Interleukin-4–induced gene 1 (IL4I1) was first described as a B-cell IL4-inducible gene and is highly expressed in primary mediastinal B-cell lymphomas. We established stable HEK293 clones expressing human and mouse IL4I1 to examine their biochemical properties and function. Both proteins were secreted into the culture medium, and we observed the secretion of endogenous human IL4I1 (hIL4I1) protein in a mediastinal lymphoma B-cell line, MedB-1. We showed that IL4I1 has l-amino acid oxidase activity, optimal at physiological pH and primarily directed toward phenylalanine. Immunohistochemical analysis of secondary lymphoid organs showed staining of germinal center macrophages and inflammatory myeloid cells. In vitro, functional enzyme was highest in mature dendritic cells (DCs), suggesting a role in antigen-presenting cell/T-lymphocyte cross-talk. Indeed, hIL4I1 inhibited the proliferation of CD3-stimulated T lymphocytes with a similar effect on CD4+ and CD8+ T cells. In contrast, memory T cells were more strongly affected by hIL4I1 and its catabolite H2O2 than naive T cells. hIL4I1 inhibitory effect was dependent on enzymatic activity and H2O2 production and associated with a transient down-regulation of TCRζ expression. Altogether these data suggest IL4I1 as a new immunomodulatory enzyme produced by DCs.
Introduction
Interleukin-4 (IL4) is the master cytokine for T-helper type 2 (Th2) lymphocyte differentiation and function. Along with its important role in B-lymphocyte stimulation by T-helper cells in germinal centers of secondary lymphoid organs, it also regulates the growth and function of many different cell types of the immune system.1 IL4 transcriptional effects are primarily mediated by the signalization and transcription factor 6 (STAT6), which is responsible for the induction and repression of multiple target genes.2
An immediate-early IL4-inducible gene called interleukin four–induced gene 1 (FIG1/IL4I1) has first been described in the mouse3 and subsequently characterized in human B cells.4 IL4I1 mRNA expression is restricted to lymphoid tissues, with the highest levels found in lymph nodes and spleen.4,5 IL4-activated murine B cells express IL4I1 within 2 hours of stimulation. We have demonstrated that high levels of expression of this gene are characteristic of primary mediastinal large B-cell lymphoma (PMBL), a specific subtype of diffuse large B-cell lymphoma.5 The high expression of IL4I1 by PMBL may result from the constitutive activation of STAT6 in these tumors.6
Both human and mouse IL4I1 mRNA sequences encode a protein containing a putative signal peptide indicative of potential secretion and a large central domain, highly homologous to flavin-containing amino acid oxidases.3,4 While sharing high similarity over the majority of the sequence (547 amino acids of the 567 in the human sequence), the human and mouse proteins diverge substantially at their C-terminal region.4 The IL4I1 protein shares the highest similarity with proteins presentingl-amino acid oxidase (LAAO) activity, such as snake venom LAAO,7,8 and mouse milk LAAO.9 Recently, Mason et al have demonstrated that mouse IL4I1 (mIL4I1) possesses an LAAO activity toward aromatic amino acids. The enzyme was mostly active at acidic pH, in contrast to other known members of the LAAO family, and its expression was restricted primarily to lysosomes.10
In this work, we have characterized the biochemical properties of the human protein and have compared them to those of the mouse enzyme. We have analyzed IL4I1 expression in secondary lymphoid tissues and in vitro in different cells of the immune system and investigated the possible role of hIL4I1 in T-cell regulation. Most interestingly, the human enzyme was expressed, secreted, and active in dendritic cells (DCs) and able to inhibit T-lymphocyte proliferation in vitro by a mechanism associated with a temporary decrease in T-cell receptor (TCR) complex ζ chain expression. This decrease was dependent on the enzymatic production of H2O2.
Materials and methods
Reagents
Chemical and cell culture reagents were purchased from Sigma Aldrich (St Quentin Fallavier, France) and GIBCO (Invitrogen, Cergy Pontoise, France) respectively. Human catalase was from Calbiochem (Darmstadt, Germany). Antibodies against CD3 (OKT3), TCRζ-PE (TIA-2) and myc (9E10) were purchased from eBioscience (San Diego, CA), Immunotech (Beckman Coulter, Marseille, France) and Oncogene (Merck, Darmstadt, Germany) respectively. Magnetic microbeads coated with anti-human CD3, CD4, CD8, CD19, CD45RA and CD45RO antibodies and LS separation columns were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). IL-4, GM-CSF, TNFα, and IFNγ were purchased from R&D Systems (Abingdon, United Kingdom); LPS, PGE2 and poly-IC from Sigma Aldrich. Soluble CD40 trimeric ligand (CD40L) was kindly provided by Immunex (Seattle, WA).
Plasmids
The full-length 1.7 kb cDNA of hIL4I1 (GenBank accession number NM 152899) without the termination codon was obtained by polymerase chain reaction (PCR) with the following primers (5′-ACAGCGGGAATTCTG-ATGGCCCCATTGGCCCTGCACCTCC-TCGTC-3′; 5′-CGTGACCTCGAGCATGCGA-GGTCCTCGTGTG-3′) from the MedB-1 reverse-transcribed cDNA and inserted into EcoRI and XhoI sites of pcDNA-mycHis3.1 (Invitrogen). The full-length hIL4I1 cDNA contained a silent mutation (serine 547: AGC>AGT). An E481A-hIL4I1 mutant was generated using the Gene Tailor Site-Directed Mutagenesis System (Invitrogen) with the following primers: 5′-CGCATCTACTTTGCCGGCGCCCACACCGCCTAC-3′ and 5′-GCCGGCAAAGTA-GATGCGGCCATAAGGGAC-3′. The mouse cDNA (GenBank accession number NM_010215) was generated by PCR from mouse spleen reverse transcribed cDNA with the following primers: 5′-CGACGTGGAATTCTGATGGCTGGGCTGGCCCTGCGTCTT-3′ and CTTGACTGCGGC CGCGGAGTGGTCCCCCACTCGGTGCAT and cloned into the EcoRI and NotI sites of pcDNA-mycHis3.1. For inducible expression, the wild-type human sequence was ligated into the pcDN4-TO-mycHis plasmid and used to transfect T-Rex 293 cells (Invitrogen). All sequences were confirmed by DNA sequencing.
Transfection and cell culture
Human embryonic kidney 293 (HEK) or T-Rex 293 cells were transfected using the Transfast kit (Promega, Charbonnières, France) as described by the manufacturer.
Stable HEK clones expressing IL4I1 were maintained in complete DMEM (10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/mL penicillin, and 10 μg/mL streptomycin) supplemented with 1.2 mg/mL G418 (mouse and E481A-hIL4I1 mutant) or 50 μg/mL blasticidin and 200 μg/mL zeocin (wt hIL4I1), respectively, at 37°C in 5% CO2. Expression in T-Rex 293 clones was induced by the addition of 1 μg/mL tetracycline. The SU-DHL-4 cell line was obtained from DSMZ (Braunschweig, Germany). The MedB-1 cell line has been described previously.11
Adherent peripheral blood mononuclear cells (PBMCs) obtained from healthy donors after informed consent were differentiated into macrophages as in Perez-Bercoff et al12 and into immature DCs (iDCs) using GM-CSF and IL4 as previously described.13 Mature dendritic cells (mDCs) were obtained by 24-hour incubation with different stimulating cocktails: 10 ng/mL TNFα + 3 μg/mL PGE2, 1.5 μg/mL CD40L with or without 1000 UI/mL IFNγ, 20 ng/mL LPS, or 10 μg/mL poly-IC. B lymphocytes from tonsils and T lymphocytes from PBMCs were obtained using anti-CD19 and anti-CD3 antibody-coated microbeads respectively, according to the manufacturer's instructions.
Antibody preparation
A polyclonal antibody against a peptide (SQDWKAERSQDPFEKC) in the N-terminal region was developed in rabbit and affinity purified against the same peptide by PickCell Laboratories BV (Amsterdam, the Netherlands).
Protein preparation
For Western blot analysis, cells were lysed as previously described,6 the proteins separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) or 7% Tris-acetate NUPAGE gels (Invitrogen), and transferred onto polyvinylidene difluoride membranes (Millipore, St Quentin en Yvelines, France). Revelation was performed using the appropriate antibodies, followed by Enhanced Chemi Luminescence detection using the Autochemi System and Labworks Analysis Software (UVP, Cambridge, United Kingdom). SU-DHL-4 and Med-B1 cell-derived extracts were immunoprecipitated using the anti-IL4I1 antibody prior to Western blot analysis.
For LAAO activity, cells recovered in PBS containing complete mini protease inhibitors (Roche, Meylan, France) were submitted to 4 cycles of freezing and thawing. After centrifugation at 13000g for 10 minutes, the soluble fraction was collected. The protein concentration was determined using the Biorad protein assay (Marnes-la-Coquette, France), with bovine serum albumin as standard.
Protein purification
His-tagged IL4I1 protein was purified from 10 mL conditioned media from IL4I1-producing HEK cells using 1 mL of Ni-NTA agarose beads (Qiagen, Courtaboeuf, France). As a control, the purification procedure was performed on HEK cells transfected with the control pcDNA4-TO-mycHis (mock beads).
Measurement of LAAO activity
LAAO activity was determined on 100 μg total cell protein or 60 μL medium by measuring the release of H2O2 according to Mason et al.10 Secreted IL4I1 activity was measured at 37°C on 3 × 105 cells incubated for 6 hours with or without 10 mM phenylalanine as substrate in PBS–5% fetal calf serum by following the release of H2O2 in the supernatant.
Quantitative RT-PCR analysis
Total RNA (2 μg) isolated from cell populations using TRIzol reagent (Invitrogen) was reverse transcribed with 200 U Superscript II (Invitrogen) in the presence of 300 ng random hexamers, according to the manufacturer's instructions. IL4I1 and TATA-binding protein (TBP) mRNA levels were measured by real-time quantitative reverse-transcriptase (RT)–PCR using, respectively, Hs00541746_m1 and Hs00427620_m1 inventoried TaqMan gene expression assays (Applied Biosystems, Courtaboeuf, France). PCR was performed in duplicate, with recommended cycling conditions. Expression of IL4I1 normalized to TBP, relative to a reference cDNA (lymph node), was determined as 2-(δCtsample-δCtref).
T-cell proliferation tests
CD3+ T cells or CD4+, CD8+, CD45RO+ and CD45RA+ populations (all at 4 × 105) were incubated in 24-well plates with mock beads or hIL4I1 beads or its mutant form at different doses (expressed as μL beads per 5 × 104 cells) in a 0.4-μm transwell. After 3- or 24-hour incubation, transwells were removed and cells transferred to 96-well plates coated with anti-CD3 antibody (5 × 104 cells/well; 4 to 6 wells for each test). In some experiments, hIL4I1 beads were added directly to anti-CD3–stimulated T cells. Phenylpyruvate or H2O2 was also added directly. Catalase (1000 U/mL) was added 30 minutes prior to and during incubation with hIL4I1 beads or H2O2. Proliferation was measured by 3H-thymidine (1 μCi (0.037 MBq)/well) incorporation during the last 8 hours of a 4-day culture. Statistical analysis was performed using the Mann-Whitney test.
TCRζ chain measurements
TCRζ chain staining was performed on fresh PBMCs permeabilized with saponin and incubated with IL4I1 beads as described in “T-cell proliferation tests”. A minimum of 104 cells was analyzed on a Beckman Coulter flow cytometer. Statistical analysis was performed using the unpaired Student t test.
Immunohistochemistry
Formalin-fixed and paraffin-embedded lymphoid tissue and cell pellet sections (3 μm for all) were stained for IL4I1 (1/500 dilution, 18 524; Abcam, Cambridge, United Kingdom) and HLA-DR (1/200 dilution, CR3/43; DakoCytomation, Trappes, France) using an indirect immunoperoxidase method (EnVision+System; DakoCytomation) after antigen retrieval with EDTA buffer at pH 8 in a water bath at 98°C for 30 minutes. Images were captured with a Zeiss Axioskop2 microscope (Zeiss, Oberkochen, Germany) and Neofluar 100 ×/0.1 NA optical lenses (Zeiss). Photographs were taken with a DP70 Olympus camera (Olympus, Tokyo, Japan). Image acquisition was performed with Olympus DP Controller 2002, and images were processed with Adobe Photoshop v7.0 (Adobe Systems, San Jose, CA).
Results
Human IL4I1 is an N-glycosylated secreted protein
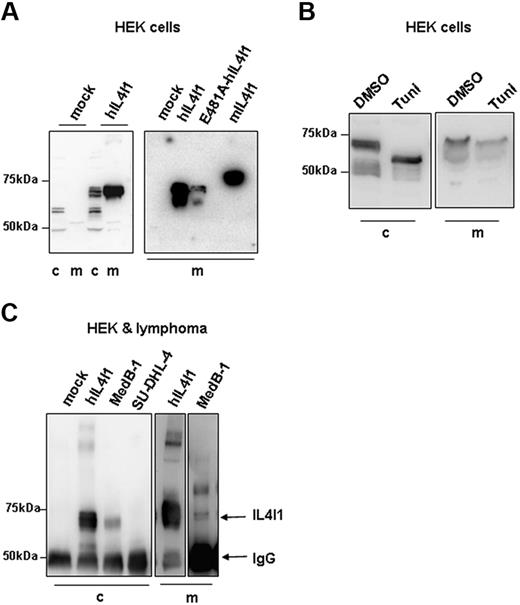
To study the biochemical characteristics and subcellular localization of hIL4I1, we generated HEK293 cell lines expressing a myc-His–tagged full-length wild-type hIL4I1 protein (567 amino acids) either constitutively or upon tetracycline induction (hIL4I1-HEK). For comparison, we expressed in the same cell line the mouse enzyme (mIL4I1, 630 amino acids) and a mutant form of the human enzyme expressing an alanine instead of a glutamic acid at position 481 of the polypeptidic chain (E481A-hIL4I1). The residue corresponding to amino acid 481 in hIL4I1 is involved in the interaction with the enzyme cofactor flavin adenine dinucleotide (FAD), in the tridimentional structure of the snake LAAO.14 No toxicity was observed and HEK293 cell proliferation was not affected by the production of the murine or human proteins (data not shown). High levels of wild-type hIL4I1 were detected both in whole hIL4I1-HEK cell lysates and in culture supernatant, when analyzed by Western blot using an antimyc antibody or an antibody specific for the N-terminal end of hIL4I1 (Figure 1A). Similar data were obtained with the constitutive and the inducible hIL4I1 clones (only data obtained with the inducible clone are shown). The mouse protein and the human mutant E481A-hIL4I1 were also secreted into the culture medium. Of note, the anti-hIL4I1 antibody revealed in the NuPAGE system several closely spaced bands possibly representing differentially glycosylated forms of the protein. The one with the highest molecular weight appeared to be secreted (Figure 1A left panel and 1C). A lower molecular weight protein was revealed by the anti-myc antibody recognizing a C-terminal epitope and probably represents a degradation product (Figure 1A right panel). Treatment with the N-glycosylation inhibitor tunicamycin for the last 24 hours of culture induced a molecular weight shift and reduced hIL4I1 secretion, indicating that hIL4I1 is an N-glycosylated protein (Figure 1B).
IL4I1 is a secreted glycosylated protein. (A) Whole-cell proteins (c) and medium (m) from HEK293 cells transfected with empty vector (mock) or with vectors expressing wild-type human IL4I1 (hIL4I1), mutant hIL4I1 (E481A-hIL4I1), or mouse IL4I1 (mIL4I1) were separated on a 7% NuPAGE and blotted onto PVDF. Blots were revealed with an anti-IL4I1 antibody (A left panel) or an anti-myc antibody (A right panel). (B) hIL4I1-HEK cells were treated with tunicamycin (Tuni) or equal volume of DMSO (DMSO) for the last 24 hours of culture and whole-cell proteins and medium analyzed as in panel A using an anti-IL4l1 antibody for revelation. (C) Cell lysates from mock-HEK, hIL4I-HEK, and the lymphoma cell lines MedB-1 and SU-DHL-4 (left panel) and culture media from hIL4I1-HEK and MedB-1 (right panel) were immunoprecipitated with an anti-IL4I1 antibody. Precipitates were analyzed by Western blot using the same antibody. Arrows show the position of IL4I1 and immunoglobulin heavy chain. Blots of media on right panel are 2 different exposures from the same blot.
IL4I1 is a secreted glycosylated protein. (A) Whole-cell proteins (c) and medium (m) from HEK293 cells transfected with empty vector (mock) or with vectors expressing wild-type human IL4I1 (hIL4I1), mutant hIL4I1 (E481A-hIL4I1), or mouse IL4I1 (mIL4I1) were separated on a 7% NuPAGE and blotted onto PVDF. Blots were revealed with an anti-IL4I1 antibody (A left panel) or an anti-myc antibody (A right panel). (B) hIL4I1-HEK cells were treated with tunicamycin (Tuni) or equal volume of DMSO (DMSO) for the last 24 hours of culture and whole-cell proteins and medium analyzed as in panel A using an anti-IL4l1 antibody for revelation. (C) Cell lysates from mock-HEK, hIL4I-HEK, and the lymphoma cell lines MedB-1 and SU-DHL-4 (left panel) and culture media from hIL4I1-HEK and MedB-1 (right panel) were immunoprecipitated with an anti-IL4I1 antibody. Precipitates were analyzed by Western blot using the same antibody. Arrows show the position of IL4I1 and immunoglobulin heavy chain. Blots of media on right panel are 2 different exposures from the same blot.
In order to exclude an artifact due to the conditions of protein overexpression, we looked for endogenous hIL4I1 expression in the PMBL-derived cell line MedB-1, previously shown to express high levels of hIL4I1 mRNA5 (Figure 1C). No hIL4I1 was detected in control (nonmediastinal) B-cell lymphoma line SU-DH-L4. Importantly, the protein was detectable both in MedB-1 cell lysates and in the culture medium, implying that hIL4I1 is naturally secreted.
Human IL4I1 has an LAAO activity
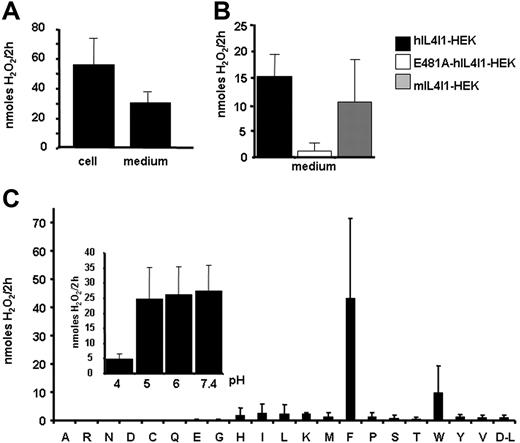
As mouse IL4I1 has been reported to have an l-amino acid oxidase (LAAO) activity,10 we tested the human protein for this activity. Significant LAAO activity was constantly detected both in cell extracts and in the culture supernatant of hIL4I1-HEK cells induced with tetracycline (Figure 2A) in comparison to mock-HEK or noninduced hIL4I1-HEK (data not shown). This activity was decreased by glycosylation inhibition (data not shown). The mouse enzyme displayed comparable activity, while that of the human mutant E481A was negligible, consistent with a putative role of residue 481 in the interaction with the enzymatic cofactor FAD (Figure 2B). The LAAO activity of hIL4I1 was optimal with phenylalanine as substrate, whereas it was consistently weaker with tryptophan, tyrosine, or leucine (Figure 2C). The human enzyme was active at all pHs tested from 5 to 7.4 (Figure 2C inset). Activity at physiological pH was also observed for the mouse enzyme (data not shown). Under typical conditions, the apparent maximum velocity (Vm) and Michaelis-Menten constant (Km) of the human enzyme were estimated to be 3.236 U/mg and 1.48 mM, respectively, and were comparable with values reported for other LAAO. These data indicate that IL4I1 is a secreted phenylalanine oxidase fully active at physiological pH.
Secreted IL4I1 presents a phenylalanine oxidase activity over a wide pH range. (A) Culture medium (60 μL) or 100 μg cell lysate from hIL4I1-HEK induced with tetracycline was used to measure LAAO activity in a colorimetric assay (“Materials and methods”) against l-phenylalanine. Results are expressed as nanomoles of H2O2 produced in a 2-hour reaction. (B) Medium (60 μL) from wild-type human (■), E481A mutant (□), and mouse IL4I1 (▩) expressing HEK cells were used to measure LAAO activity as in panel A. (C) hIL4I1-HEK cell extracts (100 μg total protein) were tested at pH 5 for LAAO activity against l-amino acids and d-leucine (one letter nomenclature) as in panel A. (Inset) LAAO activity of 100 μg total protein was measured against phenylalanine at different pHs. Mean ± SD from 3 (A-B) or 2 (C) independent experiments is shown.
Secreted IL4I1 presents a phenylalanine oxidase activity over a wide pH range. (A) Culture medium (60 μL) or 100 μg cell lysate from hIL4I1-HEK induced with tetracycline was used to measure LAAO activity in a colorimetric assay (“Materials and methods”) against l-phenylalanine. Results are expressed as nanomoles of H2O2 produced in a 2-hour reaction. (B) Medium (60 μL) from wild-type human (■), E481A mutant (□), and mouse IL4I1 (▩) expressing HEK cells were used to measure LAAO activity as in panel A. (C) hIL4I1-HEK cell extracts (100 μg total protein) were tested at pH 5 for LAAO activity against l-amino acids and d-leucine (one letter nomenclature) as in panel A. (Inset) LAAO activity of 100 μg total protein was measured against phenylalanine at different pHs. Mean ± SD from 3 (A-B) or 2 (C) independent experiments is shown.
Human IL4I1 is expressed by antigen-presenting cells
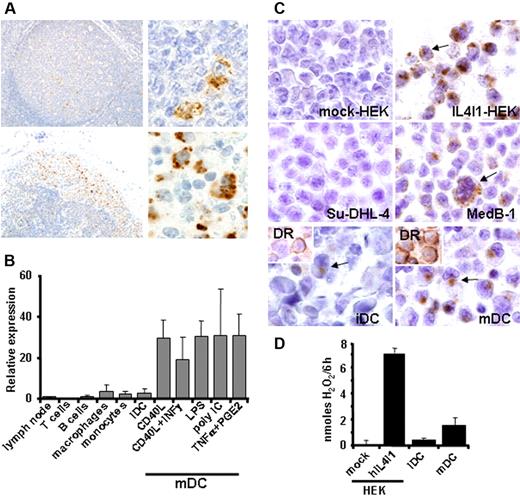
Human IL4I1 RNA has been identified in IL4-stimulated B cells,3 but the protein expression in lymphoid tissues has yet not been reported. Immunohistochemistry detection of IL4I1 in reactive lymph nodes and hyperplasic tonsils was restricted to few cells located in germinal centers that displayed the morphology of macrophages with tingible bodies. Scattered IL4I1-positive cells were also detected in interfollicular areas (Figure 3A upper row). In an inflammatory lymph node, a strong staining of histiocytes was also seen within peripheral sinusal histiocytosis. The latter constitutes a population of myeloid cells (macrophages and/or dendritic cells) coming from the inflammatory peripheral tissue drained by the lymph node (Figure 3A bottom row). We next sought to identify and quantify hIL4I1 expression in different populations of lymphocytes and antigen-presenting cells by quantitative RT-PCR (Figure 3B). Strikingly, high IL4I1 mRNA expression was detected in mDCs cultured with different combinations of maturation stimuli, in comparison to the other cell populations tested (ie, iDCs, monocytes, macrophages, B and T lymphocytes). No significant difference was found in the expression level between iDCs and the peripheral blood monocytes from which they were derived. Nonactivated macrophages presented levels of IL4I1 mRNA comparable with iDCs. Immunohistochemistry on paraffin-embedded cells consistently revealed that IL41I was localized to the Golgi compartment and intracellular granules in hIL4I1-HEK and MedB-1 cells as seen in myeloid cells from secondary lymphoid organs, while it was totally absent in mock-HEK and SU-DHL-4 cells (Figure 3C). hIL4I1 protein expression was much higher in mDCs than in iDCs and was associated with enzymatic activity against phenylalanine released in culture (Figure 3C-D). Altogether, these data show that hIL4I1 production characterizes naturally occurring myeloid cells in lymphatic organs and is strongly induced in vitro in DCs stimulated by different cocktails of immune mediators, such as cytokines, CD40L, PGE2, and Toll-like receptor ligands and that mDCs are a naturally secreting population.
IL4I1 expression in secondary lymphoid tissues and antigen-presenting cells. (A) Formalin-fixed paraffin-embedded hyperplastic tonsils (top row) or inflammatory lymph node (bottom row) tissue sections were stained with anti-IL4I1 polyclonal antibody and photographed at 20 × (left) and 100 × original magnification (right). (B) Different cell populations were isolated from blood and cultured as described in “Materials and methods” prior to mRNA extraction, and hIL4I1 RNA was measured by quantitative RT-PCR. Relative expression of hIL4I1 compared with a normal lymph node is shown. Mean ± SD from 3 independent experiments is shown. (C) Immunohistochemical analysis of mock-HEK, hIL4I1-HEK, MedB-1, and SU-DHL-4 cells, immature dendritic cells (iDCs), and mature dendritic cells (mDCs) was performed on paraffin-embedded cells using an anti-IL4I1 antibody. Arrows indicate positive cells. Note that most mDCs stained positive for IL4I1, whereas only rare iDCs disclosed a weak staining (arrow). (Inset) HLA-DR staining of the DCs indicating differential maturation. (D) HEK and DC populations were cultured in a reaction mixture with or without phenylalanine and secreted activity was evaluated by H2O2 measurement on the supernatant after 6-hour incubation at 37°C in 5% CO2. Data are expressed as H2O2 production per 6 hours after subtraction of values obtained without phenylalanine. A representative experiment (of 3) is shown.
IL4I1 expression in secondary lymphoid tissues and antigen-presenting cells. (A) Formalin-fixed paraffin-embedded hyperplastic tonsils (top row) or inflammatory lymph node (bottom row) tissue sections were stained with anti-IL4I1 polyclonal antibody and photographed at 20 × (left) and 100 × original magnification (right). (B) Different cell populations were isolated from blood and cultured as described in “Materials and methods” prior to mRNA extraction, and hIL4I1 RNA was measured by quantitative RT-PCR. Relative expression of hIL4I1 compared with a normal lymph node is shown. Mean ± SD from 3 independent experiments is shown. (C) Immunohistochemical analysis of mock-HEK, hIL4I1-HEK, MedB-1, and SU-DHL-4 cells, immature dendritic cells (iDCs), and mature dendritic cells (mDCs) was performed on paraffin-embedded cells using an anti-IL4I1 antibody. Arrows indicate positive cells. Note that most mDCs stained positive for IL4I1, whereas only rare iDCs disclosed a weak staining (arrow). (Inset) HLA-DR staining of the DCs indicating differential maturation. (D) HEK and DC populations were cultured in a reaction mixture with or without phenylalanine and secreted activity was evaluated by H2O2 measurement on the supernatant after 6-hour incubation at 37°C in 5% CO2. Data are expressed as H2O2 production per 6 hours after subtraction of values obtained without phenylalanine. A representative experiment (of 3) is shown.
Human IL4I1 inhibits T-cell proliferation with a preference for memory T cells
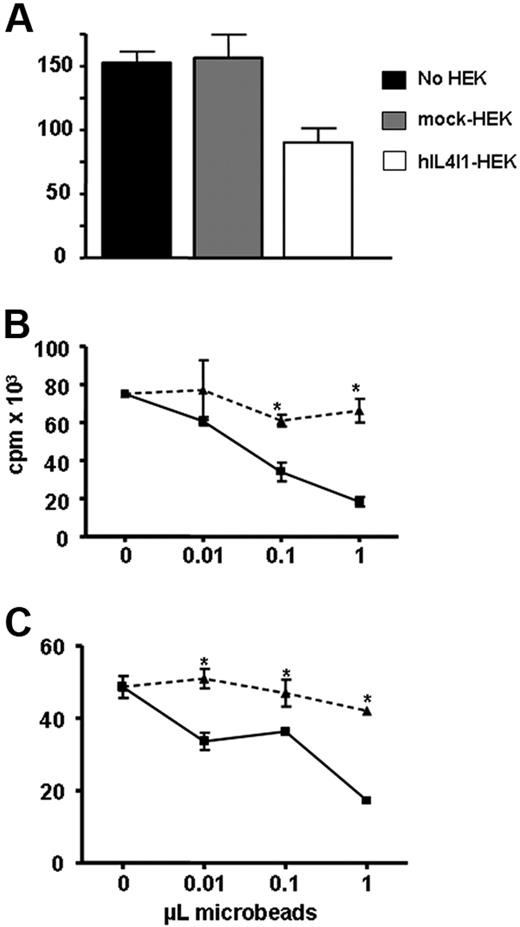
Mature DCs play a critical role in the induction and regulation of the adaptive immune response. Therefore, we wondered whether hIL4I1 participates in the regulation of T-lymphocyte proliferation. Peripheral blood lymphocytes activated via the CD3/T-cell receptor complex with an anti-CD3 antibody were cultured in the presence of irradiated mock-transfected or hIL4I1-HEK cells and proliferation measured by 3H-thymidine incorporation. Proliferation of the lymphocytes cultured in the presence of hIL4I1-expressing cells was decreased (34% ± 9.1% inhibition, mean ± SD of 3 experiments; Figure 4A). These results were confirmed using purified hIL4I1 bound to agarose nickel beads (hIL4I1 beads) with 51.3% ± 10.7% inhibition at the highest bead dose (mean ± SD of 4 experiments) compared with proliferation in the presence of control beads (mock beads). This inhibition was dependent on the amount of hIL4I1 beads used (Figure 4B).
hIL4I1 inhibits T-cell proliferation. CD3-stimulated T lymphocytes were incubated alone (■), with irradiated mock-HEK (▩), or with irradiated hIL4I1-HEK (□). Proliferation was measured by 3H-thymidine incorporation during the last 8 hours of a 4-day culture. Results are expressed as the average cpm of quadruplicates ± standard deviation (SD). A representative experiment (of 3) is shown. *P < .05. CD3-stimulated T lymphocytes were incubated with mock beads (▴) or hIL4I1 beads (■) either in direct contact (B) or through a 0.4-μm transwell (C). Proliferation was measured by 3H-thymidine incorporation during the last 8 hours of a 4-day culture. Results are expressed as the average cpm of quadruplicates ± standard deviation (SD). A representative experiment (of 4) is shown. *P < .05.
hIL4I1 inhibits T-cell proliferation. CD3-stimulated T lymphocytes were incubated alone (■), with irradiated mock-HEK (▩), or with irradiated hIL4I1-HEK (□). Proliferation was measured by 3H-thymidine incorporation during the last 8 hours of a 4-day culture. Results are expressed as the average cpm of quadruplicates ± standard deviation (SD). A representative experiment (of 3) is shown. *P < .05. CD3-stimulated T lymphocytes were incubated with mock beads (▴) or hIL4I1 beads (■) either in direct contact (B) or through a 0.4-μm transwell (C). Proliferation was measured by 3H-thymidine incorporation during the last 8 hours of a 4-day culture. Results are expressed as the average cpm of quadruplicates ± standard deviation (SD). A representative experiment (of 4) is shown. *P < .05.
To investigate whether this hIL4I1 inhibitory effect required the direct interaction between hIL4I1 and T cells, we repeated the experiment with the beads placed in transwells. Under these conditions, we still observed inhibition of T-cell proliferation (Figure 4C). A similar inhibition was achieved after a short (3 hours) incubation with the beads in transwells (46.9% ± 16.2% inhibition, mean ± SD of 5 experiments), suggesting that most of the effect of hIL4I1 takes place during this short initial period. All subsequent experiments described were thus conducted on cell populations incubated with the enzyme covered beads in transwells.
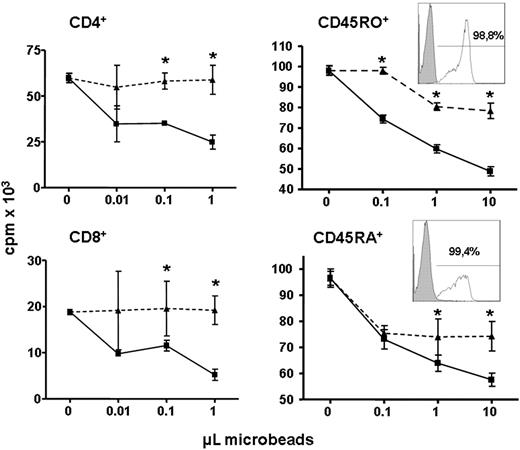
We wondered whether some T-cell populations were more sensitive to the antiproliferative effect of hIL4I1. CD4+ and CD8+ T lymphocytes, in a first set of experiments, and CD45RO+ (memory) and CD45RA+ (naive) T lymphocytes, in a second set of experiments, were purified by positive selection and incubated with hIL4I1-coated or control beads. Proliferation was similarly reduced by hIL4I1 in both CD4+ and CD8+ T-cell populations (Figure 5). However, CD45RO+ memory T cells were more sensitive than CD45RA+ T lymphocytes to inhibitory effect of hIL4I1 (Figure 5). Altogether, these data define hIL4I1 as a new T-cell proliferation regulatory molecule.
Memory T cells show the highest sensitivity to hIL4I1 activity. Positively purified CD4+, CD8+, memory CD45RO+, and naive CD45RA+ T lymphocytes were incubated for 3 hours with mock beads (▴) or hIL4I1 beads (■) through a 0.4-μm transwell and proliferation was measured by 3H-thymidine incorporation. Results are expressed as the average cpm of quadruplicates ± SD. A representative experiment (of 4) is shown. *P < .05.
Memory T cells show the highest sensitivity to hIL4I1 activity. Positively purified CD4+, CD8+, memory CD45RO+, and naive CD45RA+ T lymphocytes were incubated for 3 hours with mock beads (▴) or hIL4I1 beads (■) through a 0.4-μm transwell and proliferation was measured by 3H-thymidine incorporation. Results are expressed as the average cpm of quadruplicates ± SD. A representative experiment (of 4) is shown. *P < .05.
H2O2 mediates the antiproliferative activity of hIL4I1 by TCRζ chain down-regulation
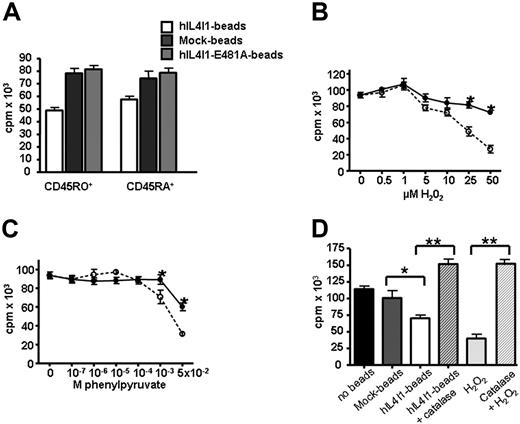
Human IL4I1 inhibition of T-cell proliferation does not require direct cell contact, suggesting that soluble(s) molecule(s) resulting from the enzymatic reaction could be responsible for this effect. This hypothesis was supported by the fact that beads coated with the inactive E481A-hIL4I1 enzyme did not affect the proliferation of naive and memory T cells (Figure 6A). Oxidative deamination of phenylalanine by IL4I1 produces H2O2 and phenylyruvate (BRENDA, EC-Number 1.4.3.2). To investigate whether these molecules participate in the effect of hIL4I1 on T-lymphocyte proliferation, CD45RO+ and CD45RA+ lymphocytes were incubated in the presence of increasing concentrations of H2O2 (Figure 6B) or phenylpyruvate (Figure 6C) before assessment of anti-CD3 induced proliferation. Both molecules inhibited the proliferation of the 2 cell populations. However, only H2O2 displayed a significant effect at micromolar doses and more significantly affected the memory T-cell population. To confirm a role for H2O2 in the inhibitory effect of hIL4I1 on T-lymphocyte proliferation, we incubated T lymphocytes with human catalase prior to the addition of hIL4I1 beads. T-cell proliferation blocked by hIL4I1 or H2O2 was fully restored by catalase (Figure 6D).
H2O2 can mediate the antiproliferative effect of IL4I1. CD45RO+ and CD45RA+ T cells were incubated through a 0.4-μm transwell for 3 hours with mock beads, hIL4I1 beads, or E481A-hIL4I1 beads (A) or in the presence of increasing concentrations of H2O2 (B) or phenylpyruvate (C) and analyzed for 3H-thymidine incorporation. Results are expressed as the average cpm of quadruplicates ± SD. (B-C) CD45RO+ (○) and CD45RA+ (●). A representative experiment (of 4) is shown. *P < .05. (D) Proliferation was assessed on T cells incubated with 50 μM H2O2 or with 10 μL mock beads or hIL4I1 beads through a 0.4-μm transwell for 3 hours with or without preincubation with 1000 U/mL catalase. Results are expressed as the average cpm of sextuplicate samples ± SD. A representative experiment is shown. *P = .04; **P < .005.
H2O2 can mediate the antiproliferative effect of IL4I1. CD45RO+ and CD45RA+ T cells were incubated through a 0.4-μm transwell for 3 hours with mock beads, hIL4I1 beads, or E481A-hIL4I1 beads (A) or in the presence of increasing concentrations of H2O2 (B) or phenylpyruvate (C) and analyzed for 3H-thymidine incorporation. Results are expressed as the average cpm of quadruplicates ± SD. (B-C) CD45RO+ (○) and CD45RA+ (●). A representative experiment (of 4) is shown. *P < .05. (D) Proliferation was assessed on T cells incubated with 50 μM H2O2 or with 10 μL mock beads or hIL4I1 beads through a 0.4-μm transwell for 3 hours with or without preincubation with 1000 U/mL catalase. Results are expressed as the average cpm of sextuplicate samples ± SD. A representative experiment is shown. *P = .04; **P < .005.
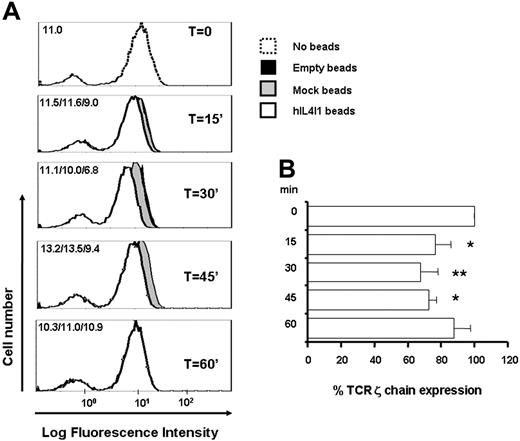
H2O2 has been shown to induce T-cell receptor ζ chain down-regulation15 and apoptosis with a preferential effect on memory T cells.16 CD3-stimulated T lymphocytes incubated in the presence of hIL4I1-covered beads did not show any increase of annexin V staining when compared with control T lymphocytes (annexin V–positive cells: medium, 31.1%; no beads, 26.1%; mock beads, 32.2%; hIL4I1 beads, 29.8%). These results indicate that the induction of apoptosis did not significantly participate in the hIL4I1 effect. In contrast, a significant and reproducible decrease of TCR CD3ζ chain expression was measured in anti-CD3–stimulated T lymphocytes 15, 30, and 45 minutes after contact with hIL4I1-covered beads when compared with incubation with control mock-HEK and empty beads (Figure 7A-B). These data suggest that the antiproliferative effect of hIL4I1 on T cells is due to a decrease in T-cell receptor signaling by the IL4I1 byproduct H2O2.
hIL4I1 down-regulates TCRζ chain levels on T lymphocytes. (A) T lymphocytes were incubated through transwells without beads (T = O, dashed line), with empty beads (black area), with mock beads (gray area), or with hIL4I1-HEK–covered beads (white area) and TCRζ chain expression at the indicated times was evaluated by flow cytometry in permeabilized cells using a monoclonal antibody. Data are expressed as mean fluorescence intensity (MFI). MFIs for each time point are shown in the upper left corner (empty beads/mock beads/hIL41I beads). (B) Inhibition of TCRζ chain expression by hIL4I1 is expressed as average TCRζ percentage expression in the presence of hIL41I compared with T cells incubated with mock beads. *P = .01; **P = .006.
hIL4I1 down-regulates TCRζ chain levels on T lymphocytes. (A) T lymphocytes were incubated through transwells without beads (T = O, dashed line), with empty beads (black area), with mock beads (gray area), or with hIL4I1-HEK–covered beads (white area) and TCRζ chain expression at the indicated times was evaluated by flow cytometry in permeabilized cells using a monoclonal antibody. Data are expressed as mean fluorescence intensity (MFI). MFIs for each time point are shown in the upper left corner (empty beads/mock beads/hIL41I beads). (B) Inhibition of TCRζ chain expression by hIL4I1 is expressed as average TCRζ percentage expression in the presence of hIL41I compared with T cells incubated with mock beads. *P = .01; **P = .006.
Discussion
In this work, we have characterized both human and mouse IL4I1 as secreted l-phenylalanine oxidases. Human and mouse IL4I1 present 79% identity over the majority of the molecule, while they share less than 11% identity in the C-terminal region.3 Not only is this region much shorter in human, but it also has a different amino acid composition that changes its overall theoretical isoelectric point. These differences led us to characterize the human enzyme and to use the mouse protein as the reference. However, when stably overexpressed in HEK293 cells both human and mouse IL4I1 displayed similar biochemical properties. First, in accordance with the presence of a signal peptide, both were detected in the culture medium. Secretion was confirmed in another transfection model (F.C., unpublished data, August 2006) and also in the PMBL-derived cell line MedB-1 and in mDCs that naturally expressed high levels of IL4I1. Thus, like all LAAO enzymes described thus far, IL4I1 is secreted.9,17 Second, IL4I1 preferentially hydrolyzed l-phenylalanine and its activity was not restricted to acidic pH, being also active at a pH approaching neutrality. Surprisingly, some of these results differed from data published by Mason et al for the mouse enzyme, although we used the same experimental conditions to test the enzymatic activity.10 In particular, these authors did not find mIL4I1 to be secreted into the medium. However, they used a different overexpression system consisting of the transient transfection of NIH3T3 cells, which may have been suboptimal and did not permit a high level of expression and the detection of secreted protein.
Human IL4I1 has a preferential LAAO activity toward the essential amino acid phenylalanine as already reported for the murine enzyme.10 This preference suggests a possible physiological role for hIL4I1 in maintaining tight control of phenylalanine metabolism and regulating cellular phenylalanine and/or the levels of its catabolites. Moreover, the secretion of active IL4I1 by mature human DCs and its in vivo expression by myeloid cells suggests a role in intercellular communication with T cells. Proliferation of human peripheral blood T cells induced by an anti-CD3 antibody was significantly inhibited in the presence of purified hIL4I1, even when hIL4I1-coated beads were separated from the T cells by transwells. Recent experimental data indicate that amino acid availability18 and catabolism play important roles in the regulation of immune functions.19 Indeed, amino acid–depleting enzymes, such as the tryptophan catabolizing enzyme indoleamine 2,3 dioxygenase (IDO) and the 2 arginine catabolizing enzymes arginase I (ASEI) and inducible nitric oxide synthase (iNOS2), expressed by a still poorly defined population of myeloid cells (named myeloid-derived suppressive cells, MDSCs), which includes macrophages and DCs, have been shown to impair T-cell activation and inhibit both in vitro and in vivo T-cell responses.20 In vivo, few IL4I1+ cells were detected in normal lymphoid tissue but inflammatory lymph nodes showed intense IL4I1+ expression in myeloid cells from peripheral sinusal histiocytosis. This pattern of staining strongly resembles IDO+ cells distribution in secondary lymphoid tissues.21 Similar to results obtained with IDO and ASEI, (1) our results obtained using the transwell system and the inactive mutant form of hIL4I1 clearly showed that T-lymphocyte proliferation was sensitive to the enzymatic activity of hIL4I1; (2) hIL4I1 is highly expressed by antigen-presenting cells, with the highest levels found in mature DCs. In mouse myeloid cells, Th1 cytokines induce IDO and iNOS2 and Th2 cytokines, such as IL4 and IL13, are potent inducers of ASEI, while both participate in concomitant induction of the 2 enzymes in MDSCs.22,–24 We suggest that IL4I1, which is induced by IL4,3 represents another member of this family of immunoregulatory enzymes. Under our culture conditions, DCs were derived from donor monocytes using IL4, which could have acted synergistically with LPS to allow the secretion of active enzyme in mature DCs. Indeed, a similar 2-step stimulation by PGE2 and TLR ligands is required by mature DCs for functional IDO production.25 Very recent microarray data have shown increased mRNA expression of IL4I1 in tumor-induced mouse MDSCs, indicating that IL4I1 may participate in the negative-feedback regulation of T-cell activation by this heterogeneous population of alternatively activated myeloid cells.24
IL4I1 oxidative deamination of phenylalanine produces H2O2 and phenylpyruvate. The reversal of the inhibitory effect of IL4I1 by incubation of T lymphocytes with catalase indicates that this effect was mediated by H2O2. Most of the previously described LAAO enzymes present proapoptotic effects that have been related to H2O2 production.8,26,27 We had no difficulties in developing stable cell lines with constitutive or inducible expression of active IL4I1 in HEK293 cells. While the enzyme is probably functionally active inside the cell, it is highly compartmentalized, restricted to vesicles (Figure 3B and data not shown) where it is likely to be physically separated from its substrate, and hence metabolically inactive. In addition, it is possible that HEK293 cells may be resistant to the effects of H2O2 and other IL4I1 catabolites and/or phenylalanine deprivation upon its secretion. However, other cells such as primary T lymphocytes may lack these protective mechanisms. Indeed, donor T-cell proliferation was significantly inhibited in the presence of purified hIL4I, while the growth of the transformed T-cell line Jurkat was not affected (data not shown). While CD4+ and CD8+ T cells displayed similar sensitivity to hIL4I1, the proliferation of memory T cells was more strongly inhibited than that of naive T cells. Memory T lymphocytes were also more sensitive to the presence of H2O2 and phenylpyruvate than naive T lymphocytes. These results, obtained with H2O2, are in accordance with a previous report,16 whereas to date phenylpyruvate is known only for its toxicity toward neural cells.28 However, the effects of H2O2 were observed at micromolar doses, while those of phenylpyruvate required millimolar amounts and thus are less likely to be compatible with a physiological effect.
Depending on the length of exposure, H2O2 has been reported to have multiple effects on T cells. Short exposure to H2O2 decreases TCRζ chain expression and tyrosine phosphorylation, ultimately resulting in the suppression of antigen-specific T-cell proliferative responses15 and cytokine secretion29 without affecting viability, while prolonged treatment with H2O2 induces T-cell apoptosis.16 In our experimental setting, we observed a transient decrease in TCRζ chain expression in the absence of apoptosis. The brief production of H2O2 due to the rapid loss of activity of hIL4I1-coated beads in culture medium (data not shown) may explain these results along with the fact that incubation of T cells with hIL4I1 for 24 hours did not increase its effect compared with a 3 hours incubation.
In conclusion, we have demonstrated for the first time that human IL4I1 is a secreted l-phenylalanine oxidase expressed by antigen-presenting cells that is able to inhibit TCRζ chain expression and T-lymphocyte proliferation via H2O2 production. We propose that it is a new member of a family of immunomodulatory enzymes that exert their inhibitory role in the immune response by modulating the levels of amino acids and their catabolites. Moreover, the fact that IL4I1 is overexpressed by certain tumors, such as primary mediastinal B-cell lymphomas, could indicate a possible role for this enzyme in tumor-induced escape from immunosurveillance. Additional work will be necessary to further elucidate the physiological role of hIL4I1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by INSERM AVENIR Program (F.C.).
We are in debt to William Hempel for critical reading of the paper. We are thankful to Valérie Guislain and Nadine Martin for helpful technical assistance. We thank Peter Macheroux for helpful discussions. M.B. was supported by ARTGIL (granted by Amgen and Roche France).
Authorship
Contribution: M.-L.B. designed and performed experiments (biochemical characterization); J.M. designed and performed experiments (T-cell proliferation); V.M.-F. designed experiments, and assisted in analyzing the data and writing the paper; P.M. generated reagents (MedB-1 cell line); P.G. assisted in writing the paper; K.L. performed experiments (Q-PCR), and assisted in designing the experiments, analyzing the data, and writing the paper; F.L. performed experiments (mutagenesis); C.G. performed experiments (cell culture); M.B. performed experiments (immunohistochemistry); C.C.-B. analyzed immunohistochemistry data; F.C. designed and performed experiments, analyzed the data, and wrote the paper.
M.-L.B. and J.M. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Flavia Castellano, INSERM U841 equipe 09, Hôpital Henri Mondor, 51 avenue du Maréchal de Lattre de Tassigny, Créteil F-94000, France; e-mail: flavia.castellano@creteil.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal