Abstract
Human mesenchymal stem cells (hMSCs) represent promising tools in various clinical applications, including the regeneration of injured tissues by endogenous or transplanted hMSCs. The molecular mechanisms, however, that control hMSC mobilization and homing which require invasion through extracellular matrix (ECM) barriers are almost unknown. We have analyzed bone marrow–derivedhMSCs and detected strong expression and synthesis of matrix metalloproteinase 2 (MMP-2), membrane type 1 MMP (MT1-MMP), tissue inhibitor of metalloproteinase 1 (TIMP-1), and TIMP-2. The ability of hMSCs to traverse reconstituted human basement membranes was effectively blocked in the presence of synthetic MMP inhibitors. Detailed studies by RNA interference revealed that gene knock-down of MMP-2, MT1-MMP, or TIMP-2 substantially impaired hMSC invasion, whereas silencing of TIMP-1 enhanced cell migration, indicating opposing roles of both TIMPs in this process. Moreover, the inflammatory cytokines TGF-β1, IL-1β, and TNF-α up-regulated MMP-2, MT1-MMP, and/or MMP-9 production in these cells, resulting in a strong stimulation of chemotactic migration through ECM, whereas the chemokine SDF-1α exhibited minor effects on MMP/TIMP expression and cell invasion. Thus, induction of specific MMP activity in hMSCs by inflammatory cytokines promotes directed cell migration across reconstituted basement membranes in vitro providing a potential mechanism in hMSC recruitment and extravasation into injured tissues in vivo.
Introduction
Human mesenchymal stem cells (hMSCs) from bone marrow are characterized by their ability of self-renewal paired with the capacity to differentiate into diverse mesodermal cell types such as osteoblasts, chondrocytes, and adipocytes.1,2 Moreover, hMSCs were shown to give rise to cells beyond the germ layers with visceral mesoderm, neuroectoderm, or endoderm characteristics.2–4 Additional functions have been reported for hMSCs in providing cytokine and growth factor support for the expansion of hematopoetic5 and embryonic stem cells,6 or by playing an immunomodulatory role.7 One of the most remarkable but least understood findings is the ability of hMSCs to migrate from bone marrow or peripheral blood into damaged tissues. Transplantation experiments in animals and patients demonstrated that mesenchymal stem cells migrate to sites of injury, where they enhance wound healing,8 support tissue regeneration following myocardial infarction,9 home to and promote the restoration of bone marrow microenvironment after damage by myeloablative chemotherapy,10 or help to overcome the molecular defect in children with osteogenesis imperfecta.11 Another interesting observation is that systemically delivered hMSCs are mobilized to and integrate into tumor tissue.12 Taken together, these exciting features have rendered hMSCs a promising tool for tissue engineering13 as well as multiple cell and gene therapy strategies.14–16 Detailed studies have demonstrated that homing of hematopoetic stem cells from blood into bone marrow or their mobilization from bone marrow into blood and tissues is mainly controlled by cytokines/chemokines, adhesion molecules, and proteolytic enzymes.17–19 However, little is known about the molecular mechanisms regulating cell movement and relocalization in hMSCs.
A key requirement for cells to reach distant target sites is the ability to traverse the protein fibers of the extracellular matrix (ECM) which is present between cells of all tissue types.20 Basement membranes represent a specialized form of the ECM that separate epithelium or endothelium from stroma by a dense layer of ECM. To overcome these matrix barriers, migrating cells require specific proteolytic enzymes. Besides some serine- and cysteine-proteinases, in particular the matrix metalloproteinases (MMPs) consisting of more than 24 zinc-dependent endopeptidases, are capable of degrading ECM components. Consequently, MMPs are found to be involved in various physiologic and pathologic processes.21 The 2 gelatinases, MMP-2 and MMP-9, preferentially cleave denatured collagens (gelatin), laminin, and collagen type IV as the major constituent of basement membranes.20,21 Biosynthesis and activity of the gelatinases are associated with the invasive capacity of various cell types such as leukocytes, endothelial cells, and metastasizing tumor cells.22–24 MMP-2 and MMP-9 are secreted from the cells as latent zymogens which are rapidly complexed by their specific endogenous inhibitors, the tissue inhibitor of metalloproteinases (TIMP)–2 and TIMP-1, respectively. Like all MMPs, the gelatinases require activation by proteolytic removal of the N-terminal proenzyme domain. Whereas secreted proMMP-9 is converted into its active form by cleavage through soluble proteinases such as MMP-3 and plasmin, proMMP-2 is activated on the cell surface by a unique mechanism implicating TIMP-2 as well as membrane-type 1 MMP (MT1-MMP) (MMP-14).25
A variety of physiologic stimuli has been described to initiate or enhance gelatinase gene expression in diverse cell types. These factors include inflammatory cytokines such as transforming growth factor β1 (TGF-β1), interleukin 1β (IL-1β), and tumor necrosis factor α (TNF-α),26–29 which are also found to be increased in damaged and inflamed tissues.30 Thus, we and others speculate that the release of certain cytokines, chemokines, and/or growth factors at sites of injury might mobilize hMSCs from bone marrow, peripheral blood, or surrounding tissues into the defective areas, a process which may be enabled via up-regulation of MMP activity in these cells.31–33 This concept is supported by own recent findings showing that Wnt3a enhances MT1-MMP–dependent migration of bone marrow–derived hMSCs through human reconstituted basement membranes.31 Moreover, previous studies of others demonstrated that stromal cell-derived factor 1 (SDF-1) and hepatocyte growth factor stimulated chemoinvasion of hMSCs across Matrigel, implicating MT1-MMP activity.32 Another inflammatory mediator, sphingosine-1-phosphate, was shown to induce chemotactic migration of hMSCs implicating MMP activity.34 Despite those intriguing findings, detailed analysis on the role of inflammatory cytokines in hMSC invasion and identification of particular MMPs essentially involved in this process have not been performed so far.
In this work, we have examined bone marrow–derived hMSCs for mRNA and protein expression of MMP-2, MMP-9, MT1-MMP, TIMP-1, as well as TIMP-2. RNA interference (RNAi) technology was performed to achieve selective knock-down of these genes to study their individual contribution in hMSC migration through human reconstituted basement membranes. For the first time, diverse inflammatory cytokines such as TGF-β1, IL-1β, and TNF-α but not SDF-1α were identified to strongly promote chemotactic invasion of hMSCs by up-regulation of MMP activity in these cells, suggesting in vivo significance of this mechanism in stem cell recruitment to sites of injury.
Materials and methods
Cell culture
hMSCs were purchased from Cambrex (Walkersville, MD). The experiments shown in this study were performed with hMSCs which had been isolated from bone marrow of 3 healthy persons under informed consent (hMSC lot no. 3F0664, no. 1F1061, and no. 4F1127). The hMSC lots had been tested by the providing company for purity by flow cytometry and for their ability to differentiate into the osteogenic, chondrogenic, and adipogenic lineage. The cells were positive for CD105, CD166, CD29, and CD44. The absence of hematopoetic cell contamination was ensured by controlling cells for negative expression of CD14, CD34, and CD45 as described.1 Cultivation of hMSCs in our laboratory was performed using the mesenchymal-stem cell-growth (MSCG)–Medium BulletKit (Cambrex) according to the supplier's instructions at 37°C in a humidified air atmosphere containing 5% CO2. Cells were passaged at a confluency of approximately 90% using stem cell Trypsin-EDTA (Cambrex). For experiments under serum-free conditions, hMSCs were washed with serum-free medium and incubated in Dulbecco Modified Eagle Medium (DMEM; PAA Laboratories, Coelbe, Germany) supplemented with 1% Nutridoma SP (Roche Applied Science, Mannheim, Germany) in the absence or presence of TGF-β, IL-1β, TNF-α, or SDF-1α (all purchased from PeproTech, Rocky Hill, NJ) at the indicated concentrations. hMSC proliferation did not significantly change during exposition to these agents for 48 hours as determined by use of the CyQuant proliferation assay (Invitrogen, Karlsruhe, Germany). All experiments were carried out with hMSCs of the fifth or sixth passage which exhibited an average cell doubling time of 96 hours. Cells were tested by us for their ability to differentiate into the adipogenic and osteogenic lineage as described.31,35
Zymography
Cell culture supernatants were analyzed for the presence of secreted gelatinases by zymography as described previously.36 As a marker for electrophoretic mobility of gelatinases in zymograms we used conditioned medium from HT1080 fibrosarcoma cells containing proMMP-9, proMMP-2, and activated forms of MMP-2.37 Densitometric quantification of gelatinolytic activity in zymograms was performed using the ImageMaster-1D Elite quantification software (GE Healthcare Life Sciences, Freiburg, Germany).
Immunoblot analysis of cell-associated MMPs/TIMPs
For hMSC lysis and protein extraction, a buffer containing 50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, and a mixture of small molecular weight inhibitors of metallo-, serine-, and cysteine-proteinases (Complete-Mini; Roche Applied Science) was added to subconfluently grown cells. The cell lysate was then vortexed and incubated for 30 minutes at 4°C. Subsequently, the supernatants containing extracted proteins were collected by centrifugation at 16 000g and stored at −20°C. SDS–polyacrylamide gel electrophoresis (PAGE) was performed under reducing conditions in precast 4% to 12% mini-gels applying the NuPAGE Bis-Tris buffer system (Invitrogen). After electrophoretic separation proteins were electroblotted on polyvinyl difluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were blocked in 10% nonfat milk and then incubated with polyclonal rabbit antibodies against MT1-MMP (1 μg/mL; Sigma, Munich, Germany) and TIMP-1 (0.4 μg/mL; Sigma), or polyclonal goat antibody against actin (0.5 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA), or 1 μg/mL monoclonal mouse antibodies against MMP-2, MMP-9 (Calbiochem, Schwalbach, Germany), and TIMP-2 (Chemicon, Temecula, CA) for 1 hour at room temperature. After washing blots were incubated for 15 minutes with anti–rabbit or anti–mouse IgG (GE Healthcare Life Sciences) were conjugated with horseradish peroxidase as secondary antibodies at a dilution of 1:1500. Detection was performed applying the enhanced chemiluminescence system (GE Healthcare Life Sciences). Recombinant protein standards (Invitrogen) were used for molecular weight determination.
Quantification of active MT1-MMP
The protein expression of MT1-MMP was quantified in cell extracts using the MMP-14 Biotrak Activity Assay according to the manufacturer's recommendations (GE Healthcare Life Sciences). After 6 hours of incubation, the optical density at 405 nm was measured using the MPP 3408 microtiterplate reader (ASYS Hitech, Salzburg, Austria).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Isolation of total RNA from hMSCs was accomplished using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and on-column DNase digestion with the RNase-free DNase-set (Qiagen) was performed according to the manufacturer's protocols. The cDNA synthesis was completed following the instructions of the First Strand cDNA Synthesis Kit for RT-PCR (AMV) (Roche Applied Science) using oligo dT primers. qRT-PCR was carried out on a LightCycler (Roche Applied Science) using LightCycler-FastStart DNA Master SYBR Green I Kit (Roche Applied Science). For amplification of specific transcripts, LightCycler Primer Sets for MMP-2, MMP-9, MT1-MMP, TIMP-1, TIMP-2 as well as for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene standard were applied according to the manufacturer's instructions (Search LC GmbH, Heidelberg, Germany). PCR was performed as described previously.31
Transfection of hMSCs with small interfering RNA (siRNA)
RNA interference (RNAi) technology was used to generate specific knock-downs of MMP- and TIMP-mRNA transcription in hMSCs. siRNAs targeted against human mRNAs of MMP-2, MT1-MMP, TIMP-1, and TIMP-2 were designed in our laboratory according to the protocol of Reynolds et al.38 Sense and antisense oligonucleotides were synthesized by Qiagen. Nonspecific siRNA which has no target in the human transcriptome was used as a negative control and was also purchased from Qiagen (catalog no. 1022076). The sequences were as follows: MMP-2 (NM_004530) target sequence, 5′-AAGGAGAGCTGCAACCTGTTT-3′; MMP-2 siRNA sense, 5′-GGAGAGCUGCAACCUGUUU-3′; MMP-2 siRNA antisense, 5′-AAACAGGUUGCAGCUCUCC-3′; MT1-MMP (NM_004995) target sequence, 5′-AACCAGAAGCTGAAGGTAGAA-3′; MT1-MMP sense, 5′-CCAGAAGCUGAAGGUAGAA-3′; MT1-MMP antisense, 5′-UUCUACCUUCAGCUUCUGG-3′; TIMP-1 (NM_003254) target sequence, 5′-AATCAACCAGACCACCTTATA-3′; TIMP-1 sense, 5′-UCAACCAGACCACCUUAUA-3′; TIMP-1 antisense, 5′-UAUAAGGUGGUCUGGUUGA-3′; TIMP-2 (NM_003255) target sequence, 5′-AAGGATCCAGTATGAGATCAA-3′; TIMP-2 sense, 5′-GGAUCCAGUAUGAGAUCAA-3′; TIMP-2 antisense, 5′-UUGAUCUCAUACUGGAUCC-3′; negative control siRNA sense, 5′-UUCUCCGAACGUGUCACGU-3′; negative control siRNA antisense, 5′-ACGUGACACGUUCGGAGAA-3′.
siRNA transfection of hMSCs was performed as previously described by us35 with some modifications. Briefly, 1 day before transfection hMSCs were plated at 5 × 103 cells/cm2 in MSCG-medium into 6-well dishes and allowed to reach 30% confluence after 24 hours of incubation. siRNA at a final concentration of 25 nM was combined with 10 μL Lipofectamine 2000 (Invitrogen) in a total volume of 500 μL DMEM and allowed to complex by incubation for 20 minutes at room temperature. The transfection mixture was then applied to the hMSCs and incubated for 6 hours at 37°C in a humidified atmosphere containing 5% CO2. Subsequently, cells were washed with PBS and maintained in MSCG-medium for 24 hours before application in experiments. Cell viability and the capacity for differentiation along the mesodermal lineage were not affected under these conditions.
Cell invasion assay
Studies on chemotactic invasion of hMSCs were performed using the Costar Transwell chamber system (24-well; Costar, Pleasanta, CA)39 with some modifications as previously described by us.31 Membrane filters with a pore size of 8 μm and a diameter of 6.5 mm (Costar) were coated with 10 μg human ECM (BD Biosciences, Bedford, MA) which is mainly composed of laminin, collagen type IV, and proteoglycans, providing a composition similar to that of human basement membranes. The coated filters were dried overnight at room temperature under sterile conditions. Prior to the experiment they were reconstituted with serum-free medium for 2 hours. The lower compartment of the invasion chamber was filled with 600 μL DMEM containing 10% human serum (PAA Laboratories) or cytokines/chemokines at the indicated concentrations as a source of chemoattractants. Then the coated filter inserts were placed into the wells forming the upper compartment. hMSCs (5 × 103) either untreated or after transfection with the respective siRNA were suspended in 200 μL serum-free medium and seeded into the upper compartment of the invasion chamber. Each invasion experiment was performed in triplicate. The invasion chambers were incubated for 48 hours at 37°C in a humidified air atmosphere with 5% CO2. After incubation cells and ECM on the top surfaces of the filters were wiped off with cotton swabs. Cells that had migrated into the lower compartment and attached to the lower surface of the filter were counted after staining with Diff Quick (Dade Diagnostika, München, Germany). Cell viability was assessed by trypan blue staining. The invasion rate was calculated from the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded in the upper compartment.
For migratory inhibition experiments hMSCs were preincubated for 30 minutes with 10 μg/mL GM6001 (Calbiochem), a synthetic broad-spectrum MMP inhibitor,40 or with the same concentration of Ro 206-0222, a highly specific inhibitor for MMP-2, MMP-9, and MT1-MMP41 (kindly provided by Dr Krell, Roche Diagnostics, Pharma Research Penzberg, Germany), before being transferred into the upper compartment. The respective inhibitors were also added to the medium in the upper and lower compartments in the same concentrations. Preceding measurements had shown that incubation of hMSCs with the inhibitors at 10 μg/mL for 48 hours resulted in maximal inhibition of cell migration without substantially affecting viability and proliferation.
Data analysis
Statistical significance was assessed by comparing mean (± SD) values with Student t test for independent groups. Significance was assumed for P value less than .05. Statistical analysis was performed using the Origin 7.5 software (OriginLab, Northampton, MA).
Results
hMSCs constitutively express various MMPs and TIMPs
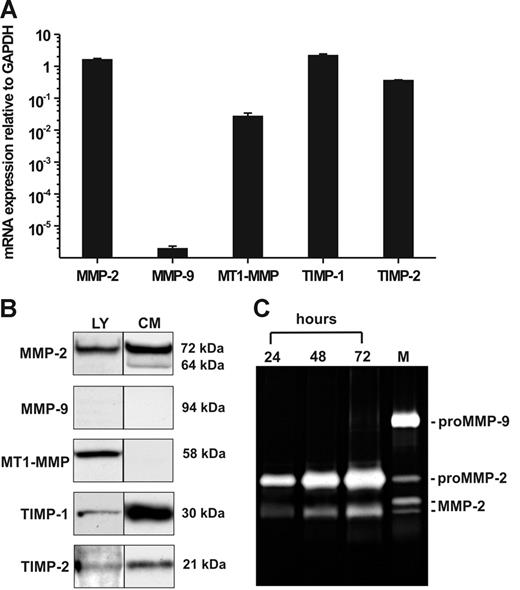
Quantitative determination of mRNA expression in hMSCs by RT-PCR revealed pronounced transcription of MMP-2, MT1-MMP, TIMP-1, and TIMP-2 when compared with that of GAPDH, whereas MMP-9 mRNA was only barely detectable (Figure 1A). To verify these findings on the protein level, we performed Western blotting analysis of hMSC lysates for the content of cell-associated MMPs and TIMPs. In addition, conditioned medium obtained from hMSCs cultivated for 72 hours under serum-free conditions was examined for the presence of secreted MMPs and TIMPs. Consistent with our mRNA data, the cell extracts contained 72-kDa proMMP-2, MT1-MMP in the form of its 58-kDa active species, TIMP-1 (30 kDa), and TIMP-2 (21 kDa) (Figure 1B). Moreover, hMSCs released substantial amounts of 72-kDa proMMP-2 into culture supernatants together with a smaller portion of its activated 64-kDa form as well as TIMP-1 and TIMP-2 (Figure 1B). MT1-MMP was not detected in the culture supernatants corresponding to its nature as a membrane-anchored proteinase. MMP-9 protein was absent in both hMSC lysates and conditioned medium (Figure 1B), which was in accordance to its extremely low mRNA expression level. The findings on gelatinase secretion were confirmed by 72-hour time-course analysis of hMSC culture supernatants by zymography, proving the accumulation of released proMMP-2 as well as of its fully activated species, whereas MMP-9 was not detectable (Figure 1C). These and all following results are representative for measurements accomplished with hMSC samples from 3 different persons (see “Cell culture”).
Constitutive expression of mRNA and protein of MMPs and TIMPs in hMSCs. (A) qRT-PCR analysis of MMP-2, MMP-9, MT1-MMP, TIMP-1, and TIMP-2 gene transcription in hMSCs cultivated in MSCG medium. The results are mean values ± SDs of mRNA expression relative to GAPDH (set as 1) from a triplicate measurement representative for 3 independent experiments with 3 different hMSC lots. (B) Western blot detection of MMP-2, MMP-9, MT1-MMP, TIMP-1, and TIMP-2 in cell lysates (LY) and conditioned medium (CM) of hMSCs cultivated for 72 hours under serum-free conditions. Aliquots (30 μL) standardized by protein content were separated by SDS-PAGE under reducing conditions, blotted, and probed with the specific antibodies. (C) Zymographic analysis of gelatinase secretion from hMSCs. Aliquots of culture supernatants (10 μL) taken at different time points during a 72-hour cultivation period of hMSCs in serum-free medium were analyzed. HT1080 conditioned medium containing proMMP-9, proMMP-2, and active forms of MMP-2 was used as a marker (M).37
Constitutive expression of mRNA and protein of MMPs and TIMPs in hMSCs. (A) qRT-PCR analysis of MMP-2, MMP-9, MT1-MMP, TIMP-1, and TIMP-2 gene transcription in hMSCs cultivated in MSCG medium. The results are mean values ± SDs of mRNA expression relative to GAPDH (set as 1) from a triplicate measurement representative for 3 independent experiments with 3 different hMSC lots. (B) Western blot detection of MMP-2, MMP-9, MT1-MMP, TIMP-1, and TIMP-2 in cell lysates (LY) and conditioned medium (CM) of hMSCs cultivated for 72 hours under serum-free conditions. Aliquots (30 μL) standardized by protein content were separated by SDS-PAGE under reducing conditions, blotted, and probed with the specific antibodies. (C) Zymographic analysis of gelatinase secretion from hMSCs. Aliquots of culture supernatants (10 μL) taken at different time points during a 72-hour cultivation period of hMSCs in serum-free medium were analyzed. HT1080 conditioned medium containing proMMP-9, proMMP-2, and active forms of MMP-2 was used as a marker (M).37
hMSCs are able to traverse human ECM: blockage by MMP inhibitors
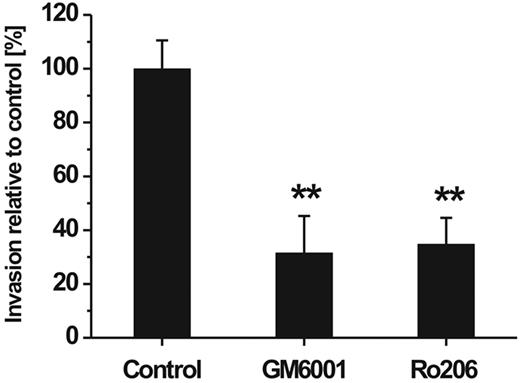
To study the invasive capacity of hMSCs, we performed an in vitro cell migration assay which was adapted to species homogeneous conditions by the use of human ECM as a cell migration barrier and a gradient of chemoattractants constituted by 10% human serum. To investigate the involvement of MMPs in the ability of hMSCs to traverse the human reconstituted basement membrane, the assay was performed in the presence and absence of synthetic MMP inhibitors. Addition of GM6001 representing a broad-spectrum inhibitor of MMP activity significantly reduced the transmigration rate of hMSCs (Figure 2). Ro 206-0222, a highly specific inhibitor of MMP-2, MMP-9, and MT1-MMP activity,41 impaired the invasive capacity of hMSCs to a similar extent (Figure 2). Cell viability was not affected by either of the 2 inhibitors at the concentration used to achieve maximal migratory inhibition (data not shown). From these data it can be concluded that MMPs play a major role in the directed traversal of hMSCs through human ECM barriers.
Influence of MMP inhibitors on hMSC invasion through a barrier of reconstituted human basement membrane. hMSCs were placed onto Transwell filters coated with human ECM and incubated for 48 hours in the absence (control) or presence of the broad-spectrum MMP inhibitor GM6001 (10 μg/mL) or Ro 206-0222 (10 μg/mL), a highly specific inhibitor of MMP-2, MMP-9, and MT1-MMP.41 Cell invasion rate was determined in the percentage relative to control (set as 100%). Data are presented as mean ± SD of 1 triplicate experiment representative of 3 independent experiments. **P < .01.
Influence of MMP inhibitors on hMSC invasion through a barrier of reconstituted human basement membrane. hMSCs were placed onto Transwell filters coated with human ECM and incubated for 48 hours in the absence (control) or presence of the broad-spectrum MMP inhibitor GM6001 (10 μg/mL) or Ro 206-0222 (10 μg/mL), a highly specific inhibitor of MMP-2, MMP-9, and MT1-MMP.41 Cell invasion rate was determined in the percentage relative to control (set as 100%). Data are presented as mean ± SD of 1 triplicate experiment representative of 3 independent experiments. **P < .01.
RNAi efficiently inhibits expression of MMPs and TIMPs in hMSCs
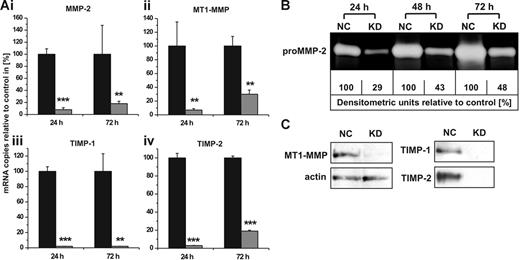
To elucidate the individual contribution of constitutively expressed MMPs and TIMPs to the cellular invasion capacity, we established conditions in hMSCs to specifically silence the gene transcription of MMP-2, MT1-MMP, TIMP-1, and TIMP-2 by RNAi. As determined by qRT-PCR 24 hours after transfection, we achieved knock-down efficiencies of 92% to 98% for these MMPs and TIMPs when compared with control cells transfected with non–target-directed siRNA (Figure 3A). The down-regulation of mRNA expression was still effective with levels between 70% and 98% when determined 72 hours after treatment with the respective siRNAs (Figure 3A). In addition, these results could also be confirmed on the protein level. Time course analysis of culture supernatants from hMSCs transfected with siRNA against MMP-2 revealed a pronounced decline of secreted proMMP-2 as determined by zymography (Figure 3B). Likewise, successful blockage of MT1-MMP production as well as of TIMP-1 and TIMP-2 release in hMSCs carrying the specific knock-downs for 72 hours was demonstrated by Western blotting analysis (Figure 3C). These data proved that the RNAi technology represented an effective tool for studies on the functions of MMPs and TIMPs in hMSCs.
MMP/TIMP knock-downs in hMSCs: effect on mRNA and protein levels. (A) hMSCs were transfected with siRNAs targeting the gene expression of MMP-2 (i), MT1-MMP (ii), TIMP-1 (iii), or TIMP-2 (iv). Control cells were transfected with non–target-directed siRNA (set as 100%). Transcription of specific mRNAs was quantified by qRT-PCR 24 and 72 hours after siRNA transfection. Data represent the mean ± SD of a triplicate measurement representative for 5 transfection experiments. ***P < .001, **P < .01. (B) hMSCs transfected with non–target-directed control siRNA (NC) or with siRNA against MMP-2 (KD) were cultivated under serum-free conditions and analyzed for secreted proMMP-2 after different time intervals by zymography. For densitometric quantification, enzyme release from control cells transfected with non–target-directed siRNA was set as 100% at each time point. (C) Western blotting analysis of protein extracts obtained from hMSCs 72 hours after transfection with control siRNA (NC) or with siRNA against MT1-MMP (KD). Cellular actin was detected on the same blot to control for application of equal amounts of protein in each lane. TIMP-1 and TIMP-2 secretion from hMSCs carrying the respective knock-downs (KD) or control siRNA (NC) was examined by Western blotting of 72-hour culture supernatants. Protein data are representative of 3 independent experiments with similar results.
MMP/TIMP knock-downs in hMSCs: effect on mRNA and protein levels. (A) hMSCs were transfected with siRNAs targeting the gene expression of MMP-2 (i), MT1-MMP (ii), TIMP-1 (iii), or TIMP-2 (iv). Control cells were transfected with non–target-directed siRNA (set as 100%). Transcription of specific mRNAs was quantified by qRT-PCR 24 and 72 hours after siRNA transfection. Data represent the mean ± SD of a triplicate measurement representative for 5 transfection experiments. ***P < .001, **P < .01. (B) hMSCs transfected with non–target-directed control siRNA (NC) or with siRNA against MMP-2 (KD) were cultivated under serum-free conditions and analyzed for secreted proMMP-2 after different time intervals by zymography. For densitometric quantification, enzyme release from control cells transfected with non–target-directed siRNA was set as 100% at each time point. (C) Western blotting analysis of protein extracts obtained from hMSCs 72 hours after transfection with control siRNA (NC) or with siRNA against MT1-MMP (KD). Cellular actin was detected on the same blot to control for application of equal amounts of protein in each lane. TIMP-1 and TIMP-2 secretion from hMSCs carrying the respective knock-downs (KD) or control siRNA (NC) was examined by Western blotting of 72-hour culture supernatants. Protein data are representative of 3 independent experiments with similar results.
Knock-down of MMP-2, MT1-MMP, and TIMP-2 impairs hMSC invasion
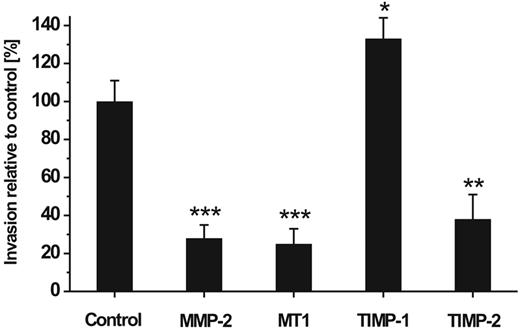
In a next step we used RNAi to elucidate the individual role of constitutively expressed MMP-2, MT1-MMP, TIMP-1, and TIMP-2 in the invasive capability of hMSCs. For this purpose, hMSCs carrying specific MMP/TIMP knock-downs were applied in the Transwell cell invasion assay and analyzed for their migratory potential. We found that down-regulation of MMP-2, MT1-MMP, and TIMP-2 significantly impaired the migration of hMSCs through the reconstituted basement membranes by 72%, 75%, and 65%, respectively, when compared with control cells that had received a non–target-directed siRNA (Figure 4). In contrast, blockage of TIMP-1 raised the invasive behavior of hMSCs (Figure 4). These findings indicate that the expression of MMP-2 and MT1-MMP as well as of TIMP-2 enable hMSCs to migrate across ECM, whereas the production of TIMP-1 exhibited a repressive effect on this process.
Effect of MMP/TIMP knock-downs on hMSC invasion. hMSCs carrying knock-downs for the expression of MMP-2, MT1-MMP (MT1), TIMP-1, and TIMP-2 were assessed for their ability to traverse human ECM. The hMSCs were applied in the Transwell invasion assay 24 hours after their transfection and incubated for 48 hours. Thereafter, cells that had migrated to the lower chamber were counted. Control cells transfected with non–target-directed siRNA were set as 100%. Data are presented as mean ± SD of 1 triplicate experiment representative of 3 independent measurements. ***P < .001, **P < .01, *P < .05.
Effect of MMP/TIMP knock-downs on hMSC invasion. hMSCs carrying knock-downs for the expression of MMP-2, MT1-MMP (MT1), TIMP-1, and TIMP-2 were assessed for their ability to traverse human ECM. The hMSCs were applied in the Transwell invasion assay 24 hours after their transfection and incubated for 48 hours. Thereafter, cells that had migrated to the lower chamber were counted. Control cells transfected with non–target-directed siRNA were set as 100%. Data are presented as mean ± SD of 1 triplicate experiment representative of 3 independent measurements. ***P < .001, **P < .01, *P < .05.
Cytokines/chemokine can modulate expression of MMPs and TIMPs in hMSCs
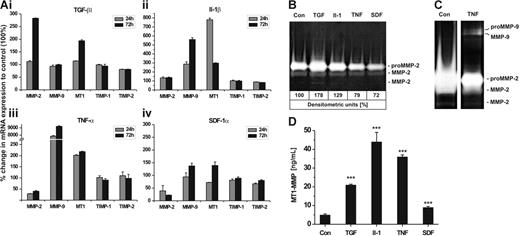
Next, we investigated whether the inflammatory cytokines TGF-β1, IL-1β, TNF-α, and the chemokine SDF-1α influenced the expression of MMP-2, MT1-MMP, MMP-9, TIMP-1, and TIMP-2 in hMSCs. The most effective cytokine/chemokine concentration on the transcription of these genes had been determined in preceding experiments (data not shown). Incubation with TGF-β1 (100 ng/mL) induced the mRNA expression of MMP-2 and MT1-MMP about 3-fold and 2-fold, respectively, when compared with untreated control cells, whereas that of MMP-9 and the TIMPs remained almost unchanged (Figure 5Ai). Addition of IL-1β (50 ng/mL) significantly stimulated the transcription of MT1-MMP (∼ 8-fold) and MMP-9 (∼ 5-fold) but did not strongly alter the mRNA levels of MMP-2 and the TIMPs (Figure 5Aii). Exposure of hMSCs to TNF-α (50 ng/mL) was found to halve the MMP-2 transcription level and concurrently duplicate that of MT1-MMP (Figure 5Aiii). Remarkably, TNF-α exhibited a strong increase in MMP-9 mRNA synthesis with values of 80-fold and 90-fold after 24 and 72 hours of incubation, respectively, whereas TIMP-1 and TIMP-2 expression was poorly affected (Figure 5Aiii). Addition of the chemokine SDF-1α (100 ng/mL) to hMSCs clearly diminished MMP-2 expression and also reduced that of TIMP-2 and TIMP-1, albeit to a less extent. Interestingly, the mRNA levels of MT1-MMP and MMP-9 declined 24 hours after exposure to SDF-1α but showed some elevation above basal transcription after 72 hours of incubation (Figure 5Aiv).
Influence of cytokines/chemokine on mRNA and protein expression of MMPs/TIMPs in hMSCs. hMSCs were incubated with TGF-β1 (100 ng/mL), IL-1β (50 ng/mL), TNF-α (50 ng/mL), SDF-1α (100 ng/mL), or left untreated (control, Con) and cultivated under serum-free conditions for up to 72 hours. (A) mRNA expression of MMP-2, MMP-9, MT1-MMP (MT1), TIMP-1, and TIMP-2 were quantified by qRT-PCR after 24 and 72 hours of incubation with cytokines/chemokine. Results are given as the percentage of change in mRNA expression relative to untreated cells set as 100%. Values represent the mean ± SD of 1 triplicate experiment from 2 independent measurements. (B-C) Aliquots of 72-hour culture supernatants were subjected to zymography. Medium samples were applied in a 1:4 dilution to allow densitometric quantification of proMMP-2 (B) or undiluted to achieve higher sensitivity in the detection of (pro)MMP-9 (C). (D) MT1-MMP protein was determined in cell lysates of hMSCs incubated for 72 hours with and without cytokines/chemokine using the MMP-14 Biotrak Activity Assay. Data are shown as mean ± SD of 1 of 2 triplicate experiments performed. ***P < .001.
Influence of cytokines/chemokine on mRNA and protein expression of MMPs/TIMPs in hMSCs. hMSCs were incubated with TGF-β1 (100 ng/mL), IL-1β (50 ng/mL), TNF-α (50 ng/mL), SDF-1α (100 ng/mL), or left untreated (control, Con) and cultivated under serum-free conditions for up to 72 hours. (A) mRNA expression of MMP-2, MMP-9, MT1-MMP (MT1), TIMP-1, and TIMP-2 were quantified by qRT-PCR after 24 and 72 hours of incubation with cytokines/chemokine. Results are given as the percentage of change in mRNA expression relative to untreated cells set as 100%. Values represent the mean ± SD of 1 triplicate experiment from 2 independent measurements. (B-C) Aliquots of 72-hour culture supernatants were subjected to zymography. Medium samples were applied in a 1:4 dilution to allow densitometric quantification of proMMP-2 (B) or undiluted to achieve higher sensitivity in the detection of (pro)MMP-9 (C). (D) MT1-MMP protein was determined in cell lysates of hMSCs incubated for 72 hours with and without cytokines/chemokine using the MMP-14 Biotrak Activity Assay. Data are shown as mean ± SD of 1 of 2 triplicate experiments performed. ***P < .001.
In correlation with the mRNA data, TGF-β1 and IL-1β induced an increase in secretion of proMMP-2 and its active forms from hMSCs, whereas TNF-α and SDF-1α evoked a reduction in the release of these enzymes as determined by zymographic analysis of diluted culture supernatants (Figure 5B). TNF-α also stimulated hMSCs to produce proMMP-9 and its active form, which, however, became detectable only if nondiluted supernatants were analyzed (Figure 5C). Next, we quantified protein synthesis of cell membrane-bound MT1-MMP in hMSCs by the use of an assay measuring the biologically active form of this enzyme which is characteristically produced immediately after its synthesis in the cell. In concordance with the mRNA data, basal MT1-MMP activity was clearly enhanced on incubation of hMSCs with TGF-β1 (∼ 5-fold), IL-1β (∼ 9-fold), TNF-α (∼ 7-fold), and SDF-1α (∼ 2-fold) (Figure 5D). In summary, these data demonstrate that TGF-β1, IL-1β, TNF-α, and SDF-1α differentially regulate the expression and protein synthesis of special MMPs and TIMPs in hMSCs.
Cytokines/chemokine promote hMSC invasion via MMP up-regulation
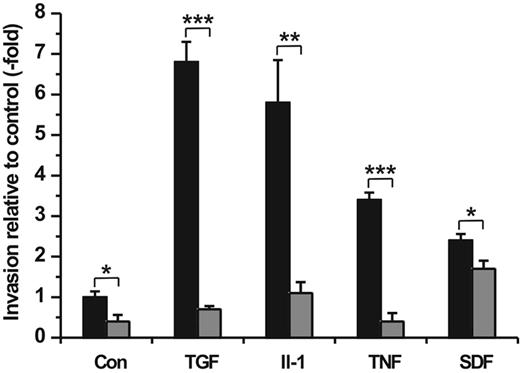
Furthermore, we performed cell invasion assays adding TGF-β1 (100 ng/mL), IL-1β (50 ng/mL), TNF-α (50 ng/mL), or SDF-1α (100 ng/mL) as chemoattractant into the lower compartment of the Transwell chamber in the presence or absence of Ro 206-0222 (10 μg/mL), the specific inhibitor of MMP-2, MT1-MMP, and MMP-9 activity. Determination of hMSC migration rates after a 48-hour incubation period revealed highly increased cell trafficking stimulated by TGF-β1 (∼ 7-fold), IL-1β (∼ 6-fold), TNF-α (∼ 3-fold), and SDF-1α (∼ 2-fold) relative to the serum-free medium alone (control) (Figure 6). By comparison, the addition of 10% human serum used as a chemoattractant under standard assay conditions elevated spontaneous hMSC migration by 14-fold (data not shown).
Chemotactic importance of cytokines/chemokine on hMSC invasion and the implication of MMPs. hMSCs were seeded in the upper compartment of Transwell cell invasion chambers. The lower compartment was filled with DMEM containing TGF-β (100 ng/mL), IL-1α (50 ng/mL), TNF-α (50 ng/mL), or SDF-1α (100 ng/mL) as chemoattractants. Control wells contained DMEM medium only (Con). Both upper and lower compartments were provided without (▪) and with Ro 206-0222 (10 μg/mL) to inhibit MMP-2, MMP-9, and MT1-MMP activity (⊡). After a 48-hour period of incubation the amount of migrated cells was quantified. Results are given in fold increase relative to spontaneous cell migration in control wells. All experiments were performed in triplicate. The mean values ± SDs of 1 of 2 separate experiments are shown. *P < .05, **P < .01, ***P < .001.
Chemotactic importance of cytokines/chemokine on hMSC invasion and the implication of MMPs. hMSCs were seeded in the upper compartment of Transwell cell invasion chambers. The lower compartment was filled with DMEM containing TGF-β (100 ng/mL), IL-1α (50 ng/mL), TNF-α (50 ng/mL), or SDF-1α (100 ng/mL) as chemoattractants. Control wells contained DMEM medium only (Con). Both upper and lower compartments were provided without (▪) and with Ro 206-0222 (10 μg/mL) to inhibit MMP-2, MMP-9, and MT1-MMP activity (⊡). After a 48-hour period of incubation the amount of migrated cells was quantified. Results are given in fold increase relative to spontaneous cell migration in control wells. All experiments were performed in triplicate. The mean values ± SDs of 1 of 2 separate experiments are shown. *P < .05, **P < .01, ***P < .001.
The strong chemotactic responses of hMSCs in trafficking through the ECM barrier toward gradients of TGF-β1, IL-1β, and TNF-α, respectively, were clearly abrogated or even diminished below the level of spontaneous cell migration by the addition of Ro 206-0222, whereas the SDF-1α–stimulated invasion was only poorly attenuated in the presence of the MMP inhibitor (Figure 6). Taken together, these findings indicate that the inflammatory cytokines TGF-β1, IL-1β, and TNF-α act as strong chemoattractants for hMSCs and enable their directed traversal through basement membranes dependent on the activity of specific MMPs.
Discussion
In this study, we demonstrate that the constitutive expression of MMP-2, MT1-MMP, and TIMP-2 but not TIMP-1 essentially contribute to the ability of bone marrow–derived hMSCs to traverse human reconstituted basement membranes. Moreover, this is the first report providing evidence that the inflammatory cytokines TGF-β1, IL-1β, and TNF-α rather than the chemokine SDF-1α chemoattract hMSCs and augment their invasive capacity by up-regulation/induction of MMP-2, MT1-MMP, and/or MMP-9 activity in these cells.
To play a decisive role in tissue repair and regeneration processes, endogenous hMSCs need to egress from bone marrow into the blood circulation with subsequent extravasation into target tissues.42 This concept is supported by the presence of hMSCs in peripheral blood and multiple tissue types.2,16,42 Moreover, recent work has shown that hMSCs intravenously transfused into mice interact with blood vessel endothelium by mechanisms similar to that of mononuclear cells or CD34+ hematopoetic progenitors during their extravasation.43 From our data, it can be assumed that hMSCs are capable of fulfilling the transmigration of the subendothelial basement membranes as a crucial step in the course of extravasation. Bone marrow–derived hMSCs were shown by us to use constitutively expressed MMP-2 and MT1-MMP activity to surmount reconstituted basement membranes in vitro. In addition, despite its MMP-2–inhibiting capacity TIMP-2 was elucidated to be indispensable for hMSC invasion. These findings are in accordance with the current understanding of MMP-2 proenzyme activation requiring both MT1-MMP and TIMP-2. Briefly, following secretion from the cells proMMP-2 and TIMP-2 reassociate with the plasma membrane by building a trimolecular complex with surface-tethered MT1-MMP. Thereafter, MT1-MMP performs N-terminal cleavage of proMMP-2 giving rise to an intermediate active form which is further converted into fully active MMP-2.44,45 The active forms of MMP-2 remain either bound or may dissociate from the cell surface46,47 as we have ascertained for the latter in hMSC culture supernatants. The role of TIMP-1 turned out to be completely different from that of TIMP-2, because TIMP-1 was not supporting hMSC invasion but rather impeded this process most probably by inhibiting MMP activity. Consistent with our results, MMP-2 mRNA expression has been previously detected in hMSCs isolated from bone marrow and umbilical cord blood.32,48,49 Others had shown a cytoplasmatic localization of MMP-2 in hMSCs and its regulation by cancer-associated genes.50 Although the investigators suspected the enzyme to be implicated in hMSC migration, they did not provide direct evidence for this assumption. Recent studies by Son et al32 had indicated the involvement of MT1-MMP in hMSC transmigration across Matrigel as deduced from experiments using an MMP inhibitor isolated from green tea. Thus, the results from our detailed analysis together with data from other investigators clearly indicate the fundamental importance of MMP-2, MT1-MMP, and TIMP-2 for hMSC invasion through ECM barriers such as basement membranes. Moreover, hMSCs differentiated into osteoblasts were shown to express several MMPs, including MMP-2 and MT1-MMP, suggesting involvement of these MMPs in the resorption and formation of bone.51
On the basis of our working hypothesis that signaling molecules overexpressed in damaged or inflamed tissues might trigger chemotactic migration of hMSCs in vivo, we next studied the influence of distinctive cytokines/chemokine on the MMP-dependent invasion capacity of these cells in vitro. TGF-β1 has been reported to be produced at elevated levels in wounds where it exhibits various functions, including the stimulation of fibroblast and leukocyte migration.30 Our result on TGF-β1–induced up-regulation of MMP-2 in hMSCs is in agreement with similar findings in other cell types such as tumor cells and keratinocytes.26,29 In contrast, MT1-MMP expression is normally under minor influence of cytokines/growth factors.52 However, the TGF-β1 induction of MT1-MMP expression in hMSCs may be explained by a unique cross-talk mechanism between the TGF-β1 and Wnt signaling pathways recently described to initiate TGF-β1–evoked effects on hMSC proliferation and differentiation via accumulation of nuclear β-catenin.53 On the basis of this finding and our recent results showing that gene expression of MT1-MMP is controlled by nuclear β-catenin levels in hMSCs,31 we postulate that the TGF-β1–induced up-regulation of MT1-MMP in hMSCs may be mediated via β-catenin. Because TGF-β1 did not considerably influence TIMP-1/-2 expression in hMSCs, its stimulatory effect on hMSC trafficking through human ECM appears to be mainly facilitated by induction of MMP-2 and MT1-MMP as we have demonstrated by the use of a specific inhibitor against these MMPs.
Furthermore, the proinflammatory and chemotactic cytokines IL-1β and TNF-α are highly expressed in wounds and inflamed tissues playing multiple roles during the early phase of healing and repair.30 Both cytokines have also been reported to influence the expression of MMP-9 and other MMPs or TIMPs in different cell types.28,29,54,55 Here, we describe for the first time that hMSCs respond with high sensitivity to IL-1β and TNF-α signaling by early and strong up-regulation of MMP-9 as well as MT1-MMP transcription in these cells. Very recent research has revealed multifunctional roles of MT1-MMP in modifying the pericellular microenvironment which are based not only on activating proMMP-2 at the cell surface but also on its ability to directly degrade ECM components such as fibronectin, vitronectin, laminin, and collagen type I.56 Thus, enhanced MT1-MMP activity in hMSCs may directly increase pericellular cleavage of ECM and thereby contribute to improved cell invasion. Moreover, MT1-MMP has been shown to be essential in monocytic transmigration through TNF-α–activated endothelium,57 demonstrating the importance of this enzyme in cellular extravasation. Despite the fact that TNF-α dramatically up-regulated the extremely low basal level of MMP-9 mRNA expression in hMSCs, a little amount of enzyme was secreted within 3 days of incubation, suggesting MMP-9 to play a minor role in TNF-α–promoted invasion of these cells. In addition, MMP-2 expression was decreased under the influence of this cytokine. Nevertheless, the early and simultaneous increase of MT1-MMP and MMP-9 might be efficient in facilitating chemoattractive invasion of hMSCs toward a gradient of TNF-α produced in inflamed tissues. The striking differences in MMP-2 and MMP-9 expression observed in hMSCs are likely to be a consequence of the distinct promoter structures of both genes and are in agreement with data reported from other cell types of mesodermal origin such as fibroblasts and endothelial cells which constitutively secrete MMP-2 but synthesize MMP-9 only on stimulation.23,26
The chemokine SDF-1α and its receptor CXCR4 control migration, growth, and differentiation of various cell types, including hematopoetic CD34+ stem cells. Modulations in SDF-1/CXCR4 levels contribute to the recruitment of leukocytes from bone marrow to injured and inflamed tissues.58,59 In hMSCs, SDF-1α stimulated chemotactic migration across human ECM, although its influence was relatively weak and barely dependent on MMP activity. Limited cellular response toward SDF-1α may be explained by the fact that only a small subpopulation of hMSCs have been shown to express functional CXCR4 receptors.60 Another reason may be potential cleavage and inactivation of SDF-1α by MMP-2 and MT1-MMP constitutively expressed in hMSCs, similar to a mechanism demonstrated in CD34+ cells.61 Very recently, Son et al32 have shown that SDF-1α stimulates transmigration through Matrigel by bone marrow– and cord blood–derived hMSCs cultivated for up to 18 passages. The investigators provide evidence for the involvement of MT1-MMP in this process, which is confirmed by our finding that SDF-1α slightly up-regulated the activity of MT1-MMP in bone marrow–derived hMSCs. In addition, we could demonstrate that exposure to SDF-1α decreased transcription and release of MMP-2 and also reduced the mRNA levels of TIMP-1 and TIMP-2 in these cells, indicating differential regulatory influence of SDF-1α on MMP/TIMP expression in hMSCs.
Taken together, our results demonstrate that hMSCs are capable of migrating through human reconstituted basement membranes. For this purpose, hMSCs use constitutively expressed MMP-2, MT1-MMP, and TIMP-2. The essential contribution of these gene products to hMSC invasion was clearly demonstrated by the use of synthetic MMP inhibitors and a detailed RNAi approach. In addition, the inflammatory cytokines TGF-β1, IL-1β, and TNF-α rather than the chemokine SDF-1α exhibit significant chemoattractive potential on hMSCs and induce cellular trafficking across human ECM barriers via up-regulation of MMP-2, MT1-MMP, and/or MMP-9. Thus, hMSCs fulfill essential requirements for homing, extravasation, and migration into tissues in response to inflammatory stimuli. Our data may be of fundamental relevance in clinical applications such as the mobilization of endogenous hMSCs from bone marrow into blood and to sites of injury by cytokine administration, or the treatment of ex vivo–expanded hMSCs with cytokines to increase their migration/homing potential after transplantation into patients.
Authorship
Contribution: C.R. designed the research, analyzed the data, and wrote the manuscript; V.E. contributed to the research design, performed the research, and analyzed the data; M.K. performed the research; H.K., M.J., and P.N. supervised the work, controlled and analyzed the data; all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
C.R. and V.E. contributed equally to this study.
Correspondence: Christian Ries, Abteilung für Klinische Chemie und Klinische Biochemie, Chirurgische Klinik der LMU München, Nussbaumstrasse 20, 80336 München, Germany; e-mail: christian.ries@med.uni-muenchen.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr H.W. Krell (Roche Diagnostics GmbH, Pharma Research Penzberg, Germany) for generously providing synthetic MMP inhibitors and Thomas Pitsch for his valuable technical assistance. V.E. is PhD candidate at the Technical University of Munich and this work is submitted in partial fulfillment of the requirement for the PhD. This article is dedicated to Professor Marianne Jochum on the occasion of her 60th birthday.
This work was supported by the Wilhelm Sander-Stiftung (grant 2002.122.1) and the German Federal Ministry of Defense (contract M/SAB1/5/A001).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal