaccharide action efficiently blocks the induction of tissue injury.5 The release of tumor necrosis factor-α or interferon-γ by activated cells or direct cellular interactions contribute significantly to DC maturation with upregulation of costimulatory molecule expression and the release of cytokines that promote T helper type 1 differentiation.72 DC expression of pattern-recognition receptors such as Toll-like receptors (TLR) makes them exquisitely sensitive to microbial stimuli. It has been proposed that TLR triggering is required in cis with adaptive immune signals (eg, ligation of the CD40 receptor by activated T cells) to induce full “licensing” of DCs that then become capable of priming full Th1 responses.73 To what extent such combinatorial signaling mediated through APC is actually required for the induction of GVHD or GVL is not known. The fact that GVHD is apparently induced in Myd88-/-mice, in which host APCs are defective in their capacity to respond to TLR signals, and also in CD40-/-mice, in which APCs cannot be activated by donor T cells expressing CD40L, suggests considerable redundancy in the requirements for APC activation.74 In this setting, inflammation or other changes (such as lymphopenia) generated as a result of irradiation may be sufficient to bypass the requirement for Myd88 pathway activation or CD4+ T cell help. Whatever the mechanism, it is likely that “licensing” of APCs provides an essential checkpoint that controls the initiation of GVHD. It follows that this process must be sufficient to overcome any regulatory mechanisms that induce peripheral tolerance. In the very early phase posttransplantation, depletion of host regulatory T cells and the generation by DC of proinflammatory cytokines (eg, IL-6) that act directly on effector T cells75 may overcome any potential downmodulatory influences on graft-versus-host reactivity.
In the second phase after myeloablative transplantation, resolution of inflammation leads to a new “steady state” in which the capacity of APCs to provoke immunity against the host may be substantially reduced because there are fewer host APCs, and these are no longer activated. In the absence of APC activation, specific targeting of model antigens to DC leads to the abortive proliferation of specific T cells that lack the capacity to generate effector cytokines.76,77 Thus, in the absence of inflammation, tolerance may be induced by “default.” In contrast, if DCs are activated artificially by coadministration of agonistic CD40 antibody or other proinflammatory stimuli, productive immunity is generated.76,77 Consistent with this model, the magnitude of the immune response (in terms of both GVHD and GVL) after delayed donor leukocyte infusions (DLI) to full allogeneic chimeras diminishes significantly with time. In the first few weeks posttransplantation, transfer of donor T cells may induce GVL with a reduced risk of GVHD. However, after 4 to 5 weeks, nearly all graft-versus-host reactivity is lost.78,79 Although there are a number of potential explanations for this phenomenon, including the loss of host APCs and the influence of regulatory cell populations,79 one factor important in the declining response might also be the lack of APC activation. It is also possible that after recovery of acute GVHD, priming APC not only become depleted, but also become “conditioned” and less able to provoke immunity.80
These temporal changes in GVH reactivity, observed after myeloablative transplantation, are not necessarily reproduced after transplantation using nonmyeloablative protocols. In the early phase, the relative lack of tissue injury may provoke less triggering of the innate immune system and hence less APC activation. If donor hematopoietic progenitor cells are given without donor T cells, host APCs are present in greater numbers than after conventional transplantation. In this setting, delayed transfer of donor T cells can induce very significant graft-versus-host reactivity with no evidence of a decay in the response according to the time of T cell transfer.81 In preclinical models, the GVH response is restricted to the lymphohematopoietic system and no GVHD is observed despite the presence of considerable numbers of host APCs at the time of donor T cell transfer.4,11,23,24 Graft-versus-host-reactive T cells become activated, expand, and acquire homing receptors for GVHD target organs yet fail to access the skin or gut.4 This failure of T cells to traffic and induce tissue injury can be readily reversed in the presence of proinflammatory stimuli. Indeed, localized GVHD can be induced after exposure of the skin to a topical TLR agonist,4 and one potential explanation is that APC activation within tissues is important in the development of wider tissue injury.
Much of the previously mentioned data are derived from preclinical models that by necessity have been designed to reduce the number of potential variables that might influence the development of an immune response after transplantation. For example, models of experimental transplantation in mice rarely incorporate any form of posttransplant immune suppression. In humans, immunosuppressive agents used to prevent GVHD may therefore blunt the effects of priming by APCs on T cells. On withdrawal of immunosuppression, surviving T cells previously activated by host APCs might then be recruited to a developing graft-versus-host response. In this case, donor APCs crosspresenting host alloantigens might have a role in driving the response, as suggested in one study.55 Drugs such as calcineurin inhibitors, glucococorticoids, and mycophenolate mofetil not only modulate T cell activation, but also influence DC functions.82,–84 For example, calcineurin inhibitors inhibit antigen uptake and processing, key elements of the crosspresentation apparatus.82 Glucocorticoids inhibit DC differentiation and cytokine production on exposure to proinflammatory stimuli.84 Granulocyte colony-stimulating factor has a number of distinct effects on APC populations, including differential mobilization of DC subsets,85 alteration of the capacity of DCs to generate cytokines in response to TLR signaling,86 and inhibition of IL-12 production by DCs.87 The precise extent to which any or all of these factors influence the development of GVL or GVHD is unknown.
Antigen-presenting cells and chronic graft-versus-host disease
After myeloablative transplantation, donor hematopoietic progenitors reconstitute the thymocyte and thymic APC compartments, whereas radioresistant cortical and medullary epithelial cells are derived from the host. In this scenario, positive selection of developing donor thymocytes will occur on host epithelia. In contrast, negative selection of cells with potential antihost reactivity may occur either on host-derived medullary epithelial cells (through, for example, autoimmune regulator (AIRE)-dependent expression of host self-antigens88 ) or on donor-derived APCs crosspresenting host antigens within the thymic medulla.89 Host-derived thymic epithelial cells may induce anergy of host-reactive thymocytes90 or positively select regulatory T cells.91 Collectively, these processes must be efficient if T cells with antihost reactivity are not to induce tissue damage within the periphery. Disruption to the thymic architecture after the development of acute GVHD92 may perturb these tolerance mechanisms and lead to the release of alloreactive T cells. Furthermore, the emergent T cell repertoire may also contain increased numbers of cells that are truly autoreactive and thus have the potential to be primed by donor APCs in peripheral tissues.93,94 These findings may help to explain the continuing tissue injury that occurs in chronic GVHD despite the majority of APCs, including DCs, being of donor origin.95 Formal testing of the role of APC populations in the development of chronic GVHD has proven somewhat difficult, because no single model completely represents the delayed onset and complex phenotype observed in human patients. Thus, although both host and donor APCs,96 including B cells,97,98 have been demonstrated to play a role in priming donor CD4+ T cells, the exact clinical relevance of these models is uncertain.
Antigen-presenting cells and graft-versus-leukemia
In experiments using in vivo tumor protection assays in which donor T cells are transferred after a delay to established allogeneic chimeras, host APCs are required to prime maximal GVL activity.23,24 Maximal GVL responses against a host-derived tumor expressing class I but not class II MHC requires expression of not only class I, but also class II MHC by host APCs.23,24 In this model, maximal expansion of graft-versus-host-reactive CD8+ T cells and GVL activity depends on the presence of both CD4+ T cells in the DLI and on host APCs23 consistent with a model in which donor CD4+ T cells “license” host APCs for priming of donor graft-versus-host-reactive T cells. Of note, host APCs do not appear to influence donor T cell effector differentiation as evaluated by cytokine synthesis. Thus, host APCs appear to maximize expansion (through effects on proliferation and/or survival) rather than influence effector functions on a per cell basis. Significantly, in freshly conditioned recipients, CD4+ T cells are not required to provide help for the CD8+ T cell-dependent GVL response.23 This suggests that the ability of conditioning-induced inflammation to activate APCs bypasses the requirement for CD4 help to maximize CD8 effector expansion.
In these and similar assays involving delayed T cell transfer and a highly aggressive tumor, GVL activity is dependent on MHC alloreactivity and little or no GVL activity against minor H antigens or tumor-associated antigens (TAA) is detected.23,99 The requirement for shared alloantigen expression by host APCs and tumor cells has been confirmed in other models, although in cases that involve T cell transfer to freshly irradiated mice, reactivity against minor H antigens is sufficient to induce GVL.25,79 For the most part, crosspresentation of TAA does not lead to any detectable GVL effects.25 However, crosspresentation of alloantigens and/or TAA by donor APCs was sufficient to induce measurable GVL activity under conditions of low tumor bulk.25 It is noteworthy that none of these studies (which involve tumor inoculation after irradiation) have tested the potential of chemotherapy/ radiotherapy during conditioning to enhance crosspresentation. In studies of established tumors, induction of apoptosis by chemotherapy is sufficient to boost crosspresentation of TAA and this area merits further investigation.100
The role of tumor cells in priming GVL activity is not known. It has been hypothesized that the sensitivity of chronic myeloid leukemia (CML) or low-grade lymphoma to GVL relates to the potential of the neoplastic cells to develop APC functions.101 This concept is attractive, because such cells could then present TAA such as proteinase 3,102 p210 (in CML),103 or idiotype (in lymphoma)104 directly to the immune system. For example, CML stem cells can differentiate into dendritic cells105 and on ligation of CD40, B cells derived from patients with follicular lymphoma develop APC functions.106 It is important to emphasize, however, that GVL responsiveness is not necessarily synonymous with direct priming by tumor cells and such responses might instead reflect increased sensitivity of these cells to the effector phase of the immune response. In fact, to date, there is no evidence from preclinical models that tumor cells can directly prime GVL directed at minor H antigens or TAA even if such cells express costimulatory molecules.25
For patients with tumors that are sensitive to GVL activity, long-term disease-free survival requires that GVH-reactive T cells eradicate all tumor cells during the initial primary response or that any residual cancerous cells are held in check by memory effectors that can mount a recall response. In many patients, antitumor responses are poorly sustained.101,107 In a model of GVL induced by delayed transfer of donor T cells (mixed with GVH-reactive T-cell receptor (TCR) transgenic CD8+ T cells) to mixed chimeras, graft-versus-host-reactive cytotoxic T lymphocytes (CTL) were shown to undergo initial expansion, followed by deletion, in association with gradual extinction of host-reactive CTL responsiveness. No recall immunity is established and mice are susceptible to tumors on subsequent rechallenge.81 The reasons for this are not clear, but it is known that the initial graft-versus-host response eradicates host APCs, leaving only donor APCs that are less efficient at eliciting GVL. Although “add back” of host DCs at the time of donor T cell transfer to full chimeras can help to prime effective GVL activity,79 it remains to be seen whether a similar strategy can rescue a secondary response. Alternatively, strategies aimed at maximizing the proliferation and/or survival of graft-versus-host-reactive memory stem cells (eg, administration of IL-15)108 in full chimeras might also permit enhancement of GVL activity even in the absence of host APCs.
Novel therapies targeting antigen-presenting cells
The concept of manipulating antigen presentation for therapeutic benefit peritransplantation or posttransplantation is an attractive one. In very general terms, changes in antigen presentation might be achieved by altering APC functions or numbers (Table 1)Preclinical data suggest that depletion of host APCs or interference with their functions would be inappropriate in settings where one is depending on GVL activity to clear residual tumor.23,–25 In contrast, where GVL is not required, such as after transplantation for nonmalignant disorders, reductions in host APC number or function might be an acceptable means of preventing GVHD. One potential advantage of this approach is that avoidance of T cell depletion would permit initial reconstitution by memory T cells and a reduced risk of infection. A potential disadvantage is that a reduction in host APCs might also limit the potential expansion of alloantigen-specific Foxp3+CD4+CD25+ T cells,109 thus negating any potential reduction in the priming of graft-versus-host-reactive effector T cells. An alternative strategy could include administration of FTY720, a novel agent that prevents GVHD induction but allows potent GVL activity.110 Potential mechanisms might include inhibition of activated DC migration to111 and/or reduced egress of effector T cells from secondary lymphoid organs.112
Potential strategies to alter APC numbers or function
| Reagent . | Comment . |
|---|---|
| CAMPATH-1H | Depletes blood DCs118,119 |
| Tissue penetration may be limited and LC (which have low CD52 expression) are not depleted significantly118,120 | |
| CMRF-44 | IgM antibody, which binds to a determinant on activated DCs and is expressed on both peripheral blood DC and migrating LC |
| Induces depletion by complement-mediated cytotoxicity in vitro121 | |
| UV | Ultraviolet (A-B) light leads to host-derived LC migration from the skin and replacement by donor-derived LC progenitors122 |
| Short wavelength ultraviolet irradiation effectively depletes host LC and prevents the induction of GVHD,41 although ultraviolet induces other immunomodulatory effects; translation to human setting has proven problematic123 | |
| Alloreactive NK | Preconditioning of recipient mice with alloreactive donor NK cells lacking inhibitory receptors for host class I MHC induces resistance to the development of GVHD; alloreactive NK cells deplete host CD11c+ DCs20 |
| Clinical applications could include choosing unrelated donors who are mismatched for inhibitory ligands or NK cells from third party donors to deplete host DCs before transplantation | |
| Costimulatory blockade | Inhibit host APC-T cell interactions (see Blazar and Taylor124 for extensive review) |
| Regulatory DC | Adoptive transfer of IL-10/TGFβ conditioned host DCs highly effective at preventing GVHD125 |
| FT720 | Sphingosine-1-phosphate (S-1-P) analog that inhibits GVHD but preserves GVL110 ; DCs express receptors for S-1-P and are therefore potential targets for this drug; administration of FTY720 inhibits DC migration and is associated with a sharp reduction in their capacity to access secondary lymphoid organs111 |
| Reagent . | Comment . |
|---|---|
| CAMPATH-1H | Depletes blood DCs118,119 |
| Tissue penetration may be limited and LC (which have low CD52 expression) are not depleted significantly118,120 | |
| CMRF-44 | IgM antibody, which binds to a determinant on activated DCs and is expressed on both peripheral blood DC and migrating LC |
| Induces depletion by complement-mediated cytotoxicity in vitro121 | |
| UV | Ultraviolet (A-B) light leads to host-derived LC migration from the skin and replacement by donor-derived LC progenitors122 |
| Short wavelength ultraviolet irradiation effectively depletes host LC and prevents the induction of GVHD,41 although ultraviolet induces other immunomodulatory effects; translation to human setting has proven problematic123 | |
| Alloreactive NK | Preconditioning of recipient mice with alloreactive donor NK cells lacking inhibitory receptors for host class I MHC induces resistance to the development of GVHD; alloreactive NK cells deplete host CD11c+ DCs20 |
| Clinical applications could include choosing unrelated donors who are mismatched for inhibitory ligands or NK cells from third party donors to deplete host DCs before transplantation | |
| Costimulatory blockade | Inhibit host APC-T cell interactions (see Blazar and Taylor124 for extensive review) |
| Regulatory DC | Adoptive transfer of IL-10/TGFβ conditioned host DCs highly effective at preventing GVHD125 |
| FT720 | Sphingosine-1-phosphate (S-1-P) analog that inhibits GVHD but preserves GVL110 ; DCs express receptors for S-1-P and are therefore potential targets for this drug; administration of FTY720 inhibits DC migration and is associated with a sharp reduction in their capacity to access secondary lymphoid organs111 |
Several clinical studies have explored the potential for augmentation of GVL after delayed DLI to mixed chimeras. Although this approach has clearly afforded the induction of major antitumor responses,113,–115 no clear relationship is apparent between the degree of myeloid chimerism (in peripheral blood) and disease responses.115 The lack of such a correlation could result from a variety of factors, but may reflect the capacity of crosspresentation of tumor-associated and minor H antigens by donor APC to prime similar antitumor responses as those induced through direct presentation. In contrast, the mouse studies showing the ability of host APC to optimize antitumor effects involved extensive MHC disparities between the donor and host,23,24 and these disparities were critical for achievement of antitumor responses.23,24 In the highly aggressive tumors studied in the mouse model, antiminor H antigen or antiTAA responses were insufficient to induce measurable GVL effects through either direct or crosspresentation to DLI T cells.23 Because GVL is not readily elicited in murine MHC-matched BMT models,23,78,99 the role of host APCs in inducing antitumor responses from DLI has proven difficult to determine. These studies highlight the potential to achieve stronger GVL effects in humans if HLA barriers could be transgressed without severe GVHD.
Even in the absence of HLA disparity, it might be feasible to augment GVL activity at the time of DLI by increasing the number of available APCs (including leukemia-derived DCs) that can present host antigens by using cytokines such as Flt3 ligand116 or GM-CSF.101 This approach could be combined with reagents that induce APC activation such as TLR agonists4,40 or by the blockade of coinhibitory pathways such as PD-1-PD-L1.117 In a similar vein, “add back” of host DCs might boost GVL responses in full allogeneic chimeras.79 It seems likely that such strategies will also increase the risk of GVHD, although this might be acceptable in individuals with few other therapeutic options.
Acknowledgments
We thank Dr. Clare Bennett for helpful review of the manuscript and Ms. Kelly Walsh for expert assistance with the manuscript.
This work was supported by NIH grants RO1 RO1 CA79989, POI CA111519, by a Senior Research Award from the Multiple Myeloma Foundation and by a Senior Bennett Fellowship from the Leukemia Research Fund, United Kingdom.
National Institutes of Health
Authorship
Contribution: R.C. and M.S. cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Megan Sykes, Transplantation Biology Research Center, Bone Marrow Transplantation Section, Massachusetts General Hospital, Harvard Medical School, Massachusetts General Hospital (MGH)-East Bldg. 149-5102, 13th Street, Boston, MA 02129; e-mail: Megan.Sykes@tbrc.mgh.harvard.edu.