CXCR3 ligands were secreted by tissue fibroblasts and peripheral blood–derived mononuclear leukocytes in response to interferon-γ (IFN-γ) and Toll-like receptor (TLR) ligands. Subsequent purification and identification revealed the presence of truncated CXCL11 variants missing up to 6 amino acids. In combination with CD26/dipeptidyl peptidase IV, the metalloprotease aminopeptidase N (APN), identical to the myeloid cell marker CD13, rapidly processed CXCL11, but not CXCL8, to generate truncated CXCL11 forms. Truncated CXCL11 had reduced binding, signaling, and chemotactic properties for lymphocytes and CXCR3- or CXCR7-transfected cells. CD13/APN-truncated CXCL11 failed to induce an intracellular calcium increase but was still able to bind and desensitize CXCR3 for intact CXCL11 signaling. CXCL11 efficiently bound to CXCR7, but CXCL11 was not able to induce calcium signaling or ERK1/2 or Akt phosphorylation through CXCR7. CD26-truncated CXCL11 failed to attract lymphocytes but still inhibited microvascular endothelial cell (HMVEC) migration. However, further processing of CXCL11 by CD13 resulted in significant reduction of inhibition of HMVEC migration. Taken together, during inflammation or cancer, CXCL11 processing by CD13 may lead to a reduced number of tumor-infiltrating lymphocytes and in a more angiogenic environment.
Introduction
Leukocyte migration during normal (eg, lymphocyte homing) and pathologic conditions (eg, inflammation) is regulated by a number of protein families including adhesion molecules, cytokines, chemokines, and proteases. A complex network of interactions between these proteins allows for the fine-tuning of the directional migration of leukocyte subfamilies. Cytokines regulate chemokine and chemokine receptor expression, chemokines activate integrins, and the chemokine gradient determines the direction of leukocyte migration. In vivo, chemotactic activity also depends on the availability of chemokines on endothelial layers by interaction with glycosaminoglycans and “silent” or rather “decoy” chemokine receptors.1,,–4 In addition, synergy between cytokines to induce chemokines or between chemokines to attract leukocytes may amplify the inflammatory response.5 Proteases are not only important for the degradation of the extracellular matrix but more and more evidence also points toward their crucial role in the regulation of chemokine activity and receptor specificity.6
The CXC chemokine ligand 11 (CXCL11) or IFN-inducible T-cell α-chemoattractant (I-TAC) belongs to the CXC chemokine family characterized by the presence of 1 amino acid in between the 2 NH2-terminal cysteines.7,8 CXCL11 is produced by a variety of cells including leukocytes, fibroblasts, and endothelial cells upon stimulation with interferons (IFNs). Simultaneous stimulation of fibroblasts or endothelial cells with IFN-γ and interleukin-1β or the TLR3 ligand double-stranded RNA resulted in a synergistic increase of CXCL11 production.9 Although on fibroblasts bacterial peptidoglycan (PGN) and lipopolysaccharide (LPS), TLR2 and TLR4 ligands, respectively, also synergized with IFN-γ to produce CXCL11, no such synergistic CXCL11 production was observed in microvascular endothelial cells. In leukocytes, bacterial LPS and PGN even inhibited interferon-induced CXCL11 production.10 CXCL11 attracts activated T-helper 1 (Th1) lymphocytes and natural killer (NK) cells. Like CXCL9 or monokine induced by IFN-γ (Mig) and CXCL10 or IFN-γ–inducible protein-10 (IP-10), CXCL11 signals through CXC chemokine receptor 3 (CXCR3). Although expression of CXCL11 is more strictly regulated, it is the most potent CXCR3 ligand.7,8 Furthermore, CXCL11 has recently been identified also as a ligand for RDC1/CXCR7.11
In addition to their role as NK-cell and lymphocyte chemoattractants, CXCR3 ligands as well as CXCL4, CXCL4-L1, and CXCL14 inhibit angiogenesis induced by CXCL8/interleukin-8 (IL-8), basic fibroblast growth factor (bFGF), or vascular endothelial cell growth factor (VEGF).12,–14 CXCR1, CXCR2, and CXCR3 were detected on microvascular endothelial cells, and neutralization of CXCR2 diminished the angiogenic activity of CXCL8 in vivo in rats and mice.15 The role of CXCR3 in the antiangiogenic activity of CXCR3 ligands is more controversial, and an alternatively spliced CXCR3 receptor (CXCR3B) has been claimed to be involved.12,16,17 Although the NH2 termini of CXCL9, CXCL10, and CXCL11 have been reported to be crucial for CXCR3-dependent calcium signaling and chemotaxis, CD26/dipeptidyl peptidase IV (DPP IV)–truncated CXCL9 and CXCL10 retained angiostatic activity despite loss of CXCR3-mediated signaling.18 Otherwise, limited information is available on natural posttranslational modification of CXCL11. Therefore, natural CXCL11 produced by fibroblasts and peripheral blood mononuclear cells (PBMCs) was purified to homogeneity. We identified different NH2-terminally truncated natural CXCL11 isoforms and direct evidence was given that some forms are derived by processing with the aminopeptidase N (APN). APN is identical to the myeloid cell marker CD13.19 CD13/APN is the principle aminopeptidase in plasma and is present as a membrane-bound enzyme on myeloid cells including mature monocytes and neutrophils.20 Moreover, CD13/APN is expressed on fibroblasts, epithelial cells, and endothelial cells of angiogenic but not normal vasculature.21 Angiogenic proteins such as bFGF and VEGF induce CD13/APN expression and CD13/APN inhibitors were reported to alter angiogenesis.22,23 Therefore, the effect of NH2-terminal processing by CD13/APN on receptor binding and signaling capacities and on the biologic activity of CXCL11 on leukocytes and endothelial cells was investigated.
Materials and methods
Reagents and enzyme incubations
Recombinant CXCL11 without carrier proteins was bought from R&D Systems (Minneapolis, MN) and CXCL8(1-77), CXCL8(6-77), and CXCL12 were from PeproTech (Rocky Hill, NJ). Protein-sequencing grade microsomal CD13/APN isolated from porcine kidney, Leu-4–nitroanilide, and the protease inhibitors phenylmethylsulfonyl fluoride (PMSF), E64, or N-(trans-epoxysuccinyl)-L-leucine-4-guanidinobutylamide and 1,10-phenanthroline were purchased from Sigma-Aldrich (St Louis, MO); chymostatin was from Roche (Indianapolis, IN); and benzamidine was from Acros Organics (Geel, Belgium). Purified soluble CD26/DPP IV was kindly provided by Dr S. Scharpé and Dr I. De Meester (University of Antwerp, Belgium).24 CD13 digests of CXCL11(3-73) were performed at 37°C in phosphate-buffered saline (PBS; pH 7.4) containing the serine protease inhibitor benzamidine (10 mM). Incubations of intact CXCL11 with CD26 (at 25 units/L) plus CD13 were carried out without benzamidine. Trifluoroacetic acid (TFA; 0.1%) was added to stop the reaction and samples were desalted on C4 ZipTips (Millipore, Bedford, MA) prior to mass spectrometry on an Esquire LC ion trap mass spectrometer (MS; Bruker, Bremen, Germany). Alternatively, acidified samples were blotted on PVDF membranes in a ProSorb cartridge prior to Edman degradation on a 491 Procise cLC protein sequencer (Applied Biosystems, Foster City, CA). Treatments with neutrophil-derived CD13/APN were carried out with the supernatant of purified neutrophils (30 × 106 cells/mL) that were kept in 50 mM Tris (pH 7.5) containing 100 mM NaCl for 2 hours at 37°C, and that was supplemented with the protease inhibitors benzamidine (10 mM), PMSF (10 mM), E-64 (10 μM), chymostatin (50 μM), and TIMP-1 (3 μg/mL) to inhibit other proteases. The amount of CD13/APN activity in the supernatant was determined with the Leu-4–nitroanilide conversion assay (according to the quality control test procedure of the manufacturer) and corresponded to the activity of 4 pmol/mL commercial porcine CD13/APN.
Cell cultures and production of natural chemokines
Human skin/muscle-derived fibroblasts (E1SM) were grown to confluence in 175-cm2 tissue culture flasks in Eagle minimal essential medium (MEM) containing 10% (vol/vol) fetal bovine serum (FBS; Cambrex Bio Science, Verviers, Belgium) and induced with 10 μg/mL of the double-stranded RNA (dsRNA) polyriboinosinic:polyribocytidylic acid (polyrI:rC; Sigma-Aldrich), 5 μg/mL of lipopolysaccharide (LPS from Escherichia coli 0111:B4; Difco Laboratories, Detroit, MI), and 20 ng/mL IFN-γ (PeproTech) for 96 hours.10 Leukocytes were obtained from fresh human buffy coats obtained from the blood transfusion centers of Antwerp and Leuven and purified as previously described.10 Purified PBMCs from 24 buffy coats were pooled and induced at 5 × 106 cells/mL with 10 μg/mL polyrI:rC and 20 ng/mL IFN-γ in RPMI 1640 containing 2% FBS for 21 hours. Conditioned media were harvested and stored at −20°C until purification. Alternatively, purified PBMCs from single donors were stimulated with phytohemagglutinin (PHA; Sigma-Aldrich; 2 μg/mL) for 3 days and washed with RPMI 1640, and activated lymphocytes were kept in culture at 2 × 106 cells/mL for 2 to 3 weeks in RPMI 1640 supplemented with 10% FBS and 50 U/mL IL-2 (PeproTech) before use in chemotaxis or binding assays.18
Chinese hamster ovary (CHO) cell lines transfected with CXCR3 (CHO-CXCR3), CXCR4 (CHO-CXCR4), or CXCR7 (CHO-CXCR7) were generated as previously described and cultured in Ham F-12 growth medium (Cambrex Bio Science) enriched with 10% FBS, 400 μg/mL G418, and 1 mM sodium pyruvate.18 CHO-CXCR7 cells were stained with rabbit anti–human CXCR7 antibodies (ab 12 870; Abcam, Cambridge, United Kingdom) and analyzed by fluorescence-activated cell sorter (FACS), which confirmed CXCR7 expression (data not shown). In contrast to CHO-CXCR4 cells, FACS analysis did not show staining of CHO-CXCR7 cells with anti-CXCR4 antibodies (12G5 antibody; Becton Dickinson, Franklin Lakes, NJ; data not shown).
Purification and identification of natural CXCL11
Natural human CXCL11 was purified to homogeneity in a 4-step purification procedure. The CXCL11 concentration in column fractions was determined by enzyme-linked immunosorbent assay (ELISA).10 Briefly, fibroblast- or leukocyte-derived conditioned media were first concentrated by adsorption to controlled pore glass and subsequently purified by heparin-Sepharose affinity chromatography and Mono S cation exchange chromatography (GE Healthcare, Diegem, Belgium).25 In order to obtain homogeneously purified chemokine, CXCL11-containing fractions were finally subjected to reversed-phase high-performance liquid chromatography (RP-HPLC; 2.1 × 220-mm Brownlee C8 Aquapore RP-300 column; PerkinElmer, Norwalk, CT). Proteins were eluted from the column in an acetonitrile gradient in 0.1% TFA and UV absorption was monitored at 214 nm.
The C8 RP-HPLC column effluent was collected in 400-μL fractions and a minor portion of the column effluent (0.7% vol/vol) was split on-line to an Esquire LC electrospray ion-trap mass spectrometer. Profile spectra were collected during the chromatographic separation. Averaged profile spectra of proteins that eluted within the UV absorption peaks that contained CXCL11 immunoreactivity were deconvoluted with the Bruker software (Data Analysis Version 3.0). The experimentally determined average relative molecular mass (Mr) of the proteins was compared with the theoretical average Mr of intact or truncated CXCL11 isoforms. Alternatively, the NH2-terminal sequence of the isolated CXCL11 immunoreactivity was determined by Edman degradation.
Peptide synthesis
Intact and NH2-terminally truncated CXCL11 missing 2, 4, or 6 amino acids were prepared by solid-phase peptide synthesis and deprotected as previously described.26 Subsequently, peptides were loaded on a 4.6 × 150-mm Source 5RPC column (GE Healthcare) and eluted with an acetonitrile gradient in 0.1% TFA. Part of the column effluent (0.7% vol/vol) was split to an ion-trap mass spectrometer, and averaged profile spectra were deconvoluted to determine the Mr. Proteins with the correct Mr were folded overnight in 150 mM Tris (pH 8.6) containing 1 M guanidinium chloride, 3 mM EDTA, 0.3 mM reduced glutathione, and 3 mM oxidized glutathione and repurified by RP-HPLC on a C8 Aquapore RP-300 column (PerkinElmer). Ion-trap mass spectrometry was used to select the fractions that contained the folded peptides. Protein concentration was determined with the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL).
In vitro chemotaxis and receptor-binding assays
Lymphocyte chemotaxis was performed in Hanks balanced salt solution (HBSS) + 0.1% wt/vol human serum albumin (HSA) at 2 × 106 cells/mL in Boyden microchambers (Neuro Probe, Cabin John, MD) with fibronectin-coated, polyvinylpyrrolidone-free polycarbonate membranes (5-μm pore size; Corning Separations Division, Acton, MA) for 2 hours at 37°C.18 Activated lymphocytes that migrated through the membrane were stained with Hemacolor staining solutions (Merck, Darmstadt, Germany) and counted microscopically in 10 oil-immersion fields (× 500 magnification). The chemotactic index was calculated as the number of lymphocytes that migrated to the sample divided by the number of cells that spontaneously migrated to the sample dilution medium (HBSS + 0.1% wt/vol HSA).
Competition for 125I-labeled CXCL11 binding was measured on activated lymphocytes or CHO-CXCR3 or CHO-CXCR7 cells in binding buffer (50 mM HEPES [pH 7.2] containing 1 mM CaCl2, 5 mM MgCl2, and 0.1% [wt/vol] bovine serum albumin). Briefly, 2 × 106 CHO cells or 4 × 106 lymphocytes were incubated for 2 hours at 4°C with 125I-CXCL11 or 125I-CXCL12 (Amersham Pharmacia Biotech, Uppsala, Sweden) and varying concentrations of unlabeled chemokine. Cells were centrifuged at 250g and washed 3 times with 2 mL of binding buffer supplemented with 0.5 M NaCl and the radioactivity present on the cells was measured in a gamma counter (Triathler Multilabel Tester; Hidex, Turku, Finland).
Signal transduction assays
Alterations in the intracellular calcium concentration ([Ca2 +] i) in CHO-CXCR3 cells in response to chemokines were monitored by fluorescence spectrometry with the fluorescent dye Fura-2-AM as previously described.18 During desensitization experiments, chemokines were added with an interval of 100 seconds. The increase in [Ca2 +] i after desensitization was compared with the increase in [Ca2 +] i after the addition of an equal volume of dilution buffer to calculate the percentage desensitization.
Phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt/protein kinase B (PKB) were evaluated as described.27 The amount of phosphorylated ERK1/2 and PKB/Akt in the supernatant (in pg phospho-ERK1/2 or phospho-Akt per mg total protein) was determined using specific ELISAs for phospho-ERK1 (T202/Y204), phospho-ERK2 (T185/Y187), or phospho-Akt (S473; R&D Systems).
In vitro wound-healing assay
Human dermal microvascular endothelial cells (HMVECs) were cultured in 24-well plates in EBM-2 medium with the EGM-2–MV Bulletkit (Cambrex Bio Science). When HMVECs were grown to confluence, the culture medium was replaced with 0.5 mL EBM-2 medium with 40 μg/mL mitomycin-C (Acros Organics) without EGM-2–MV Bulletkit. After 30 minutes at 37°C and 5% CO2, a plastic pipette tip was used to draw a linear scar in the cell monolayer of each well. The monolayers were washed twice with PBS to remove the cells that were detached from the monolayer and chemokines were added in 0.5 mL EBM-2 medium with EGM-2–MV Bulletkit. After 24 hours, HMVEC cultures were fixed and stained with Hemacolor staining solutions. In every 24-well plate, 3 control cultures were included to which no chemokine was added. The difference of the width of the individual scars before and after treatment was scored under a microscope and set at zero for control cultures. A scar that was broader compared with control scars (inhibition of HMVEC migration) received a negative score; a more narrow scar was given a positive score (stimulation of migration). Optical scores ranged from −3 (very broad) to +3 (very narrow). All samples were tested in duplicate in each 24-well plate and were scored double blind and independently by 2 investigators. Digital pictures from representative wells were taken with a Canon G3B52 camera mounted on a Carl Zeiss Axiovert 40 CFL microscope with an A-plan × 10/0.25 lens (Carl Zeiss, Göttingen, Germany) and converted to grayscale with Paint Shop Pro (Corel, Buffalo, NY).
Results
Purification and identification of natural CXCL11
Natural CXCL11 was purified from fibroblast- or PBMC-derived conditioned media by heparin-affinity chromatography and cation-exchange chromatography. Cation-exchange column fractions containing CXCL11 immunoreactivity were further purified to homogeneity by C8 RP-HPLC (Figure 1A; data not shown). The RP-HPLC column fractions that contained CXCL11 immunoreactivity were analyzed by mass spectrometry and/or Edman degradation. Various fibroblast- and PBMC-derived fractions contained NH2-terminally truncated CXCL11 isoforms (Figure 1B; Table 1).
Purification and identification of natural CXCL11 isoforms. Conditioned medium from fibroblasts was purified by heparin-affinity and cation-exchange chromatography (not shown) and finally loaded on a C8 RP-HPLC column. Proteins were eluted from the C8 column in an acetonitrile gradient (dotted line) and detected at 214 nm (full line; A). The CXCL11 concentration in the column fractions was determined by ELISA (histograms). Panel B shows the averaged MS spectrum (mass/charge [m/z] versus abundance) for the proteins that eluted between 28 and 29 minutes from the RP-HPLC column. The charges of the detected ions (+ 5 to + 8) are indicated on the averaged spectrum, and the deconvoluted spectrum that was calculated from these charged ions is given as an inset in panel B.
Purification and identification of natural CXCL11 isoforms. Conditioned medium from fibroblasts was purified by heparin-affinity and cation-exchange chromatography (not shown) and finally loaded on a C8 RP-HPLC column. Proteins were eluted from the C8 column in an acetonitrile gradient (dotted line) and detected at 214 nm (full line; A). The CXCL11 concentration in the column fractions was determined by ELISA (histograms). Panel B shows the averaged MS spectrum (mass/charge [m/z] versus abundance) for the proteins that eluted between 28 and 29 minutes from the RP-HPLC column. The charges of the detected ions (+ 5 to + 8) are indicated on the averaged spectrum, and the deconvoluted spectrum that was calculated from these charged ions is given as an inset in panel B.
Identification of natural CXCL11 isoforms
| NH2-terminal sequence* . | CXCL11 isoform . | Cellular source . |
|---|---|---|
| FKRGRXLXIGP… | CXCL11 (4-73) | Fibroblasts |
| KRGRXLXIGP… | CXCL11 (5-73) | PBMCs |
| RGRXLXIGP… | CXCL11 (6-73) | PBMCs and fibroblasts |
| GRXLXIGP… | CXCL11 (7-73) | PBMCs |
| NH2-terminal sequence* . | CXCL11 isoform . | Cellular source . |
|---|---|---|
| FKRGRXLXIGP… | CXCL11 (4-73) | Fibroblasts |
| KRGRXLXIGP… | CXCL11 (5-73) | PBMCs |
| RGRXLXIGP… | CXCL11 (6-73) | PBMCs and fibroblasts |
| GRXLXIGP… | CXCL11 (7-73) | PBMCs |
The NH2-terminal sequence of RP-MPLC purified CXCL11 isoforms was determined by Edman degradation.
Synthesis of truncated CXCL11 isoforms
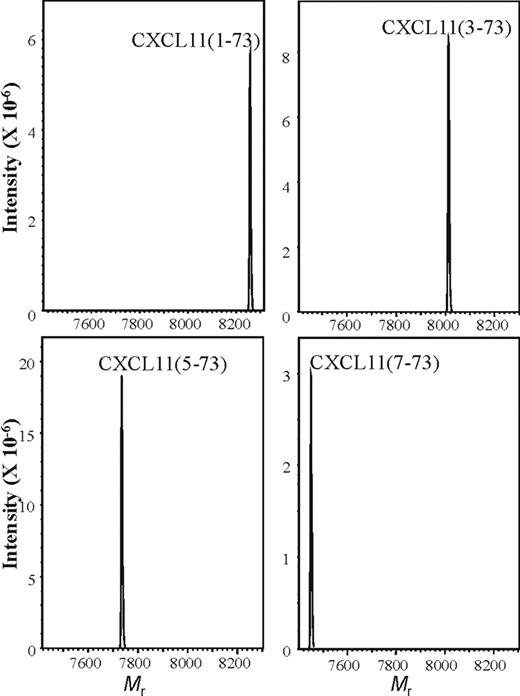
In order to obtain sufficient amounts of intact and NH2-terminally truncated CXCL11 proteins, CXCL11(1-73), CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) were chemically synthesized, deprotected, and purified by RP-HPLC on a Source 5RPC column. All 4 proteins were folded in a buffer containing oxidized and reduced glutathione and repurified by C8 RP-HPLC, and the Mr of the proteins was verified by on-line mass spectrometry (Figure 2).
Mass spectrometry of synthetic CXCL11. NH2-terminally truncated CXCL11 proteins were chemically synthesized, purified, and folded. The folded CXCL11(1-73), CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) proteins were repurified by RP-HPLC and the Mr was verified by ion-trap mass spectrometry. Spectra are averaged deconvolution spectra of the RP-HPLC fractions that were pooled for use in biochemical and biologic assays.
Mass spectrometry of synthetic CXCL11. NH2-terminally truncated CXCL11 proteins were chemically synthesized, purified, and folded. The folded CXCL11(1-73), CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) proteins were repurified by RP-HPLC and the Mr was verified by ion-trap mass spectrometry. Spectra are averaged deconvolution spectra of the RP-HPLC fractions that were pooled for use in biochemical and biologic assays.
NH2-terminal truncation of CXCL8 or CXCL11 by CD26 or CD13
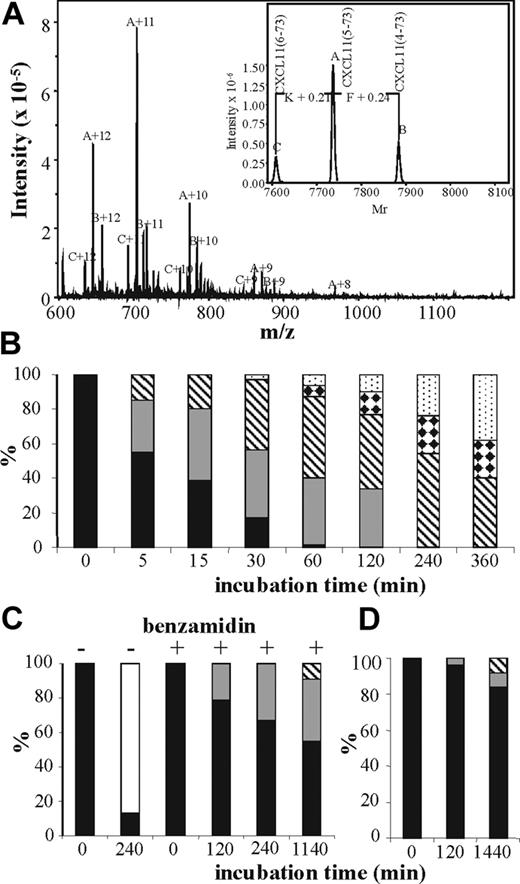
The penultimate NH2-terminal Pro protects CXCL11 from cleavage by most aminopeptidases. However, with a half-life of 2 minutes at physiologic enzyme concentrations, CXCL11 rapidly looses the 2 NH2-terminal residues upon treatment with CD26/DPP IV.28 CD13/APN is a metalloprotease that, in contrast to CD26/DPP IV, releases single amino acids from the NH2-terminus of proteins and was reported to process CXCL8.29 Incubation of CXCL11 with a combination of CD26 and CD13 generated multiple NH2-terminally truncated CXCL11 isoforms (data not shown). Incubation of synthetic CXCL11(3-73) with CD13 at an enzyme-substrate (E/S) ratio of 1:4 revealed that at 37°C, 1 to 3 NH2-terminal amino acids were removed from the NH2-terminus within 2 hours (Figure 3A). Incubation of CXCL11(3-73) with CD13 in the presence of the metalloprotease inhibitors EDTA and 1,10-phenanthroline blocked truncation (data not shown). In order to compare the susceptibility of CXCL8 and CXCL11 to CD13 truncation, CXCL8(1-77), CXCL8(6-77), or CXCL11(3-73) was incubated with CD13 at a molar E/S ratio of 1:25 and the reaction was stopped with 0.1% TFA at different time points (Figure 3). Samples were desalted on PVDF membranes and the relative amount of the different NH2-terminal CXCL11 isoforms was determined by Edman degradation. CXCL11(3-73) rapidly lost 1 to 4 NH2-terminal amino acids generating CXCL11(4-73), CXCL11 (5-73) CXCL11(6-73), and CXCL11(7-73) (Figure 3B). After 15 minutes, more than half of the chemokine was converted in a further truncated form and after 1 hour only traces of the unmodified chemokine were detected. Upon prolonged incubation for 24 hours, 77% of the chemokine was converted into CXCL11(7-73) and only 23% of CXCL11(7-73) had lost an extra Gly to generate CXCL11(8-73), probably due to the presence of the 2 adjacent cysteine bridges (data not shown). CD13 has previously been reported to process another CXC chemokine (ie, CXCL8) into CXCL8(6-77), which lacks the first 5 NH2-terminal amino acids AVLPR.29 Incubation of intact CXCL8(1-77) with CD13 at a molar E/S ratio of 1:25 for 4 hours indeed resulted in the conversion of more than 80% of this chemokine into CXCL8(6-77). However, addition of 10 nM of the serine protease inhibitor benzamidine to the incubation resulted in a complete inhibition of this CXCL8 conversion (Figure 3C). After 2 hours, all CXCL11(3-73) proteins were truncated with CD13, whereas only 21% of CXCL8(1-77) was processed into CXCL8(2-77) and no CXCL8(6-77) was detected. Even upon overnight incubation with this preparation of CD13 in the presence of benzamidine at 1:25 E/S ratio, less than 50% of CXCL8(1-77) was truncated with CD13 into CXCL8(2-77) (36%) or CXCL8(3-77) (9%). Truncation of CXCL8(6-77) (ie, the CXCL8 isoform that is typically produced by monocytes) with CD13 at a 1:25 E/S ratio provided an even slower conversion into CXCL8(7-77) and CXCL8(8-77) (Figure3D). Thus, the reported CD13-induced conversion of CXCL8(1-77)to CXCL8(6-77)29 appeared to be due to a minor contamination of the commercially available CD13 with a serine protease that cleaves after the Arg in position 5 in the CXCL8 sequence. In conclusion, compared with CD26-processed CXCL11, intact CXCL8 or monocyte-derived CXCL8(6-77) are poor CD13 substrates.
Distinct effects of CD13/APN on CXCL8 and CXCL11. Synthetic CXCL11(3-73) was incubated with CD13 and 10 mM benzamidine at a 1:4 molar E/S ratio for 2 hours at 37°C and desalted on C4 ZipTips prior to ion-trap mass spectrometry (panel A). The deconvoluted spectra of the multiple charged ions (with 8 to 12 positive charges) are shown in the inset. The 3 molecular ions A, B, and C correspond to CXCL11(5-73), CXCL11(4-73), and CXCL11(6-73), respectively. Alternatively, CXCL11(3-73) (panel B), CXCL8(1-77) (panel C), or CXCL8(6-77) (panel D) were incubated with CD13 and 10 mM benzamidine (except for the conditions without benzamidine that are indicated on panel C) at a 1:25 molar enzyme-substrate ratio for 5 minutes to 24 hours at 37°C and desalted on PVDF membranes prior to Edman degradation. The percentages of the remaining unprocessed chemokines (■) and the chemokines missing 1 (▩), 2 (▧), 3 (white bars with black diamonds), 4 (dotted bars), or 5 (□) NH2-terminal amino acids that were calculated from the molar yields for the 10 NH2-terminal amino acids are indicated in the histograms in function of the incubation time.
Distinct effects of CD13/APN on CXCL8 and CXCL11. Synthetic CXCL11(3-73) was incubated with CD13 and 10 mM benzamidine at a 1:4 molar E/S ratio for 2 hours at 37°C and desalted on C4 ZipTips prior to ion-trap mass spectrometry (panel A). The deconvoluted spectra of the multiple charged ions (with 8 to 12 positive charges) are shown in the inset. The 3 molecular ions A, B, and C correspond to CXCL11(5-73), CXCL11(4-73), and CXCL11(6-73), respectively. Alternatively, CXCL11(3-73) (panel B), CXCL8(1-77) (panel C), or CXCL8(6-77) (panel D) were incubated with CD13 and 10 mM benzamidine (except for the conditions without benzamidine that are indicated on panel C) at a 1:25 molar enzyme-substrate ratio for 5 minutes to 24 hours at 37°C and desalted on PVDF membranes prior to Edman degradation. The percentages of the remaining unprocessed chemokines (■) and the chemokines missing 1 (▩), 2 (▧), 3 (white bars with black diamonds), 4 (dotted bars), or 5 (□) NH2-terminal amino acids that were calculated from the molar yields for the 10 NH2-terminal amino acids are indicated in the histograms in function of the incubation time.
Moreover, CXCL11(3-73) (500 pmol) was incubated for 4 hours or 16 hours at 37°C in 200 μL neutrophil-derivedCD13/APN resulting in an E/S ratio of 1:600. CXCL11 isoforms were repurified by RP-HPLC and fractions that contained CXCL11 immunoreactivity were blotted on PVDF membranes and subjected to Edman degradation. Both the NH2-terminal sequences of CXCL11(3-73) (MFKRGRXLX) and CXCL11(4-73) (FKRGRXLXI) were detected. Incubation with neutrophil-derived CD13/APN in the presence of the metalloprotease inhibitors EDTA (50 mM) and 1,10-phenantroline (20 mM) resulted in the recovery of CXCL11(3-73) only.
Incubation of CHO-CXCR3 cells with CD13
In order to investigate the proteolytic effect of CD13 on CXCR3-binding and receptor-dependent activity, CHO-CXCR3 cells were incubated for 1 hour at 37°C with or without 0.7 pmol CD13 per 106 cells and subsequently prepared for Fura-2 calcium measurements. The dose of 0.7 pmol of CD13 per 106 cells was chosen since this corresponded to the amount of CD13/APN detected on 106 monocytic THP-1 cells (as determined with the hydrolysis of Leu-pNA). CHO-CXCR3 cells were treated with 0.4 nM, 1 nM, 4 nM, or 10 nM CXCL11 and the increase in [Ca2 +] i was compared between CD13-treated and control CXCR3 cells. No significant difference in increase in [Ca2 +] i was observed when cells were pretreated with CD13 or not (data not shown). Thus, even if CXCR3 was truncated by CD13, the truncation did not appear to alter the receptor-signaling capacity. This is in agreement with the presence of functional CXCR3 on CD13-expressing cells. These experiments, however, do not exclude that at different conditions there might be functional cleavage of the CXCR3 receptor.
Lack of lymphocyte chemotactic activity of truncated CXCL11
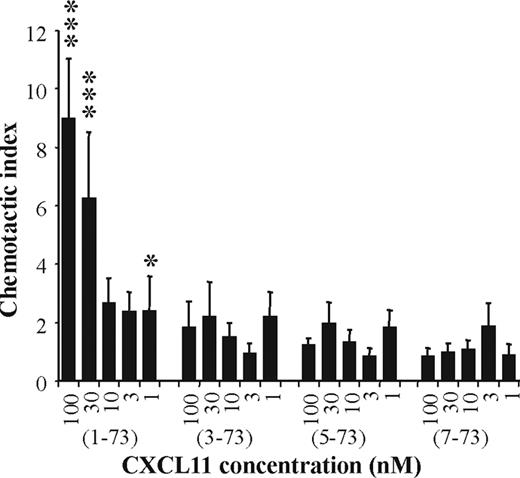
The chemotactic activity of CXCL11(1-73), CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) was compared on activated lymphocytes. A dose-dependent chemotactic effect was obtained with intact CXCL11(1-73) on PHA plus IL-2–stimulated lymphocytes (Figure 4). All 3 NH2-terminally truncated CXCL11 forms tested were devoid of significant chemotactic activity at concentrations up to 100 nM. Thus, processing of CXCL11 by aminopeptidases such as CD26/DPP IV and CD13/APN resulted in a complete loss of chemotactic activity for activated T lymphocytes.
Lymphocyte chemotactic activity of CXCL11 isoforms. CXCL11(1-73), CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) were tested for the ability to induce a chemotactic response on PHA- and IL-2–activated lymphocytes. Results represent the mean (± SEM) chemotactic index of 3 or more independent experiments. The Mann-Whitney U test was used for statistical analysis (*P < .05; ***P < .001 for a positive chemotactic response compared with buffer controls).
Lymphocyte chemotactic activity of CXCL11 isoforms. CXCL11(1-73), CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) were tested for the ability to induce a chemotactic response on PHA- and IL-2–activated lymphocytes. Results represent the mean (± SEM) chemotactic index of 3 or more independent experiments. The Mann-Whitney U test was used for statistical analysis (*P < .05; ***P < .001 for a positive chemotactic response compared with buffer controls).
Receptor-binding properties of CXCL11 isoforms
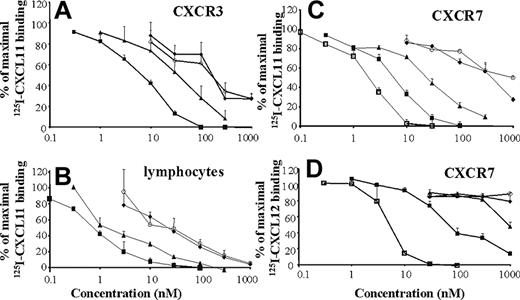
CXCL11-induced chemotaxis on lymphocytes has been reported to be CXCR3 dependent. Therefore, intact and truncated CXCL11 were tested for their ability to compete for 125I-CXCL11 binding to lymphocytes and CHO-CXCR3 cells. Intact CXCL11 competes for 125I-CXCL11 binding to CHO-CXCR3 cells in a dose-dependent manner with a minimal effective concentration of 1 nM (P < .01) and reaches complete displacement of 125I-CXCL11 at 100 nM (Figure 5A). About 3-fold more CXCL11(3-73) was required to obtain a comparable competition for binding. CXCL11(5-73) and CXCL11(7-73) were 30-fold less potent compared with intact CXCL11, whereas complete displacement could not be reached even at 1 μM. On activated lymphocytes, 0.3 nM intact CXCL11 already significantly reduced the binding of the labeled chemokine (Figure 5B). CXCL11(3-73) was again about 3-fold less potent, and the further truncated molecules CXCL11(5-73) and CXCL11(7-73)were about 30-fold less efficient than intact CXCL11 to displace intact 125I-CXCL11 from lymphocytes. The lymphocyte-binding pattern of truncated CXCL11 isoforms seemed to follow that of CXCR3-transfected CHO cells. However, the recently deorphanized receptor RDC1, tentatively renamed CXCR711,30 also binds CXCL11 in addition to CXCL12 (Figure 5C). CXCL12 competed about 3-fold more efficiently for CXCL11 binding to CHO-CXCR7 cells compared with intact CXCL11, which showed a minimal effective concentration of 1 nM. CXCL11(3-73) was again about 3-fold less potent than intact CXCL11, and CXCL11(5-73) and CXCL11(7-73) were weak CXCR7 ligands but at elevated concentrations (> 100 nM) still partially displaced 125I-CXCL11 from this receptor. CXCL11 was also able to compete for 125I-CXCL12 binding to CHO-CXCR7 cells but was 10- to 30-fold less efficient compared with unlabeled CXCL12 (Figure 5D). NH2-terminally truncated CXCL11 forms failed to compete for 125I-CXCL12 binding with the exception of CXCL11(3-73) at elevated concentrations (1 μM). Neither intact nor truncated CXCL11 forms were able to compete for CXCL12 binding to CXCR4 on CHO-CXCR4 cells (data not shown). Taken together, it can be concluded that CXCL11 binds efficiently to both CXCR3 and CXCR7 and that the truncated CXCL11 isoforms become gradually weaker to compete for intact CXCL11 binding on both CXCR3 and CXCR7.
Competition by CXCL11 isoforms for radioactive ligand binding to lymphocytes or CXCR3- or CXCR7-transfected CHO cells. Increasing concentrations of unlabeled synthetic CXCL11(1-73) (■), CXCL11(3-73) (▴), CXCL11(5-73) (♦), CXCL11(7-73) (○), or recombinant CXCL12 (□) were added together with 125I-CXCL11 to CHO-CXCR3 cells (A), lymphocytes (B), or CHO-CXCR7 cells (C) or were incubated with CHO-CXCR7 cells in the presence of 125I-CXCL12 (D). Results represent the remaining mean (± SEM) percentage specific 125I-CXCL11 or 125I-CXCL12 binding for 3 to 7 independent experiments.
Competition by CXCL11 isoforms for radioactive ligand binding to lymphocytes or CXCR3- or CXCR7-transfected CHO cells. Increasing concentrations of unlabeled synthetic CXCL11(1-73) (■), CXCL11(3-73) (▴), CXCL11(5-73) (♦), CXCL11(7-73) (○), or recombinant CXCL12 (□) were added together with 125I-CXCL11 to CHO-CXCR3 cells (A), lymphocytes (B), or CHO-CXCR7 cells (C) or were incubated with CHO-CXCR7 cells in the presence of 125I-CXCL12 (D). Results represent the remaining mean (± SEM) percentage specific 125I-CXCL11 or 125I-CXCL12 binding for 3 to 7 independent experiments.
Signaling properties of CXCL11 isoforms
CXCL11 is known to induce a CXCR3-dependent increase of the [Ca2 +] i in activated T cells.7 Therefore, CHO-CXCR3 cells were stimulated with multiple doses of intact and NH2-terminally truncated CXCL11. Synthetic and recombinant CXCL11 induced a comparable dose-dependent increase in [Ca2 +] i from 0.04 nM onwards (Figure 6A–B) and equally desensitized the receptor for a second stimulation with 0.1 nM intact CXCL11 (Figure 6B). In contrast, compared with intact CXCL11, CXCL11(3-73) was more than 1000-fold and 100-fold less efficient to induce (Figure 6B inset) and to desensitize CXCR3 signaling, respectively. CXCL11(3-73) induced a low but significant calcium response from 200 nM onwards (P < .05) and from 10 nM it desensitized the CXCR3 calcium response to 0.1 nM intact CXCL11 to reach a complete desensitization at 200 nM (Figure 6C). Although CXCL11(5-73) and CXCL11(7-73) were totally inactive in CXCR3 signaling at concentrations up to 1 μM, these forms both desensitized CXCR3 partially when minimally 40 nM of the truncated molecules was used (P < .05) but were less efficient compared with CXCL11(3-73) (P < .05). Finally, although CXCL11 (10 nM to 100 nM) bound to CXCR7, it was unable to induce an increase in [Ca2 +] i in CHO-CXCR7 cells (data not shown).
Signal transduction pathways activated by CXCL11 isoforms. Recombinant CXCL11(1-73) (□), synthetic CXCL11(1-73) (■), CXCL11(3-73) (▴), CXCL11(5-73) (♦), and CXCL11(7-73) (○) were tested for their ability to increase the [Ca2 +] i in CHO-CXCR3 cells (A-B). In panel A, a representative dose-response experiment is shown. The inset in panel B shows the average increase in [Ca2 +] i induced by CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) at an enlarged scale. For desensitization experiments (C), 0.1 nM intact CXCL11(1-73) was added as the second stimulus. Results represent the mean (± SEM) of 3 or more independent experiments. Alternatively, serum-starved CHO-CXCR3 cells were treated with different concentrations of CXCL11 isoforms. After 5 minutes, the reaction was stopped and cells were lysed. The level of phosphorylated ERK1/2 (■) or PKB/Akt (□) in the cell lysate was determined with specific ELISAs (D). The mean values and standard errors are derived from 2 to 10 experiments. Statistical analysis was performed using the Mann-Whitney U test. Significant differences in ERK1/2 or PKB/Akt phosphorylation compared with control samples are indicated as *P < .05, **P < .01, and ***P < .001.
Signal transduction pathways activated by CXCL11 isoforms. Recombinant CXCL11(1-73) (□), synthetic CXCL11(1-73) (■), CXCL11(3-73) (▴), CXCL11(5-73) (♦), and CXCL11(7-73) (○) were tested for their ability to increase the [Ca2 +] i in CHO-CXCR3 cells (A-B). In panel A, a representative dose-response experiment is shown. The inset in panel B shows the average increase in [Ca2 +] i induced by CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) at an enlarged scale. For desensitization experiments (C), 0.1 nM intact CXCL11(1-73) was added as the second stimulus. Results represent the mean (± SEM) of 3 or more independent experiments. Alternatively, serum-starved CHO-CXCR3 cells were treated with different concentrations of CXCL11 isoforms. After 5 minutes, the reaction was stopped and cells were lysed. The level of phosphorylated ERK1/2 (■) or PKB/Akt (□) in the cell lysate was determined with specific ELISAs (D). The mean values and standard errors are derived from 2 to 10 experiments. Statistical analysis was performed using the Mann-Whitney U test. Significant differences in ERK1/2 or PKB/Akt phosphorylation compared with control samples are indicated as *P < .05, **P < .01, and ***P < .001.
In addition to a calcium flux, CXCL11 induced a dose-dependent phosphorylation of ERK1/2 and Akt/PKB in CHO-CXCR3 cells (Figure 6D). Even at 300 nM, no ERK1/2 or Akt/PKB signaling was detectable with any of the 3 truncated CXCL11 forms. These findings are concordant with the calcium signaling data (Figure 6A–B). Neither CXCL11 (300 nM and 1 μM) nor CXCL12 (100 nM) induced phosphorylation of ERK1/2 or Akt/PKB in CHO-CXCR7 cells (data not shown).
Endothelial cell migration
In an in vitro wound-healing assay with endothelial cells, intact CXCL11 inhibited HMVEC migration at a concentration of 0.8 nM to 100 nM (Figure 7). CXCL11(3-73) significantly inhibited endothelial cell migration at 20 nM to 100 nM. Thus, truncation by CD26 only marginally affected the inhibitory effect of CXCL11. Although CXCL11(7-73) also significantly inhibited HMVEC migration at 20 nM to 100 nM, CXCL11(7-73) was significantly less efficient compared with intact or CD26-truncated CXCL11. In summary, although all 3 NH2-terminally truncated variants of CXCL11 retained some inhibitory properties in an in vitro HMVEC migration assay, CXCL11(7-73) was a less-potent inhibitor compared with intact or CD26-truncated CXCL11. In view of the lymphocyte chemotaxis results, these data indicate that CXCR3 as well as other receptor mechanisms might be implicated in HMVEC migration.
Inhibition of endothelial cell migration. CXCL11(1-73), CXCL11(3-73), and CXCL11(7-73) were tested for their ability to inhibit endothelial cell migration in vitro. Scars were drawn in a confluent HMVEC monolayer and the alteration in width upon addition of chemokine was scored microscopically after 24 hours. Zero scores correspond to the change in width for untreated control cultures. A scar that retained a broader width compared with control scars (inhibition of migration) received a negative inhibition score. Panels A, B, C, and D are representative examples of inhibition of migration with width scores 0, −1, −2.25, and −2.5, respectively. Results in panel E represent average changes in scores (± SEM) for 8 to 10 experiments, each tested in duplicate wells in one 24-well plate. Each well was scored double blind by 2 investigators. Statistical analysis of inhibition of HMVEC migration between different CXCL11 forms was performed with the Mann-Whitney U test [*P < .05, **P < .01, and ***P < .001 for CXCL11 isoforms compared with buffer-treated controls; ++P < .01 for CXCL11(1-77) compared with CXCL11(7-77); and X for P < .05 for CXCL11(3-73) compared with CXCL11(7-73)].
Inhibition of endothelial cell migration. CXCL11(1-73), CXCL11(3-73), and CXCL11(7-73) were tested for their ability to inhibit endothelial cell migration in vitro. Scars were drawn in a confluent HMVEC monolayer and the alteration in width upon addition of chemokine was scored microscopically after 24 hours. Zero scores correspond to the change in width for untreated control cultures. A scar that retained a broader width compared with control scars (inhibition of migration) received a negative inhibition score. Panels A, B, C, and D are representative examples of inhibition of migration with width scores 0, −1, −2.25, and −2.5, respectively. Results in panel E represent average changes in scores (± SEM) for 8 to 10 experiments, each tested in duplicate wells in one 24-well plate. Each well was scored double blind by 2 investigators. Statistical analysis of inhibition of HMVEC migration between different CXCL11 forms was performed with the Mann-Whitney U test [*P < .05, **P < .01, and ***P < .001 for CXCL11 isoforms compared with buffer-treated controls; ++P < .01 for CXCL11(1-77) compared with CXCL11(7-77); and X for P < .05 for CXCL11(3-73) compared with CXCL11(7-73)].
Discussion
More and more evidence points toward an important role for posttranslational modifications of chemokines to regulate the inflammatory response.6 Although some chemokines were reported to exist in glycosylated forms, so far no major biologic consequences have been linked with the attachment of sugars to chemokines. In contrast, several reports demonstrate that the interaction between chemokines and proteases, in particular, enzymes that modify the NH2 terminus of chemokines, affect the inflammatory, angiogenic, and antiviral activities of specific chemokines. Some chemokines (eg, platelet basic protein) require NH2-terminal processing in order to become active.31 Other chemokines acquire enhanced or reduced receptor specificity upon NH2-terminal processing. Indeed, removal of the NH2-terminal dipeptide from CCL5 by CD26/DPP IV resulted in increased CCR5 affinity but impaired CCR1 and CCR3 binding.32,33 This modification led to reduced monocyte and eosinophil chemotactic activity but increased chemotaxis of lymphocytes and enhanced anti–HIV-1 activity. CD26/DPP IV also processed the CXCR3 ligands CXCL9, CXCL10, and CXCL11, resulting in impaired CXCR3 signaling and lymphocyte chemotaxis.18 Surprisingly, CD26-processed CXCL9 and CXCL10 retained their antiangiogenic properties. In this manuscript, the identification of multiple natural PBMC- or fibroblast-derived NH2-terminally truncated CXCL11 forms generated by CD13/APN is reported and their receptor affinity, signaling capacity, and biologic activities were investigated. Although CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) retained weak CXCR3-binding properties, these forms failed to induce a chemotactic response in lymphocytes and to induce ERK1/2 or Akt/PKB phosphorylation in CXCR3-transfected cells. Only CXCL11(3-73) had weak calcium signaling properties in cells transfected with CXCR3 at almost micromolar concentrations. These results are in accordance with an earlier report that synthetic CXCL11(4-73) acted as a chemotaxis antagonist and retained some CXCR3-binding properties.34 This manuscript confirms that CXCL11 also binds to the 7-transmembrane–spanning receptor RDC1, which was recently recognized to bind CXCL12 and therefore was tentatively named CXCR7.11,30 RDC1 was initially reported to be a receptor for vasointestinal peptide, a possibility that was later set aside.35,36 In contrast to the CXCR3 gene that is located on the X chromosome, the gene for CXCR7 as well as those for the chemokine receptors CXCR1, CXCR2, and CXCR4 are located on human chromosome 2.37,38 Receptor-binding studies revealed that CXCL12 has a higher affinity for CXCR7 compared with CXCL11 (Figure 5). NH2-terminal truncation of CXCL11 decreased its affinity for CXCR7. Although CXCL11 efficiently bound to CXCR7, no calcium flux or ERK1/2 or Akt/PKB phosphorylation could be detected even with elevated concentrations of ligand. Thus CXCR7 does not appear to be activated by CXCL11 in the same manner as classical chemokine receptors. CXCR7 protein expression was detected in normal cartilage tissue, tumor cells, activated endothelial cells, fetal liver cells, monocytes, and switch memory B cells that produce immunoglobulins in vitro.11,39,40 In addition, CXCR7 RNA was up-regulated by hypoxia in human monocytes and acted as a coreceptor for specific HIV and SIV strains.41,42 The reported30 CXCL12-induced chemotaxis of T cells transfected with CXCR7 is controversial.11 However, no CXCR7-dependent calcium has been reported with CXCL12. Despite the apparent lack of classical chemokine receptor signaling pathways, CXCR7 expression promoted cell adhesion and cell survival in vitro, and blocking CXCR7 with a small molecule (CCX754) resulted in reduced tumor growth in vivo.11
The myeloid cell marker and metalloprotease CD13/APN, which is known to remove NH2-terminal residues from peptides and proteins, rapidly processed CXCL11(3-73). Since CXCR3 ligands and CD13 have both been reported to function as regulators of angiogenesis,22,23,43 intact and CD13-truncated CXCL11 forms were compared for their ability to inhibit endothelial cell migration. Although CXCL11 truncation by CD26, resulting in the removal of the NH2-terminal dipeptide, did not significantly affect the inhibitory activity in an endothelial cell migration assay, further truncation of CXCL11 with CD13 resulted in a reduction of the inhibition of endothelial cell migration by CXCL11(7-73). Thus, processing of CXCL11 by CD13 resulted not only in the loss of lymphocyte and NK-cell chemotaxis but also in an enhanced local angiogenic environment. This might have consequences in the context of inflammation and tumor development, whereby intact CXCL11 attracts activated lymphocytes and NK cells to inflamed tissue and to tumors and inhibits the generation of novel blood vessels essential for tumor growth and tissue repair. CD13 not only functions as a protease but also acts as a receptor for human coronavirus.44 Since CXCR3 and CXCL11 have been implicated in viral infection,45,46 CD13-induced inactivation of the most potent CXCR3 ligand may not only affect infection with coronavirus but may also influence other viral pathologies. Limited CD26-induced truncation of CXCL11 will reduce the infiltration of lymphocytes but only marginally affects the antiangiogenic activity of CXCL11. However, further processing by CD13 additionally resulted in a significant reduction of antiangiogenic activity.
In conclusion, this is the first report on chemokine processing by the leukocyte markers CD13 and CD26 in concert to dampen the immune response and to increase the angiogenic environment during infection, inflammation, and cancer. Together with the complexity of the chemokine family, this forms an additional manner of fine-tuning of host defense against invaders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank René Conings and Jean-Pierre Lenaerts for technical assistance.
This work was supported by the Center of Excellence (Credit no. EF/05/15) of the K.U. Leuven, the Concerted Research Actions (G.O.A.) of the Regional Government of Flanders, the Fund for Scientific Research of Flanders (F.W.O.-Vlaanderen), the Interuniversity Attraction Poles Program-Belgian Science Policy (I.A.P.), and the European Union 6FP EC contract INNOCHEM (grant LSHB-CT-2005–518167). J.V. is a research assistant and E.S. and S.S. are senior research assistants of the F.W.O.-Vlaanderen. M.G. holds a postdoctoral fellowship of the Research Fund of the K.U. Leuven.
Authorship
Contribution: P.P., A.M., T.L., J.V., M.G., I.R., E.S., W.P., and S.S. performed research and collected and analyzed data; M.P. contributed vital new reagents; and P.P. and J.V.D. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Proost, Lab Molecular Immunology, Rega Institute, K.U. Leuven, Minderbroedersstraat 10, B-3000 Leuven, Belgium; e-mail: paul.proost@rega.kuleuven.be.

![Figure 1. Purification and identification of natural CXCL11 isoforms. Conditioned medium from fibroblasts was purified by heparin-affinity and cation-exchange chromatography (not shown) and finally loaded on a C8 RP-HPLC column. Proteins were eluted from the C8 column in an acetonitrile gradient (dotted line) and detected at 214 nm (full line; A). The CXCL11 concentration in the column fractions was determined by ELISA (histograms). Panel B shows the averaged MS spectrum (mass/charge [m/z] versus abundance) for the proteins that eluted between 28 and 29 minutes from the RP-HPLC column. The charges of the detected ions (+ 5 to + 8) are indicated on the averaged spectrum, and the deconvoluted spectrum that was calculated from these charged ions is given as an inset in panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-10-049072/2/m_zh80130702950001.jpeg?Expires=1769099935&Signature=zw6I-YPam7v28IKL~rnaxcGppDtLl-NibTzT8fZMOZjvOVr1SIEwNZN9Rl6NmoRR~g4QBaLyV0LKKMCe43Q7~~GfhBv8tgdqyTsBbBO34piuDp2u0gM4ZoU~qwZs~f4bRKrMzobgNAL3GuqyNhkJiY29DZer0fpw-1620mNFRQMgp7N6Qe6zf-lEOPAGVE2Oaz~5qcIY~28WDSTRwOCqQ4gkUjkWM0bt1pdu0XvXdHof0LDDbKgtpHdwXNBROgh7pPukeMi5fJQGigOmwLRLzzc~6H4srCQfQCkc1k6ZwvXmk29GOOQTm5dTdXwxsJj8Pw6nqBOpoPJtuvbLpLA1nQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Signal transduction pathways activated by CXCL11 isoforms. Recombinant CXCL11(1-73) (□), synthetic CXCL11(1-73) (■), CXCL11(3-73) (▴), CXCL11(5-73) (♦), and CXCL11(7-73) (○) were tested for their ability to increase the [Ca2 +] i in CHO-CXCR3 cells (A-B). In panel A, a representative dose-response experiment is shown. The inset in panel B shows the average increase in [Ca2 +] i induced by CXCL11(3-73), CXCL11(5-73), and CXCL11(7-73) at an enlarged scale. For desensitization experiments (C), 0.1 nM intact CXCL11(1-73) was added as the second stimulus. Results represent the mean (± SEM) of 3 or more independent experiments. Alternatively, serum-starved CHO-CXCR3 cells were treated with different concentrations of CXCL11 isoforms. After 5 minutes, the reaction was stopped and cells were lysed. The level of phosphorylated ERK1/2 (■) or PKB/Akt (□) in the cell lysate was determined with specific ELISAs (D). The mean values and standard errors are derived from 2 to 10 experiments. Statistical analysis was performed using the Mann-Whitney U test. Significant differences in ERK1/2 or PKB/Akt phosphorylation compared with control samples are indicated as *P < .05, **P < .01, and ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-10-049072/2/m_zh80130702950006.jpeg?Expires=1769099935&Signature=ZMt9uaw6Dt-QBiVYux8nITMTT~ne8UIqWHqXP2sBAeZqF96kdZ4b2gYgrgglsUkBVZPr6eVtC1Y8ra0W-ZlJpiFzEYqfXp2-87ZHbE1Z1mmpOOvYulB9K~KG7jvFa0RSiYMaSIhOMi~zy5tagXVxUqr~wqlRwUnBFhzf9RrS4xTLzkkxW2B4~S4SCSFrj~vsyNYrRjgJ-2SMuYiMxR0oDFcSLWmy9vYHttiiIJalqr~8T779wcoEbWS0SYhm767Mo4lWpXVdl88lDrZ4svdVW3e6W2LFYn4Revqi2VRUTak0WweHzeSkXIXo7IZxu~0zQbrND0MGtEcKpH364B3iQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Inhibition of endothelial cell migration. CXCL11(1-73), CXCL11(3-73), and CXCL11(7-73) were tested for their ability to inhibit endothelial cell migration in vitro. Scars were drawn in a confluent HMVEC monolayer and the alteration in width upon addition of chemokine was scored microscopically after 24 hours. Zero scores correspond to the change in width for untreated control cultures. A scar that retained a broader width compared with control scars (inhibition of migration) received a negative inhibition score. Panels A, B, C, and D are representative examples of inhibition of migration with width scores 0, −1, −2.25, and −2.5, respectively. Results in panel E represent average changes in scores (± SEM) for 8 to 10 experiments, each tested in duplicate wells in one 24-well plate. Each well was scored double blind by 2 investigators. Statistical analysis of inhibition of HMVEC migration between different CXCL11 forms was performed with the Mann-Whitney U test [*P < .05, **P < .01, and ***P < .001 for CXCL11 isoforms compared with buffer-treated controls; ++P < .01 for CXCL11(1-77) compared with CXCL11(7-77); and X for P < .05 for CXCL11(3-73) compared with CXCL11(7-73)].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-10-049072/2/m_zh80130702950007.jpeg?Expires=1769099935&Signature=Tlk2i7C~QKzVvvSCXH2HIeWXOfWmp57bczC01cdspPetGs5TXC4xvCxX9IyOafkyK0vPm2GlbEa3tHUKUozQp47itCrHpDnGe6jhHwlUQ~l9G4DE63vhIAzFVtPwjJQZw8ItZK71BroTnCDbSAzUm-~SG~9rffQVH1eiz3DlAWEEm48y7vxnCle2ACI4BY791kb9qBNaotoO5UDIkuo0w0KnDT3E4gKo0v0p5oJeoSsA4zFDWDjHTqRKBVFnvCvZjnntWP8aAtem6Dug-DJNbd760lhrIRAZwEvlIgqrduAgefk~uqntJ5ZVPz4Ma8muRqitL9jr47WgyPLBCNiBzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal