CTLA-4 is an inhibitory molecule that down-regulates T-cell activation. Although polymorphisms at CTLA-4 have been correlated with autoimmune diseases their association with clinical outcome after allogeneic hematopoietic stem cell transplantation (allo-HSCT) has yet to be explored. A total of 5 CTLA-4 single-nucleotide polymorphisms were genotyped on 536 HLA-identical sibling donors of allo-HSC transplants. Genotypes were tested for an association with patients' posttransplantation outcomes. The effect of the polymorphisms on cytotoxic T-lymphocyte antigen 4 (CTLA-4) mRNA and protein production were determined in 60 healthy control participants. We observed a reduction in the mRNA expression of the soluble CTLA-4 isoform in the presence of a G allele at CT60 and +49. Patients receiving stem cells from a donor with at least 1 G allele in position CT60 had worse overall survival (56.2% vs 69.8% at 5 years; P = .001; hazard ratio [HR], 3.80; 95% confidence interval [CI], 1.75-8.22), due to a higher risk of relapse (P = .049; HR, 1.71; 95% CI, 1.00-2.93). Acute graft-versus-host disease (aGVHD) was more frequent in patients receiving CT60 AA stem cells (P = .033; HR, 1.54; 95% CI, 1.03-2.29). This is the first study to report an association between polymorphisms at CTLA-4 and clinical outcome after allo-HSCT. The CT60 genotype influences relapse and aGVHD, probably due to its action on CTLA-4 alternative splicing.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has been widely carried out as a curative therapy for several hematological malignancies and nonmalignant disorders such as severe aplastic anemia, thalassemia, or immune deficiencies. Disease relapse and graft-versus-host disease (GVHD) are the major complications after allo-HSCT, with the occurrence of GVHD the main cause of morbidity and mortality.1,2 A variety of genetic and nongenetic factors affect transplantation success by favoring or hindering alloimmune responses. Polymorphisms in minor histocompatibility antigens, cytokine-coding genes, and genes involved in drug metabolism, among others, affect the incidence of GVHD.3,,,–7
Donor T lymphocytes play a critical role in alloimmune recognition, and their ability to detect non–self-antigens can lead to GVHD or contribute to relapse prevention through recognition and subsequent elimination of minimal residual disease.8 Transplantation outcome may be influenced not only by the ability of T cells to recognize antigens but also by their capacity to become activated or inactivated.
In the immune recognition process, 2 signals are required for T lymphocyte expansion and differentiation: the T-cell receptor (TCR) binding to the HLA molecule-peptide complex and an antigen-independent costimulatory signal provided by the B7 (CD80 and CD86)/CD28 interaction. The cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a homologous molecule of CD28 that is a competitive antagonist for B7. CTLA-4 has greater affinity and avidity for B7 than does CD28, and its translocation to the cell surface after T-cell activation results in B7 sequestration and transduction of a negative signal, responsible for T-cell inactivation.9,,–12
The CTLA-4 gene is translated into 2 proteic isoforms: a full-length protein (flCTLA-4) and a soluble counterpart (sCTLA-4), which lacks exon 3 (responsible for coding the transmembrane domain) due to alternative splicing. flCTLA-4 down-regulates T-cell responses by inducing cell-cycle arrest and blocking cytokine production.13,14 It may also be involved in maintaining peripheral tolerance to self-antigens.15 Moreover, there is evidence that CTLA-4 is crucial in both solid organ transplantations and HSCT. Many studies have examined the effect of CTLA-4-Ig, and a number of drugs that induce tolerance and prevent allograft rejection have been developed.16,17 In contrast, little is known about sCTLA-4 function. Although sCTLA-4 was thought to be expressed by nonstimulated human T cells, no evidence of this soluble isoform has been found in the serum of healthy volunteers.18,19
The human CTLA-4 gene is located on 2q33, in a susceptibility region for autoimmune disease. Certain polymorphisms in the CTLA-4 gene correlate with different autoimmune disorders and with some malignancies.20,,,,,–26 The thymine-to-cytosine nucleotide transitions at positions −1722 and −318, and the adenine-to-guanine changes at −1661,+49, and CT60 have been widely investigated. Many studies have identified cytosine and adenine alleles as protective, whereas thymine and guanine are considered risk-associated or susceptibility alleles for several autoimmune diseases.
However, there are no studies regarding the effect of CTLA-4 polymorphisms on alloimmune recognition after allo-HSCT.
Here, we attempt to determine whether CTLA-4 gene polymorphisms −1722, −1661, −318, +49, and CT60 influenced the clinical outcome of patients receiving an allo-HSCT from an HLA-identical sibling donor. We also aimed to elucidate the involvement of the 5 polymorphisms in the transcription and protein expression levels of sCTLA-4 and flCTLA-4.
Patients, materials, and methods
Patients
DNA isolated from peripheral blood from 536 healthy HSC donors was analyzed for the presence of polymorphisms in the CTLA-4 gene. These samples were collected between years 1993 to 2006 from 19 Spanish transplantation centers. The study was performed on DNA samples from donors whose HLA-identical siblings underwent allogeneic HSCT without T-cell depletion after receiving myeloablative-conditioning regimens for the treatment of several hematologic malignancies. All patient-donor pairs meeting the selection criteria were included in the study unless there was no donor DNA availability. Patient characteristics are summarized in Table 1. This study was approved by the ethical committee from the Hospital Universitari de Bellvitge (L'Hospitalet, Barcelona, Spain) and met the recommendations of the Declaration of Helsinki.
Clinical characteristics of the patients included in the study
| No. of patients | 536 |
| Median age (range), y | 34 (0-59) |
| Sex (male:female) | 311:225 |
| Diagnosis | |
| Acute lymphoblastic leukemia | 124 |
| Acute myelogenous leukemia | 163 |
| Chronic myeloid leukemia | 158 |
| Multiple myeloma | 11 |
| Non-Hodgkin lymphoma | 35 |
| Hodgkin disease | 2 |
| Myelodysplastic syndrome | 34 |
| Other | 9 |
| Disease stage (early:advanced) | 326:175 |
| Sex mismatch, n (%) | 132 (24.8) |
| Sensitized donor, n (%) | 96 (20.1) |
| Source of stem cells (PB:BM) | 270:266 |
| Conditioning regimens | |
| Cyclophosphamide + TBI | 206 |
| Busulfan + cyclophosphamide | 308 |
| Other | 20 |
| GVHD prophylaxis | |
| CSA | 29 |
| CSA + MTX | 485 |
| CSA + MTX + corticosteroids | 2 |
| CSA + corticosteroids | 20 |
| No. of patients | 536 |
| Median age (range), y | 34 (0-59) |
| Sex (male:female) | 311:225 |
| Diagnosis | |
| Acute lymphoblastic leukemia | 124 |
| Acute myelogenous leukemia | 163 |
| Chronic myeloid leukemia | 158 |
| Multiple myeloma | 11 |
| Non-Hodgkin lymphoma | 35 |
| Hodgkin disease | 2 |
| Myelodysplastic syndrome | 34 |
| Other | 9 |
| Disease stage (early:advanced) | 326:175 |
| Sex mismatch, n (%) | 132 (24.8) |
| Sensitized donor, n (%) | 96 (20.1) |
| Source of stem cells (PB:BM) | 270:266 |
| Conditioning regimens | |
| Cyclophosphamide + TBI | 206 |
| Busulfan + cyclophosphamide | 308 |
| Other | 20 |
| GVHD prophylaxis | |
| CSA | 29 |
| CSA + MTX | 485 |
| CSA + MTX + corticosteroids | 2 |
| CSA + corticosteroids | 20 |
Advanced disease was considered for patients with acute leukemia beyond first complete remission, chronic myeloid leukemia beyond first chronic phase, and progressive disease for patients with myelodysplastic syndrome, multiple myeloma, or lymphoma. Sensitized donor corresponds to donors with previous pregnancies or transfusions. PB indicates peripheral blood; BM, bone marrow; TBI, total body irradiation; CSA, cyclosporin A; and MTX, methotrexate.
In order to evaluate whether the presence of CTLA-4 polymorphisms affected the CTLA-4 mRNA and protein expression levels, 10 mL of whole blood from 60 healthy blood donors was collected from the Blood Bank of the Hospital Universitari de Bellvitge (BST [Banc de Sang i Teixits]), Barcelona, Spain. All donors gave their written informed consent.
CTLA-4 genotyping
DNA was obtained from 200 μL of whole blood using the QIAamp DNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany).
Polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) was used to genotype 5 biallelic polymorphisms of CTLA-4: −1722TC, −1661AG, −318CT, +49AG, and CT60. The primers and restriction enzymes (New England Biolabs, Beverly, MA) used were: −1722 (BbvI) and −1661 (MseI) forward 5′-CTA AGA GCA TCC GCT TGC ACC T-3′, reverse 5′-TTG GTG TGA TGC ACA GAA GCC TTT T-3′; −318 (MseI) forward 5′-AAA TGA ATT GGA CTG GAT GGT-3′, reverse 5′-TTA CGA GAA AGG AAG CCG TG-3′; +49 (BbvI) forward 5′-GTC AAG GGA CCA TTA GAA G-3′; and CT60 (NcoI) forward 5′-CAC CAC TAT TTG GGA TAT ACC-3′, reverse 5′-AGC TCT ATA TTT CAG GAA GGC-3′. PCR reactions were performed with 100 ng DNA, as previously described,27,28 and 3 μL of the amplified products were digested for 1 hour at 37°C with 1 U of the corresponding restriction enzyme in a total reaction volume of 10 μL. The digestion products were resolved in 2.5% agarose gels stained with ethidium bromide.
mRNA isolation and RT
Whole blood (8 mL) was centrifuged in a Ficoll-Hypaque density gradient and the peripheral blood mononuclear cell (PBMC) layers were collected and pelleted. Total RNA was isolated using the Ultraspec RNA Isolation System (Biotecx Laboratories, Houston, TX). Purified RNA (500 ng) was retrotranscripted using the First Strand cDNA Synthesis Kit for RT-PCR (AMV; Roche Applied Science, Penzberg, Germany), according to the manufacturer's protocol. The reverse transcription (RT) reaction consisted in incubation for 1 hour at 37°C and a reverse transcriptase–denaturing step at 95°C for 5 minutes.
Quantification of sCTLA-4, flCTLA-4, and β-2-microglobulin mRNA expression
Specific primers for sCTLA-4, flCTLA-4, and β-2-microglobulin mRNA were used to amplify fragments of 93 bp, 79 bp, and 89 bp, respectively.20 PCR products were purified with the JETquick PCR Product Purification Spin Kit (Genomed GmbH, Löhne, Germany) and then subcloned into a pCR4-TOPO vector (Invitrogen, Carlsbad, CA). The constructs were verified by direct sequencing.
A standard curve ranging from 10 to 107 copies of each construct was established to quantify sCTLA-4, flCTLA-4, and β-2-microglobulin mRNA copies. β-2-microglobulin was used as housekeeping control transcript.
The quantitative real-time PCR (QRT-PCR) reaction consisted of 1 μL cDNA, 0.2 μL of each primer (10 mM), 0.4 μL of the corresponding probe (previously described;20 4 mM), 5 μL of Premix Ex Taq (Perfect Real Time; Takara Bio, Otsu, Shiga, Japan), and distilled water up to a final volume of 10 μL. Amplification was performed in a LightCycler 2.0 instrument (Roche; Penzberg, Germany). A reference to the standard curve was included in each run, and all samples were replicated once. The average of the replicates was used to determine absolute levels of sCTLA-4, flCTLA-4, and β-2-microglobulin mRNA copies of each sample. Comparison between samples was performed after standardization with β-2-microglobulin, and the sCTLA-4/flCTLA/4 mRNA ratio was also calculated.
Flow cytometric analysis
PBMCs from healthy volunteers were stained with mAbs conjugated with APC, PE, PE-Cy7, FITC, and PerCP fluorochromes (Becton Dickinson, San Jose, CA) against surface CD3, CD8, CD19, CD25, and CD152. Appropriate isotype controls were included for each sample. A total of 100 000 events were acquired for each sample using a FacsCanto flow cytometer (Becton Dickinson, San Jose, CA). The results were expressed as the proportion of CD3+, CD3+/CD8+, CD3+/CD25+, and CD19+ cells expressing CD152 antigen, depending on the CTLA-4 genotype. The samples were also analyzed after 24 hours of stimulation with 50 ng/mL phytohemagglutinin (PHA) and 1 μg/mL PMA (phorbol myristate acetate).
ELISA
Sera (10 μL) was used to quantify sCTLA-4 with a commercially available kit (MedSystems Diagnostics GmbH, Vienna, Austria). sCTLA-4 levels were also determined after 24 hours of PHA and PMA stimulation. A standard curve ranging from 0.156 to 10 ng/mL was established following the manufacturer's protocol. All samples and standards were analyzed in duplicate, and the average between duplicates was calculated. A total of 50 μL of diluted biotin conjugate was added to all wells. After 2 hours of incubation, 100 μL of streptavidin–horseradish peroxidase was added and incubated for 1 hours. Tetramethyl-benzidine (100 μL) was added and incubated for 15 minutes, followed by the addition of the stop solution. The optical density of each well was determined immediately at 450 nm. Absolute levels of sCTLA-4 were extrapolated from the standard curve.
Statistical analysis
The sample size consisted of 536 patients, providing 99% power for comparison of overall survival (OS) between CT60 AA and CT60 AG/GG genotypes. The statistical power calculation was based on a 2-sided significance level of .05, assuming 850 days median survival time on control patients and a ratio of CT60 AG/GG to CT60 AA of 1:3 according to the genotype distribution. The accrual time during which patients were recruited was 1825 days.
Concerning acute GVHD and relapse, the sample size was of 515 and 534 evaluable patients, respectively, which provided a statistical power of 99% to detect differences between both genotypes.
The chi-squared test was used to estimate linkage disequilibrium among pairs of alleles, while haplotype frequencies were determined using the estimating haplotype (EH) frequencies program from Rockefeller University (ftp://linkage.rockefeller.edu/software/eh). CTLA-4 allele frequencies, genotypes, and haplotypes were also formulated by direct counting. Univariate analyses were performed to evaluate the association between the presence of polymorphisms at −1722, −1661, −318, +49, and CT60 of CTLA-4 and OS, acute GVHD (aGVHD), and relapse. Two study groups were established for each polymorphism regarding the presence (in either homozygosis or heterozygosis) or absence of the susceptibility allele, except for −1722. In this case, due to the low frequency of the protective allele in homozygosis, heterozygotes were compared with homozygous subjects for the risk allele.
The Kaplan-Meier method was applied for the analysis of OS. Curves were compared using the Breslow test, which favors the detection of differences in the early events. Statistical incidence estimates were used to determine the cumulative incidence of aGVHD and relapse in the presence of polymorphisms. Death without signs of aGVHD was considered a competing risk in the analysis of aGVHD incidence. The competing risk for relapse was death in complete remission. Acute GVHD was diagnosed and graded according to standard criteria.29 Only grades II and higher of aGVHD were considered for the analysis of aGVHD incidence.
Multivariate Cox regression models using a backward stepwise procedure with the likelihood ratio criterion (inclusion/exclusion criteria: P ≤ .05/P < .10, respectively) were applied to analyze the combined effects of CTLA-4 polymorphisms and other factors on OS, relapse, and aGVHD. All variables in the univariate analysis with a P value at or below .2 were included in the multivariate analysis.
In the analysis of mRNA expression levels of flCTLA-4 and sCTLA-4 according to the 3 different genotypes of the 5 polymorphisms, univariate and multiple regression analyses relating CTLA4 expression to the G allele numbers were performed. P values were 2-sided, and those lower than .05 were considered statistically significant.
Results
Allele, genotype, and haplotype frequencies in the Spanish population
Allele and overall genotype frequencies at the 5 CTLA-4 loci for the 536 allo-HSCT donors are listed in Table 2. Genotypes corresponding to the 5 CTLA-4 polymorphisms did not significantly deviate from the Hardy-Weinberg equilibrium.
Allele and genotype frequencies at the CTLA-4 loci (n=536)
| Polymorphism . | n (%) . |
|---|---|
| No. of donors | 536 |
| −1722 | |
| TT | 372 (83.8) |
| TC | 70 (15.8) |
| CC | 2 (0.4) |
| T allele | 814 (91.7) |
| C allele | 74 (8.3) |
| −1661 | |
| AA | 400 (75.8) |
| AG | 128 (24.2) |
| GG | 0 (0) |
| A allele | 928 (87.9) |
| G allele | 128 (12.1) |
| −318 | |
| TT | 5 (1.1) |
| TC | 82 (18.1) |
| CC | 366 (80.8) |
| T allele | 92 (10.2) |
| C allele | 814 (89.8) |
| +49 | |
| AA | 208 (46.6) |
| AG | 189 (42.4) |
| GG | 49 (11.0) |
| A allele | 605 (67.8) |
| G allele | 287 (32.2) |
| CT60 | |
| AA | 153 (24.1) |
| AG | 248 (47.0) |
| GG | 127 (24.1) |
| A allele | 554 (52.5) |
| G allele | 502 (47.5) |
| Polymorphism . | n (%) . |
|---|---|
| No. of donors | 536 |
| −1722 | |
| TT | 372 (83.8) |
| TC | 70 (15.8) |
| CC | 2 (0.4) |
| T allele | 814 (91.7) |
| C allele | 74 (8.3) |
| −1661 | |
| AA | 400 (75.8) |
| AG | 128 (24.2) |
| GG | 0 (0) |
| A allele | 928 (87.9) |
| G allele | 128 (12.1) |
| −318 | |
| TT | 5 (1.1) |
| TC | 82 (18.1) |
| CC | 366 (80.8) |
| T allele | 92 (10.2) |
| C allele | 814 (89.8) |
| +49 | |
| AA | 208 (46.6) |
| AG | 189 (42.4) |
| GG | 49 (11.0) |
| A allele | 605 (67.8) |
| G allele | 287 (32.2) |
| CT60 | |
| AA | 153 (24.1) |
| AG | 248 (47.0) |
| GG | 127 (24.1) |
| A allele | 554 (52.5) |
| G allele | 502 (47.5) |
Previous studies have described a region of approximately 100 kb between CD28 and the 5′ part of ICOS (the CTLA-4–flanking genes) as being a strong linkage disequilibrium (LD) zone.20 We analyzed the segregation pattern of −1722, −1661, −318, +49, and CT60 single-nucleotide polymorphisms (SNPs) on 780 chromosomes and identified 6 major haplotypes (Table 3).
CTLA-4 most frequent haplotypes
| −1722 . | −1661 . | −318 . | +49 . | CT60 . | n chromosomes (%) . |
|---|---|---|---|---|---|
| T | A | C | A | A | 344 (44.0) |
| T | A | C | G | G | 166 (21.3) |
| T | G | T | A | G | 62 (8.0) |
| T | G | C | A | G | 59 (7.6) |
| C | A | C | G | G | 53 (6.8) |
| T | A | C | A | G | 49 (6.3) |
| −1722 . | −1661 . | −318 . | +49 . | CT60 . | n chromosomes (%) . |
|---|---|---|---|---|---|
| T | A | C | A | A | 344 (44.0) |
| T | A | C | G | G | 166 (21.3) |
| T | G | T | A | G | 62 (8.0) |
| T | G | C | A | G | 59 (7.6) |
| C | A | C | G | G | 53 (6.8) |
| T | A | C | A | G | 49 (6.3) |
CTLA-4 polymorphisms influence clinical outcome after allo-HSCT
The presence of susceptibility alleles at the −1722, −1661, and −318 promoter positions of CTLA-4 did not show significant correlations with OS, aGVHD incidence, or disease recurrence (Table 4).
Univariate analysis for CTLA-4 polymorphisms and HSCT outcome
| . | Overall survival . | aGVHD II-IV . | Relapse . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | |
| −1722 CC/CT | .454 | 1.16 | 0.78-1.73 | .112 | 0.74 | 0.52-1.07 | .523 | 1.16 | 0.73-1.85 |
| −1661 AG/GG | .538 | 1.11 | 0.79-1.55 | .679 | 1.07 | 0.76-1.50 | .268 | 1.26 | 0.84-1.88 |
| −318 CT/TT | .393 | 0.83 | 0.55-1.26 | .882 | 0.97 | 0.65-1.44 | .854 | 0.96 | 0.61-1.50 |
| +49 AG/GG | .019 | 1.47 | 1.06-2.02 | .072 | 0.75 | 0.55-1.02 | .276 | 1.22 | 0.85-1.75 |
| CT60 AG/GG | .016 | 1.57 | 1.09-2.26 | .108 | 0.77 | 0.56-1.06 | .099 | 1.41 | 0.93-2.12 |
| . | Overall survival . | aGVHD II-IV . | Relapse . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | |
| −1722 CC/CT | .454 | 1.16 | 0.78-1.73 | .112 | 0.74 | 0.52-1.07 | .523 | 1.16 | 0.73-1.85 |
| −1661 AG/GG | .538 | 1.11 | 0.79-1.55 | .679 | 1.07 | 0.76-1.50 | .268 | 1.26 | 0.84-1.88 |
| −318 CT/TT | .393 | 0.83 | 0.55-1.26 | .882 | 0.97 | 0.65-1.44 | .854 | 0.96 | 0.61-1.50 |
| +49 AG/GG | .019 | 1.47 | 1.06-2.02 | .072 | 0.75 | 0.55-1.02 | .276 | 1.22 | 0.85-1.75 |
| CT60 AG/GG | .016 | 1.57 | 1.09-2.26 | .108 | 0.77 | 0.56-1.06 | .099 | 1.41 | 0.93-2.12 |
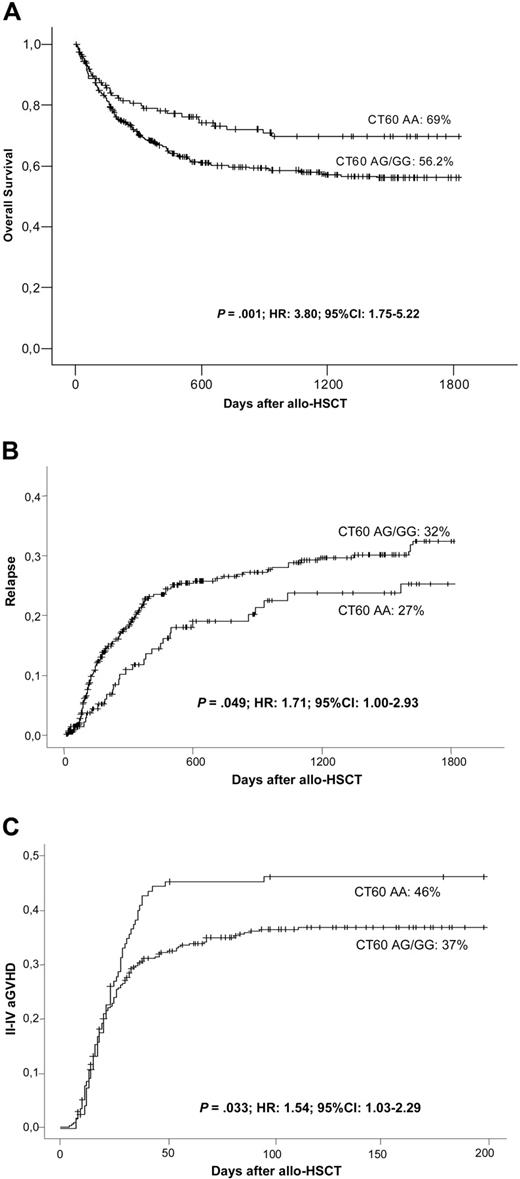
Concerning the polymorphism +49 at exon 1, higher OS at 5 years was observed for patients receiving stem cells from an AA-homozygous donor (+49 AA: 67.7% vs +49 AG/GG: 55.3%; P = .019). In addition, the donor's CT60 genotype showed a significant association with a reduction in OS at 5 years (CT60 AA: 69.8% vs CT60 AG/GG: 56.2%; Figure 1A). Multivariate analysis confirmed that the CT60 AG/GG genotype was an independent risk factor for worse OS (P = .001; hazard ratio [HR], 3.80; 95% confidence interval [CI], 1.75-8.22), whereas the polymorphism at position +49 was not (Table 5).
Clinical outcome after allo-HSCT and OT60 genotypes. OS (A), cumulative incidence of relapse (B), and grades II to IV aGVHD (C) according to the donor's CTLA-4 genotype at CT60 among allogeneic transplant recipients.
Clinical outcome after allo-HSCT and OT60 genotypes. OS (A), cumulative incidence of relapse (B), and grades II to IV aGVHD (C) according to the donor's CTLA-4 genotype at CT60 among allogeneic transplant recipients.
Multivariate analysis for transplantation outcome
| Variables . | P . | HR (95% CI) . |
|---|---|---|
| Overall survival | ||
| Early disease | <.001 | 0.33 (0.22-0.50) |
| CT60 AG/GG | .001 | 3.80 (1.75-8.22) |
| +49 AG/GG | .564 | 0.88 (0.56-1.37) |
| Donor CMV+ | .468 | 1.21 (0.72-2.04) |
| Sensitized donor | .538 | 1.18 (0.70-1.98) |
| Stem cell source | .124 | 1.40 (0.91-2.14) |
| Diagnosis | .741 | 1.08 (0.69-1.68) |
| Age younger than 30 y | .113 | 1.42 (0.92-2.18) |
| aGVHD II-IV | ||
| Stem cell source | .041 | 1.49 (1.01-2.20) |
| CT60 AG/GG | .033 | 0.62 (0.40-0.96) |
| +49 AG/GG | .587 | 0.87 (0.54-1.41) |
| Donor CMV+ | .276 | 1.28 (0.81-2.03) |
| Sex mismatch | .543 | 1.15 (0.73-1.79) |
| 1722 CC/CT | .055 | 1.63 (0.99-2.67) |
| Relapse | ||
| Age younger than 30 y | .019 | 0.61 (0.41-0.92) |
| Early disease | <.001 | 0.37 (0.25-0.56) |
| Patient CMV+ | .008 | 2.08 (1.20-3.57) |
| CT60 AG/GG | .049 | 1.71 (1.00-2.93) |
| Donor CMV+ | .158 | 1.48 (0.86-2.53) |
| Conditioning regimen | .958 | 0.99 (0.64-1.52) |
| Diagnosis | .643 | 1.10 (0.73-1.68) |
| Variables . | P . | HR (95% CI) . |
|---|---|---|
| Overall survival | ||
| Early disease | <.001 | 0.33 (0.22-0.50) |
| CT60 AG/GG | .001 | 3.80 (1.75-8.22) |
| +49 AG/GG | .564 | 0.88 (0.56-1.37) |
| Donor CMV+ | .468 | 1.21 (0.72-2.04) |
| Sensitized donor | .538 | 1.18 (0.70-1.98) |
| Stem cell source | .124 | 1.40 (0.91-2.14) |
| Diagnosis | .741 | 1.08 (0.69-1.68) |
| Age younger than 30 y | .113 | 1.42 (0.92-2.18) |
| aGVHD II-IV | ||
| Stem cell source | .041 | 1.49 (1.01-2.20) |
| CT60 AG/GG | .033 | 0.62 (0.40-0.96) |
| +49 AG/GG | .587 | 0.87 (0.54-1.41) |
| Donor CMV+ | .276 | 1.28 (0.81-2.03) |
| Sex mismatch | .543 | 1.15 (0.73-1.79) |
| 1722 CC/CT | .055 | 1.63 (0.99-2.67) |
| Relapse | ||
| Age younger than 30 y | .019 | 0.61 (0.41-0.92) |
| Early disease | <.001 | 0.37 (0.25-0.56) |
| Patient CMV+ | .008 | 2.08 (1.20-3.57) |
| CT60 AG/GG | .049 | 1.71 (1.00-2.93) |
| Donor CMV+ | .158 | 1.48 (0.86-2.53) |
| Conditioning regimen | .958 | 0.99 (0.64-1.52) |
| Diagnosis | .643 | 1.10 (0.73-1.68) |
Patient/donor CMV+ indicates positive IgG cytomegalovirus serology. Stem cell source was stratified as peripheral blood versus bone marrow. Diagnosis was stratified in acute leukemia/MDS versus other. Conditioning regimen was stratified in total body irradiation containing regimens versus chemotherapy alone.
Disease relapse was higher in the CT60 AG/GG group (32% vs 27% at 5 years; Figure 1B). Multivariate analysis demonstrated that CT60 was an independent risk factor for relapse (P = .049; HR, 1.71; 95% CI, 1.00-2.93; Table 5).
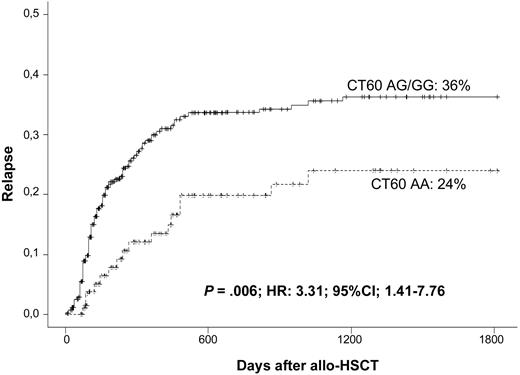
Given that the relapse pattern differs depending on the diagnosis, a highly homogeneous cohort was selected for further analysis of the effect of CTLA-4 polymorphisms on disease relapse. A total of 321 patients diagnosed with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndrome (MDS) (the main indications for allo-HSCT) were included in this analysis. In this cohort, the CT60 G allele was associated with increased relapse rates (CT60 AA: 24% vs CT60 AG/GG: 36% at 5 years; Figure 2). The HR for relapse in the presence of a G allele at CT60 was 3.31 (P = .006; 95% CI, 1.41-7.76), leading to worse OS (CT60 AA: 71% vs CT60 AG/GG: 48.6%; P = .005). Disease relapse was not affected by polymorphisms at −1722, −1661, −318, or +49.
Association of CT60 genotypes and relapse in a homogeneous cohort consisting of patients who received an allogeneic HSC transplant for the treatment of AML, ALL, and MDS.
Association of CT60 genotypes and relapse in a homogeneous cohort consisting of patients who received an allogeneic HSC transplant for the treatment of AML, ALL, and MDS.
Risk of grades II to IV of aGVHD was also increased by the CT60 genotype when considering all 536 patients. Interestingly, for GVHD, the susceptibility alleles were not those previously defined as such for survival and relapse in this study or for other autoimmune diseases. The A allele at CT60 was associated with an increased risk of developing aGVHD, while the G allele was protective. Nearly half (46%) of patients with the CT60 AA genotype developed aGVHD, but the disease incidence decreased to 37% in the presence of a G allele (Figure 1C). Multivariate analysis detected that the donor's CT60 AA genotype was an independent risk factor for developing grades II to IV aGVHD (P = .033; HR, 1.54; 95% CI, 1.03-2.29; Table 5). The incidence of severe aGVHD grades (III-IV) was not significantly affected by CT60 (AA: 21.3% vs AG/GG: 15.8%; P = .335). The SNP +49 showed a trend toward association with grades II to IV aGVHD, although statistical significance was not reached (AA: 43% vs AG/GG: 34%; P = .07). Severe grades of the disease were not influenced by +49.
CTLA-4 haplotypes
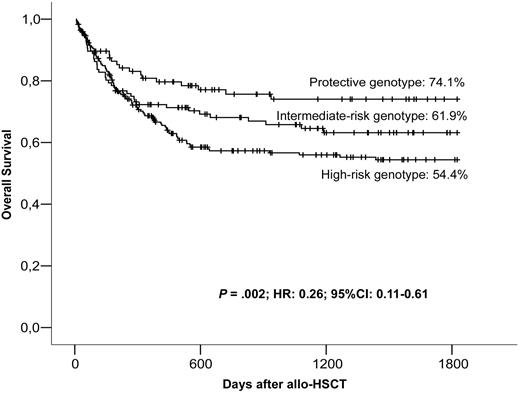
The presence of LD among the 5 CTLA-4 polymorphic sites results in the nonrandom distribution of the alleles. We identified 6 major blocks of inheritance or haplotypes for the CTLA-4 gene, with frequencies higher than the 6% in our population (Table 3). There is linkage disequilibrium between CT60 and +49 SNPs, with only 3 haplotypes observed at reasonable frequencies. In the first, CT60A is linked to +49A (the A-A haplotype), the second involves CT60G and +49G (the G-G haplotype), and in the third, CT60G is linked to +49A (the G-A haplotype). Among the 6 resulting genotypes, OS at 5 years was higher for patients receiving an A-A homozygous graft (74.1%) than for those heterozygous for it (A-A/G-A and A-A/G-G; 58.8%), as well as for those without A-A (G-A/G-A, G-A/G-G, and G-G/G-G; 55.2%; P = .034). Interestingly, patients who received transplants from donors with genotypes other than CT60AA/+49AA, regardless of whether they presented one copy of the protective haplotype or not, had similar survival rates at 5 years, suggesting that the presence of a G allele at CT60 and/or at +49 was sufficient to worsen the transplantation outcome. Given these observations, we examined whether there were differences between having just one polymorphism with the risk allele or presenting the G allele at both CT60 and +49. To this end, patients were stratified into 3 groups according to the absence of risk alleles at CT60 and +49 (protective genotype: CT60AA/+49AA), the presence of a risk allele at CT60 only (intermediate risk genotypes: CT60AG/+49AA and CT60GG/+49AA), and the presence of, at least, a risk allele in both polymorphisms (high-risk genotypes: CT60AG/+49AG, CT60GG/+49AG, and CT60GG/+49GG). Patients receiving grafts carrying the protective CTLA-4 CT60AA/+49AA genotype had a 74.1% OS rate compared with 61.9% among those with the intermediate-risk genotypes. The presence of the susceptibility alleles in both positions resulted in a dramatic decrease of OS to 54.4% (Figure 3). Multivariate analysis confirmed that the CTLA-4 protective genotype was an independent protective factor for OS (P = .001; HR, 0.22; 95% CI, 0.08-0.55). After accounting for the significant protective effect on OS of the CTLA-4 protective genotype compared with all other genotypes combined, there was no further significant variation of OS among CT60/+49 genotype combinations.
OS of patients depending on the donor's CT60/+49 genotype combinations.
Relapse was also evaluated according to the CT60/+49 combination. We found that relapse was higher in patients who received transplants from donors with the intermediate-risk and high-risk genotypes. The CT60AA/+49AA combination was identified in the multivariate analysis as protective for relapse (P = .05; HR, 0.39; 95% CI, 0.15-1.00).
Univariate analysis for grades II to IV of aGVHD and with respect to the CT60/+49 genotype was also statistically significant. The genetic combination was considered protective for OS and relapse was, in this case, the one that conferred higher incidence of aGVHD (49%). In contrast, the presence of a G allele resulted in a reduction of GVHD incidence to 39.1% when it was present at one locus, and to 33% when both CT60 and +49 displayed at least a G allele (P = .035).
Dominant effect of the CT60 polymorphism
The results previously reported indicate that the presence of a risk allele at both CT60 and +49 conferred worse OS than when this risk factor was only present at CT60, suggesting that the polymorphism at +49 also influences outcome. However, when a multivariate analysis adjusted for CT60 was performed, +49 was not identified as an independent risk factor for OS (P = .613), whereas the involvement of CT60 remained significant. When the analysis included both the CT60 genotype and the CT60/+49 haplotype, the CT60/+49 combination was no longer significant. The same was observed in the multivariate analysis for aGVHD and relapse. These results support the hypothesis that CT60 itself has a dominant effect on outcome, and the results observed for the CT60/+49 haplotype can thus be attributed to the LD between these 2 loci.
Expression of sCTLA-4 and flCTLA-4
QRT-PCR was performed to quantify sCTLA-4 and flCTLA-4. Full-length CTLA-4 transcription levels were not affected by the presence of polymorphisms at either −1722, −1661, −318, +49, or CT60. These observations were confirmed by flow cytometry. No differences in surface protein expression according to the polymorphisms were observed on CD3+, CD3+CD25+, CD3+CD8+, or CD19+ cell subsets.
No differences in the percentage of either CD19+CD152+ cells or T-cell subsets (CD3+CD8+CD25+, CD3+CD4+CD25+, CD3+CD8+CD25+CD152+, and CD3+CD4+CD25+CD152+cells) were observed considering the CTLA-4 polymorphisms, even after PHA and PMA stimulation.
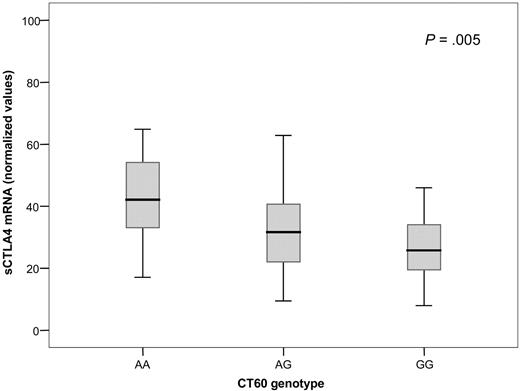
It has previously been suggested that CT60 may determine the efficiency of the alternative splicing of CTLA-4, which is responsible for the production of the soluble isoform of the protein.20 We observed that depending on the CT60 polymorphism, the amount of sCTLA-4 mRNA was reduced in a G allele dose-dependent manner. The mean quantity of sCTLA-4 transcript in individuals carrying the CT60AA genotype was 24.7% higher than in heterozygous patients and 38.5% higher than in homozygotes for the G allele (P = .005; Figure 4). We also observed a significant decrease in sCTLA-4 when the +49 genetic combinations were compared (P = .009). The presence of polymorphic alleles at −318, −1661, and −1722 did not influence the production of sCTLA-4 mRNA.
Normalized values of sCTLA-4 mRNA according to the CTLA-4 genotype at CT60. Median values ± standard deviation for each genotype are represented within the box. P value is obtained by regression analysis test relating sCTLA4 to the G allele number (0, 1, or 2) for CT60.
Normalized values of sCTLA-4 mRNA according to the CTLA-4 genotype at CT60. Median values ± standard deviation for each genotype are represented within the box. P value is obtained by regression analysis test relating sCTLA4 to the G allele number (0, 1, or 2) for CT60.
We also measured sCTLA-4 production according to CT60/+49 genetic combinations. The sCTLA-4 mRNA levels observed for individuals with the protective genotypes were considered as a reference. The expression of the soluble isoform was 31% lower in the intermediate-risk group and 34.5% lower in the high-risk group (P = .018). When regressing sCTLA-4 on the G allele numbers for both CT60 and +49 in a multiple regression model, CT60 remained statistically significant (P = .005), but +49 did not (P = .156). These results agree with our previous observations on the CT60/+49 genotypes, supporting the hypothesis that the polymorphism at CT60 is responsible for the effect observed in allo-HSCT outcome.
We also performed enzyme-linked immunosorbent assays (ELISAs) on the 60 healthy participants, and no sCTLA-4 was detected, regardless of the CT60 genotype.
Discussion
The results of this study highlight the importance of the regulatory molecule CTLA-4 in the outcome of patients who receive allo-HSCT from an HLA-identical sibling for the treatment of several hematologic malignancies.
The effect of some CTLA-4 polymorphisms on the onset and evolution of several autoimmune diseases, such as lupus erythematosus, type-1 diabetes, and Graves disease, has been widely investigated.20,,,,,,,–28 However, to our knowledge, there are no references in the literature about the role of CTLA-4 variants in alloimmunity. This study is therefore the first to show that SNPs at CTLA-4 are predictors of allogeneic transplantation outcome.
The most widely studied polymorphisms are the CT60 and +49 A-to-G transitions. Traditionally, the A allele has been identified as protective, whereas the G allele is considered to be associated with greater susceptibility to autoimmune disease. However, the results reported by different groups have often been controversial.25,30,–32 The CTLA-4 exon 1, where the +49 polymorphism is located, encodes the leader peptide of the protein, which is responsible for CTLA-4 trafficking to the endoplasmic reticulum. The reason why the G allele at +49 confers susceptibility to autoimmune disease remains unknown, although a number of hypotheses have been proposed.33 However, our findings do not support the hypothesis that the +49 polymorphism is involved in transplantation outcome. In contrast, we observed that CT60 had a dominant effect on transplantation outcome. CT60 maps at the 3′ untranslated region of CTLA-4, and it could play a role in the alternative splicing by which sCTLA-4 is generated.
Based on a small number of patients, Ueda et al suggested that the G allele of CT60 produced less sCTLA-4 mRNA than did the A allele. Given these observations, the authors concluded that sCTLA-4 expression is the functional basis for the observed genetic association between autoimmune diseases and the CTLA-4 gene. If this conclusion was correct, one would expect patients with autoimmune disease to have lower levels of sCTLA-4 than healthy counterparts. However, although sCTLA-4 was first thought to be produced by nonstimulated T cells and that its expression down-regulated after T-cell activation,18,34 no evidence of this soluble isoform has been found in sera of healthy volunteers.19 The results obtained from our ELISAs are also at odds with the data reported by Magistrelly et al. In contrast, many studies have reported increased protein levels of sCTLA-4 in patients with autoimmune disease, suggesting that this isoform of the protein is responsible for pathogenesis and reflects T-cell activation.35,36 Our results from the sCTLA-4 mRNA analysis of the 60 healthy blood donors also identified the A allele at CT60 as being responsible for a greater production of sCTLA-4, and we found that patients receiving grafts with CT60 AA genotypes had more alloimmune recognition processes, as indicated by the higher incidence of aGVHD observed in this group.
We identified the A allele at CT60 as an independent risk factor for developing moderate to severe grades of aGVHD, whereas its G counterpart conferred protection against disease recurrence. Our findings on alloimmunity, along with other results previously reported on autoimmunity, suggest that sCTLA-4 blocks the B7–flCTLA-4 interaction, thereby enhancing T-cell reactivity by preventing the transduction of inhibitory signals that lead to lymphocyte inactivation. Despite the highest incidence of aGVHD in the CT60 AA group, no reduction in OS was observed, suggesting an enhanced graft-versus-leukemia (GVL) effect, which protected these patients from relapse.
In addition, the low sCTLA-4 production associated with the G allele may explain why patients with G alleles at CT60 had increased relapse rates and, therefore, higher mortality. We hypothesize that in the absence of significant levels of sCTLA-4 competing with flCTLA-4 for the B7 ligand, T cells are more likely to be inactivated and less capable of detecting neoplastic antigens, thus allowing residual tumoral cells to escape from lymphocytic control.
In conclusion, the donor's CT60 AA genotype has been identified as a less tolerogenic genotype associated with a higher incidence of moderate to severe grades of aGVHD and better transplantation outcome through an increased GVL effect.
The clinical interest of these findings resides in the identification of patients diagnosed primarily with acute leukemia and MDS and at high risk of relapse after allo-HSCT based on their donor's CTLA-4 genotype at CT60. These patients could potentially benefit from a rapid withdrawal of immunosuppressive therapy.
Thorough knowledge of the CTLA-4 molecule and its role in the allo-HSCT setting is necessary for the better understanding of the alloreactive responses, as is the case for other non-HLA molecules such as cytokines and minor histocompatibility antigens. This information could improve the pretransplantation risk assessment process and serve as a reference for treatment planning.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Sònia Ramon for her technical assistance. We are also grateful to all members of the Laboratori de Recerca Translacional (ICO Duran i Reynals) for their useful comments and collaboration in the experimental process. We also thank Dr Antonio Agudo for his useful comments.
This study has been performed at the Alloreactivity Unit, Laboratori de Recerca Translacional and Clinical Hematology Department from the Institut Català d'Oncologia - IDIBELL, Hospital Duran i Reynals, L'Hospitalet, Barcelona, Spain.
This work was supported by grant FIS PI050939 from the Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo.
Authorship
Contribution: A.P.-G. and D.G. designed the research, analyzed data, and wrote the manuscript. M.E. provided statistical support. A.P.-G. performed research helped by M.H. and J.M.P. R.D.l.C., J.R.-G., A.J.-V., J.B.N., J.d.l.R., A.U.-I., S.B., A.I., M.G., D.S., I.E., C.S., and J.M.R. provided donors' samples and clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the GVHD/Immunotherapy Committee of the Spanish Group of Hematopoietic Stem Cell Transplantation is provided in Document S1 as a data supplement to the online version of this article.
Correspondence: David Gallardo. Clinical Haematology Department, Institut Català d'Oncologia, Hospital Duran i Reynals, Av. Gran Vía s/n, km 2.7, 08907 L'Hospitalet de Llobregat, Barcelona, Spain; e-mail: 25732dgg@comb.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal