Pleiotrophin (PTN) is an important developmental cytokine that is highly expressed during embryogenesis but shows very limited expression in adult tissues, where it is largely restricted to the brain. High PTN serum levels are associated with a variety of solid tumors. We recently showed that patients with multiple myeloma (MM) also have elevated serum levels of this protein and the amount of PTN correlated with the patients' disease status and response to treatment. In this study, we demonstrate that MM cell lines and the malignant cells from MM patients' bone marrow produced PTN and secreted PTN protein into the supernatants during short-term culture. Moreover, Ptn gene expression correlated with the patients' disease status. Inhibition of PTN with a polyclonal anti-PTN antibody reduced growth and enhanced apoptosis of MM cell lines and freshly isolated bone marrow tumor cells from MM patients in vitro. Importantly, this antibody also markedly suppressed the growth of MM in vivo using a severe combined immunodeficiency (SCID)-hu murine model. This represents the first study showing the importance of PTN in the growth of any hematological disorder. Because the expression of this protein is very limited in normal adult tissues, PTN may represent a new target for the treatment of MM.
Introduction
Multiple myeloma (MM) is characterized by the accumulation of malignant monoclonal plasma B cells in the bone marrow (BM). The overall survival of patients with MM varies greatly, with a mean of approximately 3 years.1 In addition to conventional and high-dose chemotherapy followed by autologous hematopoietic stem cell transplant,2 newer antimyeloma agents including thalidomide, lenalidomide, bortezomib, liposomal doxorubicin, and arsenic trioxide have proven to be potent inhibitors of myeloma cell growth.3,–5 They have been effective when used as single agents and in combination therapies for myeloma patients.4,5 Despite these improvements in antimyeloma therapy, the disease remains incurable.1,4,5
A variety of cytokines contribute to the survival, vascularization, chemo-resistance, and proliferation of myeloma cells.6 Although IL-6 was considered to be an essential autocrine growth factor for myeloma,7 it was later found to be insufficient for myeloma proliferation.8 Moreover, IL-6 is primarily produced by the BM stromal cells that have been stimulated by other factors, including insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), tumor necrosis factor alpha, or CD40.6 Tumor necrosis factor alpha is not sufficient for myeloma proliferation or drug resistance.9 In contrast, IGF-1 promotes myeloma growth more effectively than IL-6 and also contributes to MM drug resistance and survival.10 In addition to enhancing microvessel density, VEGF also directly induces myeloma cellular proliferation.11
Pleiotrophin (PTN) is an 18-kDa secreted heparin and chondroitin-binding protein that is a regulatory cytokine critical to the early development of blood vessels and neurons.12,–14 PTN proteins isolated from human, mouse, and rat are nearly identical at the amino acid level and share approximately 50% amino acid similarity to the only other family member, midkine (MK).12,15 Expression of the Ptn gene is tightly regulated in a temporal and tissue-specific manner during development. Ptn is induced in early embryogenesis, expression peaks near the time of birth and sharply declines thereafter, showing a very restricted expression pattern in adults.16 PTN protein is, however, highly re-expressed in the astrocytes, macrophages, and microvasculature of the adult rat cerebrum after ischemic injury,14 and the introduction of this protein into myocardial tissue increases angiogenesis.17
Aberrant expression of PTN has been found in many solid tumors.16,18,19 Deletion of the tumor suppressor phosphatase and tensin homologue deleted on chromosome 10 (PTEN) increases Ptn gene transcription and tumorigenicity in mouse models.20 Introduction of the Ptn gene into NIH 3T3 fibroblasts is sufficient to transform these cells,21 identifying Ptn as an oncogene. When Ptn-transformed fibroblasts are implanted into nude mice, aggressive and highly vascularized tumors with cytoskeletal abnormalities develop, suggesting that PTN contributes not only to the proliferation of malignant cells but also to tumor vascularization and possibly metastasis.22 PTN serum levels in patients have been found to correlate with tumor size in a variety of gastrointestinal cancers18 and with progression and metastasis in melanoma patients,23 suggesting that PTN serum levels may be a useful prognostic marker in a variety of cancers. Specific ribozyme interference of PTN mRNA in melanoma cells implanted into athymic nude mice inhibited tumor growth, vascularization, and metastasis in vivo.24,25 Thus, PTN may be a new target for cancer treatment by inhibiting both the proliferation of malignant cells as well as tumor vascularization.
PTN binds to heparin and chondroitin sulfate on the plasma cell marker CD138 (syndecan 1).26 This receptor, however, lacks cytoplasmic catalytic domains,27 thus CD138 is likely to be a coreceptor for PTN that interacts with other receptor kinases or phosphatases.28,29 PTN binds to chondroitin sulfate on the receptor protein tyrosine phosphatase beta/zeta (RPTPβ/ζ) on the surface of glioblastoma cell lines.30 Association of PTN with RPTPβ/ζ results in inhibition of the phosphatase activity leading to accumulation of tyrosine phosphorylated Fyn tyrosine kinase and β-catenin, and activation of Wnt and nuclear factor κB (NFκB)-regulated genes.31 Excess β-catenin has been found in the cytoplasm of MM cells relative to the BM and plasma B cells from healthy donors.32 We have shown increased NFκB activation in the MM cells and found that NFκB dysregulation is associated with chemoresistance.33 We discovered that PTN is increased in the serum from MM patients and that the levels correlate with disease status.34 Here we report that the malignant plasma cells from MM patients as well as MM cell lines produce and secrete PTN. Moreover, we demonstrate that a polyclonal anti-PTN antibody reduces MM cell growth and enhances apoptosis in vitro and inhibits the growth of human myeloma tumors in immunodeficient mice.
Patients, materials, and methods
Cells, cell culture, and collection of blood and BM samples
The human MM cell lines RPMI 8226, U266, and MM-1S, and the human monocyte cell line THP-1 were obtained from American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured with RPMI 1640 supplemented with 10% fetal bovine serum, nonessential amino acids, 2 mM glutamine, 1 mM sodium pyruvate, 25 mM HEPES, 200 units/mL penicillin, and 200 μg/mL streptomycin at 37°C and 5% CO2. Peripheral blood and BM aspirates were collected from MM patients and peripheral blood from healthy age- and gender-matched control subjects after obtaining institutional review board approval (Western Institutional Review Board [IRB]) and informed consent in accordance with the Declaration of Helsinki. Patients were defined as showing indolent, responsive, active, refractory, or relapsed disease according to the European Group for Blood and Bone Marrow Transplantation35 and the International Response Criteria36 guidelines. Serum was isolated by centrifugation and stored at −80°C. Peripheral blood mononuclear cells (MCs) and BMMCs were isolated by density-gradient centrifugation using Histopaque-1077 (Sigma, St Louis, MO, manufacturer's directions).
Enzyme-linked immunosorbent assay for PTN serum concentration
Serum samples from 74 consecutive MM patients and 33 healthy control donors were analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described.34 Serum samples (100 μL/well) were incubated in flat-bottomed 96-well microtiter plates overnight at 4°C. Plates were washed 3 times with phosphate-buffered saline (PBS), and blocked with PBS with 0.1% Tween-20 (PBST) supplemented with 1% bovine serum albumin at room temperature for 2 hours. Plates were then incubated with 100 μL/well of biotinylated anti-PTN (0.5 μg/mL, R&D Systems, Minneapolis, MN) at 4°C overnight, washed 3 times with PBST, incubated with 100 μL alkaline phosphatase-conjugated streptavidin room temperature 1 hour, (200 mg/mL, 1:200; KPL, Gaithersburg, MD) and washed 4 times with PBST. Bound proteins were detected using BluePhos Microwell Phosphatase substrate (KPL, manufacturer's directions) and analyzed using a μQuant (Biotek Industries, Winooski, VT) plate reader at 450 nm with KC Junior software. Values represent the mean of triplicate experiments. The standard curve for PTN concentration was determined using 0.01-100 ng/mL recombinant human PTN (R&D Systems).
Immunohistochemical and immunofluorescent analysis of PTN in MM BMMCs
BMMCs (106 cells) were spun down onto polylysine-coated microscope slides (VWR, San Dimas, CA) and then fixed with cold acetone for 5 minutes. The slides were blocked with Tris-buffered saline with 0.05% Tween[b]-20 (TBST) and 3% bovine serum albumin for 1 hour at room temperature; 100 μL of biotinylated goat antihuman PTN antibody or control pre-immune goat serum (both from R&D Systems) diluted 1:500 were added and incubated at 4°C overnight. The slides were washed 3 times with TBST and treated with ARH-conjugated antigoat antibody (KPL) diluted 1:500 in TBST at room temperature for 2 hours. The slides were washed 3 times in TBST, placed in AEC buffer for 5 minutes, and the color was detected using an AEC kit (Dako, Carpenteria, CA, manufacturer's directions). For immunofluorescent analysis, slides were incubated with anti-CD138 conjugated to phycoerythrin (PE) (1:100; BD Biosciences, San Diego, CA) and goat anti-PTN (250 μg/mL, R&D Systems) antibodies at 4°C overnight. The slides were washed 3 times with PBS for 15 minutes at room temperature and incubated with FITC-conjugated swine antigoat antibody (1:200; Biosource, Camarillo, CA) for 2 hours at room temperature. The slides were washed as before and mounted with aqueous mounting media (Biomeda, Foster City, CA).
Nonradioactive in situ hybridization: riboprobe synthesis
Full-length human PTN cDNA was cloned into pCRII-TOPO (Invitrogen, Carlsbad, CA). For synthesis of the sense control probe, the vector was linearized with StuI and digoxigenin-labeled sense RNA in vitro transcribed from the SP6 promoter; the antisense probe was synthesized from linearizing the vector with StuI followed by in vitro transcription from the T7 promoter. Both probes were transcribed using a DIG RNA Labeling Kit (Roche Applied Science, Indianapolis, IN, manufacturer's directions).
In situ hybridization
Myeloma or healthy human BM (AllCell LLC, Berkeley, CA) cells were fixed in PBS, pH 7.0, containing 4% paraformaldehyde for 30 minutes at room temperature on poly-L-lysine-coated microscope slides. The slides were washed twice in PBST and dehydrated through a series of PBST-methanol washes (25, 50, 70, and 100%). The slides were rehydrated through the methanol series and treated with 10 μg/mL proteinase K in PBST at room temperature for 7 minutes. The slides were washed once in freshly prepared PBST containing 2 mg/mL glycine and then washed twice with PBST. The slides were fixed with 2% glutaraldehyde in 4% paraformaldehyde/PBST for 20 minutes and washed twice in PBST at room temperature and treated with hybridization buffer (50% formamide, 5X NaCl, sodium chloride-sodium citrate [SSC], 1% SDS, 50 μg/mL heparin, 50 μg/mL tRNA) containing 3 μg/mL digoxigenin-labeled PTN or control riboprobes and hybridized overnight in a humidified chamber at 50°C. The slides were washed twice in solution I (50% formamide, 5X SSC) at 60°C for 30 minutes, once in 1:1 mixture of solution I and solution II (0.5 M NaCl, 10 mM Tris, pH 7.5, and 0.1% Tween-20) at 60°C for 10 minutes, and washed 3 times with solution II at 37°C for 5 minutes. The slides were incubated with 50 μg/mL RNase A in solution II for 30 minutes at 37°C. The slides were washed once in solution II at 37°C, twice in solution III (50% formamide, 2X SSC pH 5.2) at 65°C for 30 minutes, and 3 times in TBST and blocked with TBST containing 10% normal goat serum for at least 1 hour at room temperature. The slides were incubated overnight with antidigoxigenin antibody (Roche, Indianapolis, IN) and washed 3 times in TBST containing 2 mM levamisole for 5 minutes each, followed by 6 additional washes with rocking for 1 hour each at room temperature. The slides were washed twice in NTMT (100 mM NaCl, 100 mM Tris, pH 9.5, 50 mM MgCl2, 0.1% Tween-20) for 20 minutes each at room temperature. Colorimetric reactions were performed by adding 4.5 μg/mL nitro-blue tetrazolium and 3.5 μg/mL 5-bromo-4-chloro-3-indolyl phosphate (BCIP) (both Promega, Madison, WI) in NTMT to the slides and incubating in the dark 30 minutes at room temperature. Color development was stopped by washing the slides 3 times in PBST. The slides were mounted in aqueous mounting media (Biomeda).
RNA extraction and PTN reverse-transcription polymerase chain reaction
Total RNA was isolated from the BMMCs of MM patients and a healthy donor, PBMCs from a plasma cell leukemia patient, MM cell lines (MM-1S, U266 and RPMI 8226), or THP-1 cells using Trizol (Invitrogen, manufacturer's directions). RNA was resuspended in 0.1% diethyl pyrocarbonate-treated water, digested with DNase I (Sigma) to remove contaminating DNA and extracted with phenol/chloroform, followed by ethanol precipitation. Total RNA (1 μg) was reverse-transcribed to cDNA and amplified using the ThermoScript reverse-transcription polymerase chain reaction (RT-PCR) System (Invitrogen, manufacturer's directions). PCR was then performed again using the ThermoScript RT-PCR System (Invitrogen, manufacturer's directions) and a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA) for one cycle at 94°C 2 minutes, followed by 35 cycles at 94°C 30 seconds, 58°C 30 seconds, 72°C 1 minute and one cycle at 72°C 5 minutes. PTN was amplified using forward (5′-TGCAACAAAGGCAGACTGAG-3′) and reverse primers (5′-TGTTCCACAGGTGACATCTTTT-3′); GAPDH was amplified using forward (5′-AGCCACATCGCTCAGACACC-3′) and reverse primers (5′-GTACTCAGCGGCCAGCATCG-3′) under the same conditions as a loading control.
ELISA for PTN secretion
MM cell lines or BMMCs from monoclonal gammopathy of undetermined significance (MGUS), myeloma patients, and normal donors (2 × 106 cells) were cultured in RPMI 1640 containing supplements (described above) and 5% fetal bovine serum for 48 hours. Culture supernatants were collected by centrifugation and PTN and MK protein concentrations determined by ELISA as described. Each experiment was performed in triplicate.
In vitro MM growth inhibition by polyclonal anti-PTN antibody
The myeloma cell lines MM-1S, RPMI 8226, and U266, or BM cells from a MM patient, were seeded at 106 cells/100 μL/well in RPMI 1640 containing 10% fetal bovine serum, in flat-bottomed 96-well microtiter plates, and incubated for 24 hours. Cells were cultured in anti-PTN antibody (3, 30, or 300 μg/mL, R&D Systems) or 0.3 μg/mL affinity purified goat antihuman-Fc5μ control IgG (R&D Systems) for an additional 24 hours. Cell viability was assessed with a MTT assay (performed in quadruplicate) by adding 10 μL 5 mg/mL MTT solution (diluted in PBS; Sigma) to each well and incubating for 4 hours, then solubilizing the formazan with 100 μL/well acid alcohol (24:1 isopropanol/1 M HCl) and analyzed using a μQuant (BioTek Instruments, Winooski, VT) plate reader at 570 nm (primary wavelength) and 630 nm (reference wavelength) with KC Junior software. Cell recovery and viability was performed by the trypan blue dye exclusion method by light microscopy. Data are the mean of experiments performed 4 times. Statistical significance was determined using a 2-tailed Student t test.
BrdU assay for cell proliferation
MM cell proliferation (MM-1S, U266 and fresh human bone marrow cells) was further assessed using bromodeoxyuridine (BrdU). Cells were plated in microtiter plates, at a density of 2 × 106 cells/well for 12 hours. Triplicate wells were treated with 3, 30, or 300 μg/mL anti-PTN antibody (R&D Systems) or control IgG (R&D Systems) for 72 hours at 37°C. The media was discarded, and cells from each well were fixed in 70% ethanol at 4°C for 30 minutes and resuspended in 1 mL chilled 0.1 M HCL containing 0.5% Triton X-100 for 10 minutes. The acid/cell solutions were diluted to 5 mL/tube by distilled water centrifuged at 200g for 10 minutes. The cells were resuspended in distilled water. Cellular DNA was denatured by submerging the suspension into a boiling water bath for 10 minutes and then quickly cooled by placing the samples into an ice slurry for 10 minutes. The cells were washed in 1X PBS, 0.5% Triton X-100, and resuspended in 100 μL of anti-BrdU antibody diluted in 1X PBS containing 0.1% bovine serum albumin. The cells were incubated for 60 minutes at room temperature and washed with PBS. The cells were resuspended in 100 μL of diluted goat antimouse IgG-FITC (Invitrogen) for 30 minutes and then washed with PBS for flow cytometry using a Beckman-Coulter FC500 cytometer with Cytomics CXP software (Beckman Coulter, Fullerton, CA).
In vitro induction of apoptosis by anti-PTN antibody
Myeloma cell lines (MM-1S and U266) were seeded at a density of 106 cells/mL/well in triplicate in RPMI 1640 containing 10% fetal bovine serum and incubated at 37°C 5% CO2 for 24 hours. Anti-PTN antibody (3, 30, or 300 μg/mL, R&D Systems) or normal goat IgG (R&D Systems) was added and the cells cultured for an additional 48 hours. Cells were then washed twice with PBS, resuspended (106 cells/mL) in binding buffer (100 mM HEPES/NaOH, pH 7.5 containing 1.4 M NaCl and 25 mM CaCl2), and stained with 5 μL/mL Annexin V-FITC and 10 μL/mL propidium iodide (PI) (both Sigma) for 15 minutes in room temperature, in the dark. The samples were analyzed by flow cytometry (as described). Data are the mean of experiments performed 3 times. Statistical significance was determined using a 2-tailed Student t test.
In vivo MM growth inhibition by anti-PTN antibody
Human LAGλ-1 myeloma tumors were passaged in 6- to 8-week-old homozygous C.B-17 SCID/SCID (severe combined immunodeficient) mice obtained from Harlan Sprague Dawley (Indianapolis, IN) as previously described.37 All animal studies were conducted according to protocols approved by the University of California at Los Angeles (UCLA) Animal Research Committee.
Mice bearing LAGλ-1 tumors were treated with polyclonal goat antihuman PTN antibody or control pre-immune goat IgG (3 mg/kg, both R&D Systems), receiving twice-weekly intraperitoneal injections at 0.1, 0.3, 3.0, and 10 mg/kg. Tumors were measured weekly using calipers and their volumes calculated with the following formula: 4/3π × (width/2)2 × (length/2), portraying the 3-dimensional volume of an ellipsoid. Human IgG (hIgG) produced by the myeloma tumor was assessed by weekly retro-orbital bleeding followed by centrifugation at 15 700g for 30 minutes to collect serum. The hIgG subclass 1 (the isotype of the LAGλ-1 tumor) was measured by ELISA (Zymed Laboratories, South San Francisco, CA, manufacturer's directions). Absorbance at 450 nm with a reference wavelength of 550 nm was determined on a μQuant microplate spectrophotometer with KC Junior software. Tumor growth and hIgG secretion curves were analyzed in terms of treatment group means and standard error. Statistical significance of differences observed in anti-PTN antibody treated mice versus control mice was determined using a 2-tailed Student t test. The minimal level of significance was P < .05.
Results
PTN serum protein expression by myeloma patients
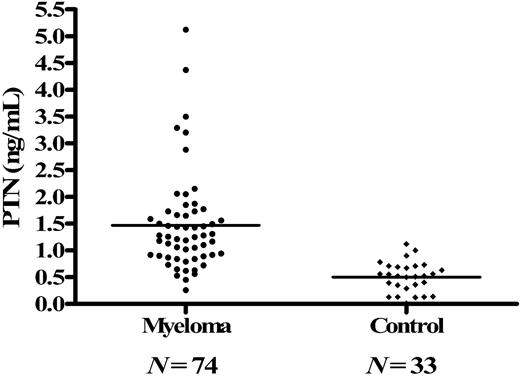
We examined PTN concentrations in the serum from 74 MM patients and 33 healthy human age- and gender-matched controls by ELISA and found that the levels of PTN in the MM patients were elevated compared with the healthy control group (P < .01; Figure 1). Specifically, the median serum concentration of PTN in myeloma patients was 1.5 ng/mL (range = 1.01 ng/mL-3.44 ng/mL), whereas the median serum levels in control subjects was only 0.50 ng/mL (range = 0.01 ng/mL-0.93 ng/mL; Figure 1).
PTN is elevated in the serum from myeloma patients. PTN serum levels were analyzed in consecutive myeloma patients (N = 74) and healthy donors (N = 33) by ELISA. PTN concentrations were markedly elevated in myeloma patient serum compared with the serum from healthy donors (P < .01).
PTN is elevated in the serum from myeloma patients. PTN serum levels were analyzed in consecutive myeloma patients (N = 74) and healthy donors (N = 33) by ELISA. PTN concentrations were markedly elevated in myeloma patient serum compared with the serum from healthy donors (P < .01).
Myeloma cells produce PTN
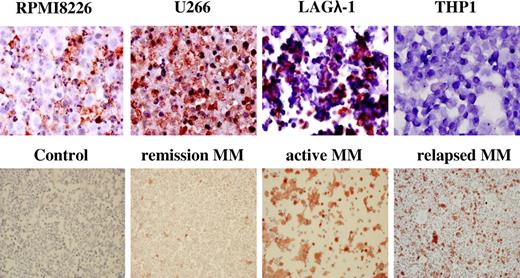
The myeloma cell lines RPMI 8226, U266, the human myeloma tumor LAGλ-1,37 and the human monocytic cell line THP-1 were analyzed for PTN protein expression by immunohistochemical (IHC) staining (Figure 2). All 3 myeloma samples showed intense PTN staining, whereas the THP-1 cells lacked PTN protein expression (Figure 2). BMMCs from a healthy donor and MM patients with varying disease status were similarly analyzed (Figure 2). PTN was not detected in the BM from the healthy subject (Figure 2 top left). In contrast, BM from the MM patients showed strong PTN staining (Figure 2 top right). BMMCs from MM patients in remission stained minimally for PTN (Figure 2 bottom left), whereas relapsing MM patients showed much stronger PTN staining (Figure 2 bottom right). These results demonstrated that PTN was produced in MM bone marrow and suggested that the level of PTN protein expression correlates with disease status, similar to our recent findings evaluating serum PTN levels in a large cohort of MM patients.34
PTN protein is produced by myeloma cells. PTN protein expression was assessed in myeloma cell lines, the BMMCs from myeloma patients, and healthy donors by IHC. The myeloma cell lines RPMI 8226 and U266, and the human myeloma tumor LAGλ-1, expressed high levels of PTN protein. In contrast, the monocytic cell line THP-1 showed no PTN staining. BMMCs from a healthy donor (control) did not produce PTN. PTN was highly expressed in the BM from an MM patient with active disease and in a relapsing patient but not in an MM patient in remission.
PTN protein is produced by myeloma cells. PTN protein expression was assessed in myeloma cell lines, the BMMCs from myeloma patients, and healthy donors by IHC. The myeloma cell lines RPMI 8226 and U266, and the human myeloma tumor LAGλ-1, expressed high levels of PTN protein. In contrast, the monocytic cell line THP-1 showed no PTN staining. BMMCs from a healthy donor (control) did not produce PTN. PTN was highly expressed in the BM from an MM patient with active disease and in a relapsing patient but not in an MM patient in remission.
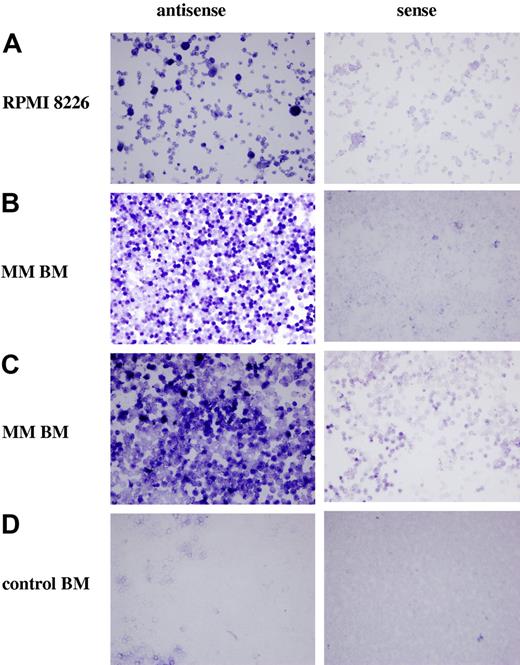
To further establish that PTN is expressed in MM cells, in situ hybridization was performed on a myeloma cell line, on freshly isolated MM BMMCs and a healthy donor (Figure 3). An antisense probe to PTN RNA, but not the negative control sense construct, detected high levels of PTN transcripts in the RPMI 8226 MM cell line (Figure 3A). When these probes were hybridized to BMMCs from the 2 MM patients, only the antisense probes detected PTN expression (Figure 3B,C). Finally, when BMMCs from a healthy donor were probed for PTN expression, none was detected (Figure 3D).
Ptn gene expression is up-regulated in myeloma cells. In situ hybridization demonstrated that Ptn was transcribed by the myeloma cell line RPMI 8226 (A right) and the BMMCs from 2 different myeloma patients (B and C right). In contrast, staining of the BMMCs from a healthy donor (D right) was equivalent to the staining by the negative control sense probes (A-D left).
Ptn gene expression is up-regulated in myeloma cells. In situ hybridization demonstrated that Ptn was transcribed by the myeloma cell line RPMI 8226 (A right) and the BMMCs from 2 different myeloma patients (B and C right). In contrast, staining of the BMMCs from a healthy donor (D right) was equivalent to the staining by the negative control sense probes (A-D left).
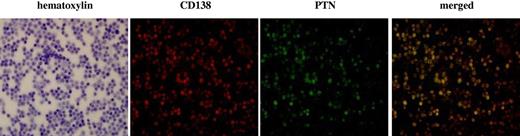
We also confirmed that the malignant cells in the BM were the source of the PTN using immunofluorescent analysis to colocalize PTN expression with that of the plasma cell marker CD138 (Figure 4). The merged fluorescent image clearly shows that PTN was produced by the CD138+ MM cells in the myeloma BM (Figure 4 bottom right).
PTN protein is expressed by CD138 + cells in the BM from myeloma patients. BMMCs were stained for PTN73 and CD138 (red). The merged image (right) localized PTN to the CD138 + cells.
PTN protein is expressed by CD138 + cells in the BM from myeloma patients. BMMCs were stained for PTN73 and CD138 (red). The merged image (right) localized PTN to the CD138 + cells.
MM cell lines and MM BM cells secrete PTN during short-term culture
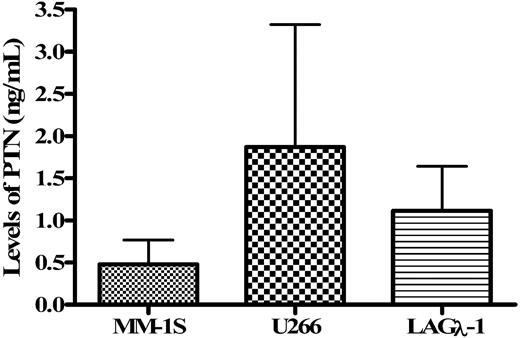
To further define the myeloma cell as the source of PTN in the serum from MM patients, we determined whether PTN was secreted by MM cell lines and myeloma BM cells during short-term culture. The MM cells lines MM-1S and U266, the human MM tumor LAGλ-1, and negative control THP-1 monocytes were cultured in media containing reduced serum, culture supernatants were isolated, and the levels of PTN in these supernatants were measured by ELISA. All 3 MM cell lines analyzed produced and secreted PTN (range = approximately 0.47-1.84 ng/mL) into the culture supernatant after 48 hours of culture (Figure 5). PTN was not found in the culture supernatants obtained from the monocytic cell line THP-1, a cell type that also did not stain for PTN by IHC (Figure 2) or show amplified product using RT-PCR with PTN-specific primers (Fig 6). The BM cells from 2 healthy donors, 1 MGUS patient, and 3 myeloma patients were similarly cultured and PTN secretion assessed (data not shown). Samples from MM patients scored positive for secreted PTN but a specific concentration value could not be obtained because of the limitations of our standard curve. PTN was not detected in the supernatants obtained from culturing the BM sample from a healthy donor (data not shown) consistent with the inability to detect PTN in these control BM samples by IHC, in situ hybridization, and RT-PCR (Figures 2 and 6). PTN secretion was also not detected in the patient with MGUS (data not shown). These findings, therefore, demonstrate that PTN was both produced and secreted by MM BM.
Myeloma cells secrete PTN into the supernatant during short-term culture. The myeloma cell lines MM-1S, U266, and the myeloma tumor LAGλ-1 were cultured for 48 hours and the culture supernatants analyzed for PTN protein concentration by ELISA. Concentration of PTN detected in the 3 myeloma cell lines was 0.5 ng/mL or less of PTN protein. Data are the average of experiments performed in triplicate.
Myeloma cells secrete PTN into the supernatant during short-term culture. The myeloma cell lines MM-1S, U266, and the myeloma tumor LAGλ-1 were cultured for 48 hours and the culture supernatants analyzed for PTN protein concentration by ELISA. Concentration of PTN detected in the 3 myeloma cell lines was 0.5 ng/mL or less of PTN protein. Data are the average of experiments performed in triplicate.
Ptn gene expression correlates with disease status in myeloma patients. Mononuclear cells from BM of a MM patient or a healthy donor, PBMCs from a plasma cell leukemia patient, and the myeloma cell lines RPMI 8226, U266, and MM-1S, and the human monocytic cell line THP-1 were analyzed for Ptn gene expression by RT-PCR. Ptn transcripts were not detected in THP-1 cells, or healthy control BM, and only minimally in a patient with indolent MM. In contrast, Ptn was expressed more highly by myeloma patients with active, refractory, or relapsed disease. The highest level of Ptn expression was detected in the malignant cells from a patient with plasma cell leukemia and the myeloma cell lines RPMI 8226, U266, and MM-1S.
Ptn gene expression correlates with disease status in myeloma patients. Mononuclear cells from BM of a MM patient or a healthy donor, PBMCs from a plasma cell leukemia patient, and the myeloma cell lines RPMI 8226, U266, and MM-1S, and the human monocytic cell line THP-1 were analyzed for Ptn gene expression by RT-PCR. Ptn transcripts were not detected in THP-1 cells, or healthy control BM, and only minimally in a patient with indolent MM. In contrast, Ptn was expressed more highly by myeloma patients with active, refractory, or relapsed disease. The highest level of Ptn expression was detected in the malignant cells from a patient with plasma cell leukemia and the myeloma cell lines RPMI 8226, U266, and MM-1S.
Ptn gene expression correlates with MM disease status
As shown here and recently reported by our group,34 serum levels of PTN as assessed by ELISA and myeloma BM staining by IHC suggested that the level of PTN expressed by myeloma patients correlated with their disease status. To further confirm and extend these results, we isolated RNA from the BMMCs from a spectrum of MM patients and analyzed Ptn gene expression by RT-PCR. Expression could not be detected in the BM from a healthy donor (Figure 6). When the BM from patients with indolent disease was analyzed, the gene was found to be only minimally expressed in one of these patients, whereas Ptn gene expression was easily detected in the BMMCs from patients with active or refractory disease and in the majority of relapsed patients (Figure 6). Ptn was also highly expressed by the myeloma cell lines RPMI 8226, U266, and LAGλ-1, and the PBMCs from a plasma cell leukemia patient (Figure 6 and data not shown). Thus, the results of RT-PCR analysis of Ptn gene expression in MM BM further supports our hypothesis that PTN production is related to disease status in MM patients.
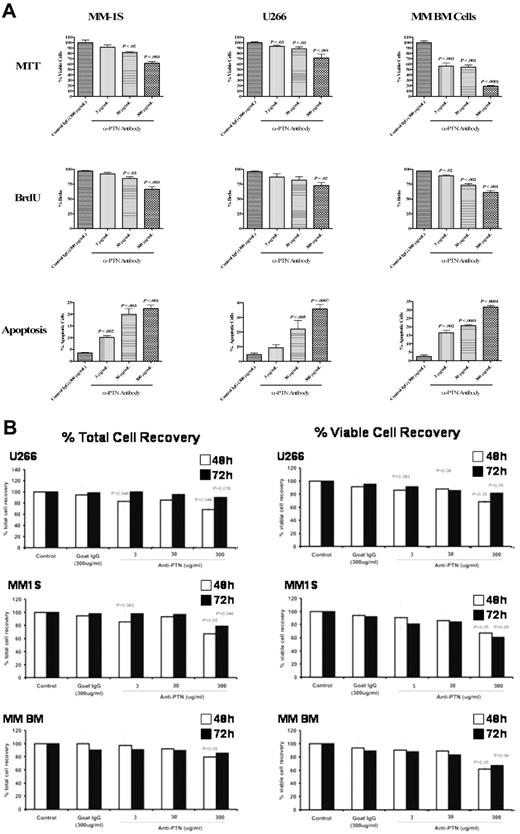
Next, we extended our studies to determine whether PTN also contributes to the growth of myeloma cells. As previously reported,38,39 we found that the recombinant PTN lacked bioactivity (data not shown). Thus, we used a polyclonal anti-PTN antibody to inhibit the activity of endogenous PTN in myeloma cells. The MM cell lines MM-1S and U266 were incubated with or without 3, 30, or 300 μg/mL polyclonal anti-PTN antibody or 300 μg/mL control IgG and metabolically active cells were assessed by the MTT assay. The activity of MM cells was significantly decreased by 300 μg/mL anti-PTN (Figure 7, MM-1S P < .003, U266 P < .001), and the metabolic activity of MM-1S cells was reduced with an even lower antibody concentration (3 and 30 μg/mL; Figure 7, P < .02). To confirm that anti-PTN antibody inhibits MM growth, we evaluated freshly isolated mononuclear cells from the BM of a MM patient heavily infiltrated with malignant plasma cells (< 90%). The metabolic activity was again reduced at all anti-PTN concentrations (Figure 7A, 3 μg/mL P < .003; 30 μg/mL P < .001; 300 μg/mL P < .001).
Anti-PTN antibody inhibits MM growth and induces apoptosis in vitro, and affects total cell recovery and the total viable cell recovery. (A) The myeloma cell lines MM-1S and U266 and BM cells from a MM patient were assessed for cell viability, proliferation, and apoptosis. MM cells were incubated with anti-PTN antibody or control IgG for 24 hours and cellular viability assessed by MTT assay. 300 μg/mL anti-PTN reduced the viability of all MM cells tested here, compared with cells incubated without antibody (media) or with control IgG. To assess cell proliferation, MM cells were treated with increasing concentrations of anti-PTN antibody and then measured for BrdU incorporation by flow cytometry. All 3 MM samples treated with anti-PTN antibody showed decreased BrdU levels compared with cells treated with control antibody. Apoptosis was measured using Annexin V/PI staining followed by flow cytometry. The anti-PTN antibody at all 3 concentrations induced apoptosis in these 3 MM samples in a concentration-dependent fashion. (B) For the evaluation of inhibition of cell growth, an aliquot of each of these cell suspensions was mixed with an equal volume of trypan blue dye and total number of viable cells recovered was determined using light microscopy. Untreated (control) cells were considered as 100%. The samples were set up in triplicates. The results shown are the means of data from triplicate samples. P values were based on comparing results with IgG control antibody vs anti-PTN antibody.
Anti-PTN antibody inhibits MM growth and induces apoptosis in vitro, and affects total cell recovery and the total viable cell recovery. (A) The myeloma cell lines MM-1S and U266 and BM cells from a MM patient were assessed for cell viability, proliferation, and apoptosis. MM cells were incubated with anti-PTN antibody or control IgG for 24 hours and cellular viability assessed by MTT assay. 300 μg/mL anti-PTN reduced the viability of all MM cells tested here, compared with cells incubated without antibody (media) or with control IgG. To assess cell proliferation, MM cells were treated with increasing concentrations of anti-PTN antibody and then measured for BrdU incorporation by flow cytometry. All 3 MM samples treated with anti-PTN antibody showed decreased BrdU levels compared with cells treated with control antibody. Apoptosis was measured using Annexin V/PI staining followed by flow cytometry. The anti-PTN antibody at all 3 concentrations induced apoptosis in these 3 MM samples in a concentration-dependent fashion. (B) For the evaluation of inhibition of cell growth, an aliquot of each of these cell suspensions was mixed with an equal volume of trypan blue dye and total number of viable cells recovered was determined using light microscopy. Untreated (control) cells were considered as 100%. The samples were set up in triplicates. The results shown are the means of data from triplicate samples. P values were based on comparing results with IgG control antibody vs anti-PTN antibody.
To further confirm the effects of this anti-PTN antibody on MM cell growth, we used the BrdU assay. We treated MM-1S, U266, and MM BM cells with the same polyclonal anti-PTN antibody and analyzed the cells for the induction of apoptosis by Annexin V/PI staining followed by flow cytometry (Figure 7A). We found that even the lowest concentration of anti-PTN antibody (3 μg/mL) induced apoptosis in a significant portion of these MM cells (MM-1S, P < .002; MM BM cells, P < .002).
Because anti-PTN antibody inhibited MM cell activities as determined by both the MTT and BrdU assays and also induced MM cell apoptosis, we examined the effect of anti-PTN antibody on total cell recovery (including both viable and dead cells) as well as total viable cell recovery by trypan blue dye exclusion. The total cell death after 48 and 72 hours of culture was no different from that observed with the control goat anti-IgG at all 3 anti-PTN concentrations evaluated (3, 30, and 300 μg/mL). There was a significant inhibition of total cell recovery for both U266 and MM1S at 48 hours and 72 hours, whether calculated for total cell recovery or for total viable cell recovery (Figure 7B). Similar findings were obtained for BM-derived cells except that the total cell recovery at 72 hours was not significant, whereas at 48 hours both the total cell recovery and total viable cells recovery were significantly inhibited.
These findings suggest that PTN is not only produced and secreted from the MM cells, but that blocking its activity with an anti-PTN antibody inhibits cell growth and induces apoptosis of MM cells.
Antibody interference with PTN reduces MM proliferation in vivo
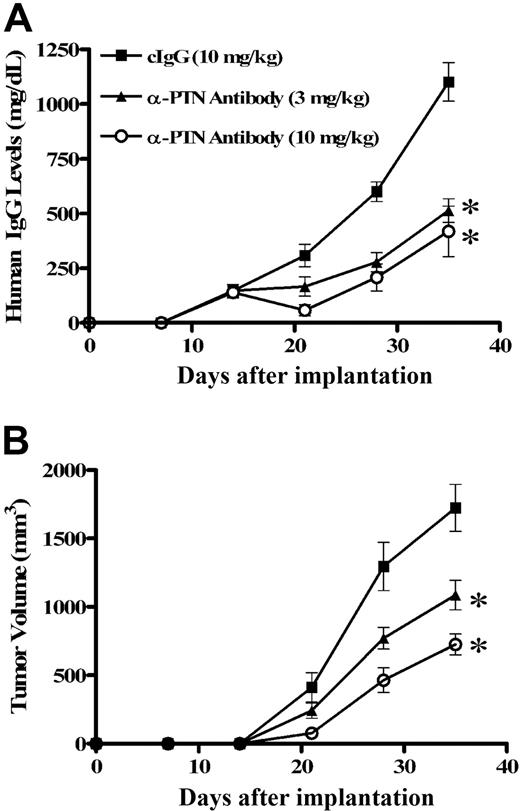
To confirm that PTN is a growth factor for myeloma cells in vivo, we treated SCID mice bearing human myeloma LAGλ-137 tumors with the polyclonal anti-PTN antibody. Fourteen days after implantation with the LAGλ-1 tumor, hIgG was detected in the mouse serum (Figure 8A). The animals were randomized into 6 treatment groups (n = 5 each) and anti-PTN antibody or pre-immune goat IgG (vehicle) was administered intraperitoneally twice weekly. Animals treated with anti-PTN antibody at doses of 1.0 mg/kg or below showed similar tumor growth to those animals receiving the control antibody (data not shown). Mice receiving 3 or 10 mg/kg anti-PTN showed reduced hIgG production (P < .005) and tumor growth (P < .05) after 1 week of anti-PTN therapy (Figure 8A,B). Continued administration of polyclonal anti-PTN antibody resulted in greater myeloma growth inhibition at 5 weeks when the study was terminated because of the large tumor burden present in the untreated animals (Figure 8A,B). Thus, administration of the polyclonal anti-PTN antibody suppressed growth of a human myeloma tumor growing in SCID mice.
Anti-PTN antibody inhibits MM proliferation in the SCID-hu myeloma model, LAGλ-1. The human myeloma tumor LAGλ-1 was implanted intramuscularly into SCID mice and hIgG production was measured by ELISA. When hIgG was detected, anti-PTN antibody (3 or 10 mg/kg) or control IgG (cIgG, 10 mg/kg) was administered intraperitoneally twice weekly. (A) Anti-PTN treatment markedly reduced hIgG production and the volume of the myeloma tumor (B) in vivo. *P < .005, **P < .05.
Anti-PTN antibody inhibits MM proliferation in the SCID-hu myeloma model, LAGλ-1. The human myeloma tumor LAGλ-1 was implanted intramuscularly into SCID mice and hIgG production was measured by ELISA. When hIgG was detected, anti-PTN antibody (3 or 10 mg/kg) or control IgG (cIgG, 10 mg/kg) was administered intraperitoneally twice weekly. (A) Anti-PTN treatment markedly reduced hIgG production and the volume of the myeloma tumor (B) in vivo. *P < .005, **P < .05.
Discussion
We report that PTN, an oncogene21 and autocrine growth factor for glioblastoma, melanoma, breast, and pancreatic cancers,39,,–42 is produced by myeloma cells and that inhibiting its activity decreases MM growth. Although PTN has previously been shown to be produced by many different solid tumors,19 these are the first studies on the role of PTN in any hematological malignancy.
We found in these studies and previous investigations34 that PTN, but not the related MK, was elevated in the serum from myeloma patients. Using IHC and in situ hybridization, we demonstrated that PTN was expressed by MM cell lines and freshly isolated myeloma BMMCs, but not from healthy donors. Immunofluorescent analysis colocalized PTN protein to CD138+ plasma cells in myeloma BM, further supporting this conclusion and eliminating BM stromal cells as the primary source of PTN. Moreover, ELISA analysis of the supernatants from myeloma cell lines and the BMMCs from MM patients, cultured for 48 hours, showed the presence of PTN, whereas BM from healthy donors did not secrete PTN into the culture medium. We also showed that the Ptn gene was expressed in myeloma cells by RT-PCR and that expression of this gene correlated with MM disease status, consistent with our previous report.34
We then investigated whether PTN could act as an autocrine growth factor as has been shown for a broad range of solid tumors.43 In vitro cell growth of myeloma cell lines was inhibited by anti-PTN antibody. Anti-PTN antibody-treated MM cells were analyzed by Annexin V/PI staining and flow cytometry. We found that apoptosis was induced in myeloma cells within 48 hours. To discriminate between cell growth inhibition and induction of apoptosis, we evaluated the effect of PTN antibody on both the total cell recovery and the total viable cell recovery. There was significant inhibition of cell growth irrespective of cell death. Notably, when this antibody was administered to SCID mice implanted with LAGλ-1 myeloma tumors, tumor growth was also inhibited. PTN is pro-angiogenic and PTN interference using specific ribozymes decreased melanoma tumor microvessel density.40 Thus, it is possible that anti-PTN antibody interferes with myeloma growth both directly by inhibiting growth and inducing death of the tumor cells, as well as indirectly by inhibiting angiogenesis. In MM, BM angiogenesis correlates with disease status44 and poor prognosis.45 We are currently investigating whether anti-PTN antibody reduces the vascularity of MM tumors in murine models.
Thus, we have identified PTN as a new autocrine growth factor for myeloma. In glioblastoma, PTN was found to augment proliferation,46 probably via the PTN receptor RPTPβ/ζ, which is expressed on glioblastoma cells.30 Binding of PTN to RPTPβ/ζ inhibits its activity, resulting in the accumulation of tyrosine phosphorylated proteins, including Fyn tyrosine kinase,47 β-catenin,29 and the activation of protein kinase C.48 We have found that in addition to the PTN receptor CD138, a subset of myeloma cells express RPTPβ/ζ49 (and unpublished results, M.S.G., January 2006). Signaling by Fyn, β-catenin, and protein kinase C in response to PTN stimulation may regulate myeloma proliferation, adherence, migration, and metastasis.32,48,50,–52 PTN-induced cell signaling may also contribute to cell adhesion-mediated drug resistance, as well as drug resistance caused by NFκB dysregulation. The α4β1 integrin was recently reported to bind the closely related protein MK in a receptor complex containing RPTPβ/ζ.53 PTN binds, with higher affinity, to all known MK receptors26,54,,–57 ; thus, PTN is likely to also bind to α4β1 integrin. In myeloma, α4β1 integrin mediates adhesion of the malignant cells to BM stroma leading to MM proliferation, metastasis, and cell adhesion-mediated drug resistance,58,–60 as well as enhanced bone resorption.
PTN is pro-angiogenic, as has been shown in some solid tumors,24,46 and reducing its levels using specific ribozymes decreased melanoma tumor microvessel density.40 Our recent discovery that PTN induces the transdifferentiation of monocytes into vascular endothelial cells and the incorporation of these cells into functional blood vessels within myeloma tumors in vivo61 suggests that PTN may also enhance MM vascularity.
Another inhibitor of angiogenesis, bevacizumab, a monoclonal antibody against VEGF, has been approved for the treatment of metastatic colorectal cancer.62 Recently, we have shown inhibition of MM growth using an antibody that binds both murine and human VEGF in several different human MM tumors growing in SCID mice.63 Whether the combination of anti-VEGF and anti-PTN antibodies will show enhanced anti-MM activity in our preclinical SCID-hu models is currently undergoing investigation.
Increased MK expression leads to chemoresistance in neuroblastoma and osteosarcoma cell lines, possibly through Akt activation.64 Some myeloma growth factors also contribute to chemoresistance, such as IL-6 (through activation of STAT3 and MAPK) and IGF-1 (via IRS-1, MAPK, and Akt signaling and downstream induction of antiapoptotic genes and NFκB).8 VEGF may also produce myeloma drug resistance through the induction of Mcl-1.65 PTN has been shown to activate the Akt, MAPK, and NFκB signaling pathways,39,66 thus PTN may also orchestrate myeloma drug resistance. Our preliminary data suggest that inhibition of PTN enhances the efficacy of chemotherapy (results not shown).
New therapies targeting other myeloma growth factors are undergoing investigation with mixed results. Monoclonal antibodies directed against IL-6 or its receptor, or an IL-6 receptor antagonist, resensitized drug-resistant myeloma cells to chemotherapy and induced apoptosis in vitro;65 however, when tested in patients there was no sustained clinical benefit.67 Preclinical studies using monoclonal anti-IGF-1 receptor antibodies, antagonists, or kinase inhibitors have been more promising. These agents inhibited proliferation and reversed the drug resistance of myeloma and solid tumors in vitro and in murine xenograft models.68
In solid tumors, PTN has been found to activate a broader spectrum of signaling pathways, including IRS-1, Akt, NFκB, STAT3, protein kinase C, MAPK, and Wnt signaling,30,56,69,,–72 than other myeloma growth factors. Activation of these signaling pathways contributes not only to myeloma tumor growth, vascularization, and metastasis but also to resistance to chemotherapy. Thus, interfering with PTN is likely to be effective against myeloma tumor growth and enhance the efficacy of other anti-MM therapies. PTN, unlike these other MM growth factors, is only minimally expressed in adults. PTN inhibition is unlikely to significantly affect the function of normal cells, making PTN an ideal target for antimyeloma therapy. We are currently developing a neutralizing monoclonal anti-PTN antibody designed to be used therapeutically for a broad range of tumor types.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Regina Swift for helpful discussions and Christine Pan James for her assistance with the preparation and editing of this manuscript.
This work was funded by the non-profit Annenberg, Kramer, Peninsula Community and Skirball Foundations and the Myeloma Research Fund.
Authorship
Contribution: H.C. designed and performed research, analyzed data, and wrote the manuscript. M.S.G. and B.B. designed research, analyzed data, and edited the manuscript. R.A.C. performed research and analyzed data. M.L., O.T., C.S.W., and H.J.L. performed research. S.J.M., H.S.Y., D.S., and J.S. analyzed data. D.G. provided new reagents and techniques and analyzed data. T.F.D. analyzed research and edited the manuscript. J.R.B. (principal investigator and corresponding author) designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James R. Berenson, Institute for Myeloma & Bone Cancer Research, 9201 W. Sunset Blvd, Suite 300, West Hollywood, CA 90069; e-mail: jberenson@imbcr.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal