Abstract
Reactive oxygen species are known to be involved in several cellular processes, including cell signaling. SOD2 is a key enzyme in the conversion of reactive oxygen species and has been implicated in a host of disease states, including cancer. Using an integrated, whole-cell approach encompassing epigenetics, genomics, and proteomics, we have defined the role of SOD2 in multiple myeloma. We show that the SOD2 promoter is methylated in several cell lines and there is a correlative decrease in expression. Furthermore, myeloma patient samples have decreased SOD2 expression compared with healthy donors. Overexpression of SOD2 results in decreased proliferation and altered sensitivity to 2-methoxyestradiol–induced DNA damage and apoptosis. Genomic profiling revealed regulation of 65 genes, including genes involved in tumorigenesis, and proteomic analysis identified activation of the JAK/STAT pathway. Analysis of nearly 400 activated transcription factors identified 31 transcription factors with altered DNA binding activity, including XBP1, NFAT, forkhead, and GAS binding sites. Integration of data from our gestalt molecular analysis has defined a role for SOD2 in cellular proliferation, JAK/STAT signaling, and regulation of several transcription factors.
Introduction
Oxygen-free radicals, including the highly reactive superoxide radical (O2·−), called reactive oxygen species (ROS), have been of increasing interest in cancer research in the past 2 decades.1,2 ROS are formed in the cell by many normal processes including (1) generation in microsomes and peroxisomes; (2) during cytochrome P450 metabolism; and, most significantly, (3) in the mitochondria by electron transport reactions.3 Considered primary ROS, O2·− generated through metabolic processes or by physical irradiation reacts further to generate secondary ROS. The effects of ROS can be both harmful and beneficial. At low levels, ROS can induce a mitogenic effect4 and participate in cellular signaling pathways.5 High concentrations of ROS leads to damaged proteins, lipids, and both nuclear and mitochondrial DNA.2 These harmful effects of ROS are protected against by both enzymatic and nonenzymatic antioxidants.1
Enzymatic antioxidants include superoxide dismutases (SODs), catalses, and glutathione peroxidases. The SOD family includes cytoplasmic SOD1 (Cu, Zn-SOD), mitochondrial SOD2 (Mn-SOD), and extracellular SOD3 (Ec-SOD). SODs convert the O2·– to hydrogen peroxide and molecular oxygen. Hydrogen peroxide, also deleterious to the cell, is further converted to water and molecular oxygen by catalases and glutathione peroxidases. Mitochondria produce most superoxide radicals, generating 2 to 3 nmol of superoxide per minute per milligram of protein.6 Since O2·− can lead to cellular damage, the greatest need for the depletion of superoxide is in the mitochondria.
Altered ROS levels are implicated in a wide variety of disease states, including arteriosclerosis, arthritis, neurodegenerative disorders such as Alzheimer and Parkinson disease, and cancer (reviewed in Gutteridge and Halliwell1 , Das,2 and Valko et al7 ). More specifically, altered SOD2 activity has been reported in the aforementioned diseases. In pancreatic cancer, the SOD2 overexpression leads to decreased growth, anchorage-independent growth, and tumor growth in nude mice,8 hinting at a tumor suppressor role for SOD2. Furthermore, there is loss of SOD2 heterozygocity in both melanoma and glioma, and mice lacking SOD2 die neonatally of cardiomyopathy, increased lipid accumulation in the liver, and metabolic acidosis.9 Van Rummen et al showed a 2-fold increase in tumor formation at age 26 to 28 months in SOD2+/− mice (approximately 50% reduced activity) compared with wild-type (WT) mice.10 In particular, there is an increase in the tumors typical of old C57BL/6 mice with a 3-fold increase in lymphomas, indicating an important tumor suppressor function for SOD2 in B cells.
Multiple myeloma (MM) is a neoplasm of terminally differentiated B cells, plasma cells, that exhibit extensive genomic instability contributing to the progression of the disease.11 Genomic instability occurs in most neoplasms and has been shown to be the result of several inappropriate tumor-associated mechanisms, including the formation of ROS.12–14 In MM, a role for ROS has been suggested but not fully investigated. Small cohorts of patients have been reported to have increased lipid peroxidation,15,16 an indication of increased ROS. Given the increase in B-cell tumor formation in SOD2-deficient mice, the role of ROS in genetic instability, and our previous report of SOD2 promoter methylation in KAS-6/1,17 we investigated the molecular consequences of altered SOD2 expression in MM.
We show that SOD2 is methylated in several MM cell lines, and that this is correlated with expression levels. We also show that SOD2 expression is decreased in patients with myeloma compared with healthy donors. Furthermore, we show that decreased SOD2 expression has important physiologic consequences for the cells. First, it renders the cells sensitive to compounds that induce an oxidative burst such as 2-methoxyestradiol (2ME2). Second, it leads to increased proliferation and a host of molecular changes such as perturbations in gene expression, changes in cellular signaling pathways such as the JAK/STAT pathway, and altered activity of several transcription factors including NFAT, forkhead proteins, and XBP1. This is the first paper to systematically determine, through the use of genomics, proteomics, and profiling of activated transcription factors, the effects of the loss of SOD2 enzymatic activity.
Materials and methods
Genomic DNA isolation, bisulfite modification, and PCR amplification
Genomic DNA was isolated using Qiagen's DNA isolation kit (QIAGEN, Valencia, CA). Sodium bisulfite sequencing was preformed as previously described.17 Details can be found in Document S1, available on the Blood website (see the Supplemental Materials link at the top of the online article).
Quantitation of SOD2 mRNA by quantitative reverse transcription–PCR
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed with SuperScript III (Invitrogen, Carlsbad, CA) and an oligo-dT primer. cDNA (100 ng) was used in the real-time polymerase chain reaction (PCR) containing Universal Master Mix (Applied Biosystems, Foster City, CA) and gene specific Assays-on-Demand mix for 18S (Hs99999901_s1; Applied Biosystems) or for SOD2 (Hs00167309_m1). Each assay was performed in triplicate and each cell line was normalized to the measurement obtained for the respective 18S ribosomal RNA.
Construction of expression and shRNA SOD2 retroviruses
Adenoviral SOD2 construct (kind gift of J. J. Cullen, University of Iowa, Iowa City) was EcoRI digested, and SOD2 cDNA was cloned into the retroviral vector pLZRS-BMN-EGFP containing an internal ribosome entry site (IRES) and enhanced green fluorescent protein (eGFP) cDNA. The shRNA oligos used for knock-down of SOD2 are as follows: 5′-GATCCCGGGGTTGGCTTGGTTTCAATATTCAAGAGATATTGAAACCAAGCCAACCCCTTTTTA-3′ and 5′-AGCTTAAAAAGGGGTTGGCTTGGTTTCAATATCTCTTGAATATTGAAACCAAGCCAACCCCGG-3′. The oligos were annealed and cloned into the BglII/HindIII sites of pRetroSuper.18
Generation of cell lines with altered SOD2 expression levels
The Phoenix Amphotropic cells (kind gift of Gary Nolan, Stanford University, Stanford, CA) were transfected using FuGENE 6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Following transfection (2 days), 3 mL of viral supernatant was used to infect 1 to 3 × 106 myeloma cells. For overexpression of SOD2, cells were flow-sorted for high GFP expression (≥ 98% pure). For knock-down of SOD2, cells were selected with 1 μg/mL puromycin 48 hours after infection. In all cases, an empty vector was independently infected, selected as indicated for SOD2 constructs, and used as the control. Expression of SOD2 was determined by Western blot (anti-SOD2, ab16954; Abcam, Cambridge, MA).
Determination of superoxide dismutase activity in cell lines
Superoxide dismutase activity was measured using Calbiochem's Superoxide Dismutase Assay Kit II according to the manufacturer's directions (Calbiochem, San Diego, CA). Briefly, 2 × 106 cells were lysed by sonication in 20 mM HEPES buffer containing 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose.
Lysate (10 μL) was mixed with 200 μL of radical detector (tetrazolium salt) containing 1 mM potassium cyanide to inhibit Cu/Zn-SOD and Fe-SOD and 20 μL xanthine oxidase. The reaction was incubated at room temperature for 20 minutes and read in a Genios (Tecan, San Jose, CA) plate reader at 450 nM. One unit of SOD activity is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
Measurement of proliferation by tritiated thymidine uptake and MTT assay
Cells were seeded in triplicate at 2 × 104 per well in a 96-well plate. The MTT assay was performed in accordance with the manufacturer's instructions (Chemicon, Temecula, CA) (Document S1). For 3H-thymidine incorporation, cells were incubated with 0.5 μCi (0.0185 MBq) of 3H-thymidine and incubated at 37°C for 4 hours, after which cells were harvested and read by scintillation counting.
2ME2 treatment and comet assay
Cells (106) were treated with 3 μM 2ME2 for 48 hours. DNA damage was assessed using the CometAssay kit (Trevigen, Gaithersburg, MD). Document S1 contains a brief description of the procedure. Comet Assays were visualized and photographed using an Olympus IX70 microscope (Olympus, Center Valley, PA) at 20×/10× magnification.
Oligonucleotide expression microarrays
Oligonucleotide microarrays were prepared by the National Cancer Institute's microarray core facility using Operon's Human Version 3.0 oligo set (Operon Biotechnologies, Huntsville, AL) printed on UltraGAPS slides (Corning, Corning, NY). This set contains 34 580 oligos, representing approximately 25 000 unique genes. Labeling, hybridization, and scanning were performed as described in Document S1.
RPAs
Protein lysates were prepared for printing on reverse-phase protein arrays (RPAs) by lysis of 10 000 cells in T-PER (Pierce, Rockford, IL). Serial dilutions (5 2-fold) were made from each lysate. RPAs were printed as previously described19 and briefly described in Document S1.
DNA/protein arrays
Panomics DNA/protein combo arrays containing 345 consensus-binding sites were hybridized according to the manufacturer's instructions (Panomics, Fremont, CA). Briefly, nuclear protein was extracted from OPM-2 containing (1) an empty vector, (2) a SOD2 overexpression vector, and (3) short hairpin RNA–targeting SOD2 (shSOD2) using Panomics' Nuclear Protein extraction kit following the manufacturer's instructions. Protein lysates (15 μg of each) was used in the binding reaction. Following cleanup, the probe was hybridized at 45°C overnight. Data were quantitated using ImageQuant (Amersham Biosciences, Arlington Heights, IL), and a binding site was considered to be regulated only if it showed reciprocal regulation in the overexpression and the shRNA cell lines.
Results
SOD2 is methylated in several MM cell lines
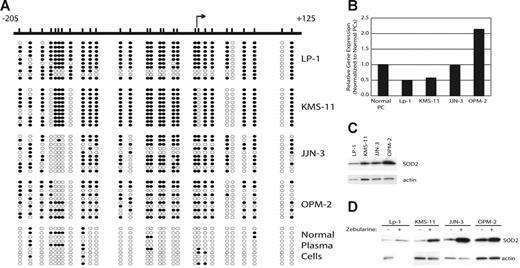
Previously, we reported that the SOD2 promoter is methylated in the MM cell lines KAS6/1 and IM-9. To ascertain the prevalence of SOD2 promoter methylation in MM, we determined the methylation density by sodium bisulfite sequencing of the SOD2 promoter in 4 additional cell lines. SOD2 is methylated in 66.7% (4 of 6, including KAS6/1 and IM-9) of MM cell lines, including KMS-11 and LP-1 (77% and 68% methylated, respectively), as shown in this report (Figure 1A). In both JJN-3 and OPM-2 the overall methylation density is much lower (48% and 52%, respectively), and there is a region from −157 to −139 that is unmethylated in nearly all clones sequenced (Figure 1A). Both mRNA (Figure 1B) and protein (Figure 1C) expression correlates with the density of methylation for these cells lines. Furthermore, expression of SOD2 could be increased by treatment with the demethylating agent zebularine (Figure 1D).
The SOD2 promoter is methylated in MM and correlates with decreased expression of SOD2. (A) The SOD2 promoter from −205 to +125, relative to the transcriptional start site, was screened by sodium bisulfite sequencing to determine methylated cytosines. The relative positions of the methylated cytosines are indicated by slash marks. Sequncing results from 10 clones are shown where • represents a methylated cytosine, whereas ○ represents an unmethylated cytosine. (B) The expression level of SOD2, as measured by DNA microarrays, is shown normalized to the expression of SOD2 in normal plasma cells. (C) Western blot showing expression of SOD2 in myeloma cell lines. Actin serves as the loading control. (D) Expression levels of SOD2 in cell lines treated with the demethylating agent zebularine. Actin serves as the loading control.
The SOD2 promoter is methylated in MM and correlates with decreased expression of SOD2. (A) The SOD2 promoter from −205 to +125, relative to the transcriptional start site, was screened by sodium bisulfite sequencing to determine methylated cytosines. The relative positions of the methylated cytosines are indicated by slash marks. Sequncing results from 10 clones are shown where • represents a methylated cytosine, whereas ○ represents an unmethylated cytosine. (B) The expression level of SOD2, as measured by DNA microarrays, is shown normalized to the expression of SOD2 in normal plasma cells. (C) Western blot showing expression of SOD2 in myeloma cell lines. Actin serves as the loading control. (D) Expression levels of SOD2 in cell lines treated with the demethylating agent zebularine. Actin serves as the loading control.
SOD2 expression is decreased in myeloma patient samples
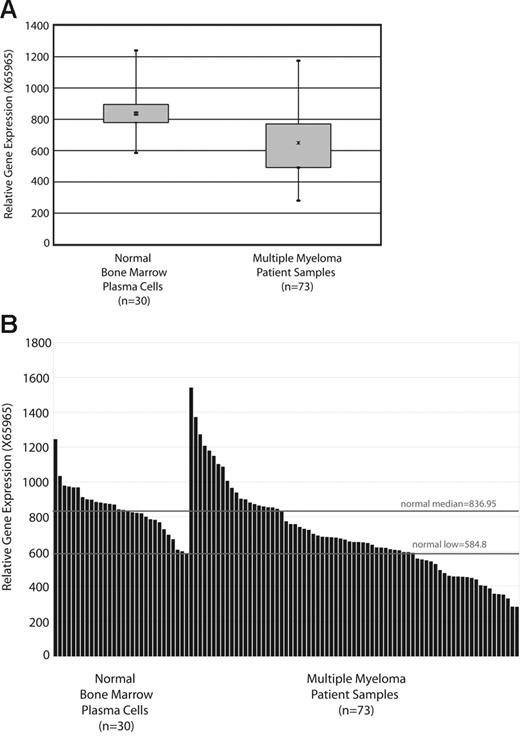
To determine if SOD2 is down-regulated in patients with myeloma, we surveyed the most extensive microarray data on myleoma patient samples collected to date, by Zhan et al20 (GSE755; Gene Expression Omnibus database21 ). Figure 2A shows a box plot of SOD2 expression mined from this database. There is a statistically significant (P < .001) decrease in SOD2 expression between patients with myeloma and healthy donors, although the range of patients with myeloma does encompass the normal range. The individual expression values (for element X65965; SOD2) obtained for each sample is shown in Figure 2B. One-third of patients have SOD2 expression levels below the lowest value obtained for healthy donors, and two-thirds of patients have an expression value below the median of the healthy donors.
Patients with myeloma have lowered SOD2 expression compared with that of healthy donors. (A) A box plot of SOD2 expression in samples from patients with myeloma and healthy donors. The SOD2 data (Affymetrix microarray element X65965; Santa Clara, CA) is from the database available at http://www.myeloma.uams.edu/lambertlab/software.asp (datasets “Affymetrix HuGeneFL*.txt for 74 newly diagnosed multiple myelomas” and “Affymetrix HuGeneFL*.txt for 31 normal bone marrow–derived plasma cells”).20 (B) The individual expression levels for each sample are plotted, and lines indicate the median and lowest SOD2 expression level of the healthy donor samples. The difference in SOD2 expression in statistically significant (P < .001).
Patients with myeloma have lowered SOD2 expression compared with that of healthy donors. (A) A box plot of SOD2 expression in samples from patients with myeloma and healthy donors. The SOD2 data (Affymetrix microarray element X65965; Santa Clara, CA) is from the database available at http://www.myeloma.uams.edu/lambertlab/software.asp (datasets “Affymetrix HuGeneFL*.txt for 74 newly diagnosed multiple myelomas” and “Affymetrix HuGeneFL*.txt for 31 normal bone marrow–derived plasma cells”).20 (B) The individual expression levels for each sample are plotted, and lines indicate the median and lowest SOD2 expression level of the healthy donor samples. The difference in SOD2 expression in statistically significant (P < .001).
Alteration of SOD2 expression and enzymatic activity
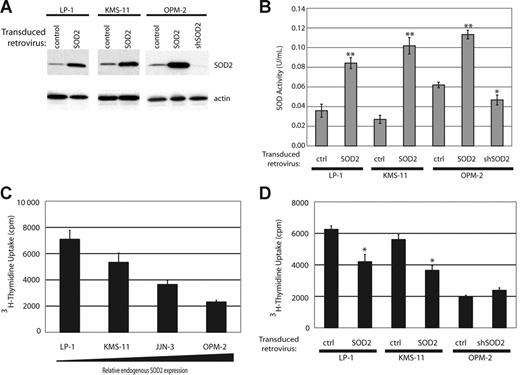
To determine the consequences of SOD2 expression, we engineered cell lines with either overexpressed SOD2 or knocked-down SOD2 expression. We overexpressed full-length SOD2 using a bicistronic retroviral construct expressing SOD2 and eGFP in cell lines that showed low protein expression, KMS-11 and LP-1, as well as in OPM-2. Following infection, all cell lines were sorted for eGFP expression and were more than 98% pure (data not shown). We knocked down SOD2 in OPM-2, the MM cell line tested with the highest expression of SOD2, by using a retroviral construct containing shSOD2 and the puromycin resistance gene. The introduction of these constructs lead to the expected change in SOD2 expression (Figure 3A), with a nearly complete abrogation of SOD2 expression in the OPM2-shSOD2 cell line. We also determined the enzymatic activity of SOD2 in our engineered cell lines by assaying for superoxide dismutase activity in the presence of 1 mM potassium cyanide, which selectively inhibits SOD1 and SOD3 but not SOD2. Figure 3B shows increased dismutase activity in the lysates from cell lines overexpressing SOD2 and a decrease in activity in the OPM2-shSOD2 lysate.
Generation of cell lines with altered SOD2 expression and enzymatic activity results in changes of proliferation rates. (A) Western blot showing SOD2 expression in cell lines with SOD2 overexpression (LP-1, KMS-11, and OPM-2) and in OPM-2 infected with an shRNA directed against SOD2. Actin serves as the loading control. (B) Measurement of SOD enzymatic activity in the engineered cell lines. The experiment was preformed in triplicate with standard error shown. *P < .05; **P < .005 as determined by Student t test. (C) Proliferation rates of the myeloma cell lines were measured by tritiated thymidine incorporation. There is an inverse correlation with proliferation rates and SOD2 expression. The experiment was preformed in triplicate and standard error is shown. (D) Proliferation rates in the altered cell lines were determined by thymidine incorporation. The experiment was preformed in triplicate and standard error is shown. *P < .005.
Generation of cell lines with altered SOD2 expression and enzymatic activity results in changes of proliferation rates. (A) Western blot showing SOD2 expression in cell lines with SOD2 overexpression (LP-1, KMS-11, and OPM-2) and in OPM-2 infected with an shRNA directed against SOD2. Actin serves as the loading control. (B) Measurement of SOD enzymatic activity in the engineered cell lines. The experiment was preformed in triplicate with standard error shown. *P < .05; **P < .005 as determined by Student t test. (C) Proliferation rates of the myeloma cell lines were measured by tritiated thymidine incorporation. There is an inverse correlation with proliferation rates and SOD2 expression. The experiment was preformed in triplicate and standard error is shown. (D) Proliferation rates in the altered cell lines were determined by thymidine incorporation. The experiment was preformed in triplicate and standard error is shown. *P < .005.
Expression of SOD2 leads to a decrease in proliferation. Analysis of the proliferation rates of the various MM cell lines showed that there was an inverse correlation with the expression level of SOD2 and proliferation rates, as measured by tritiated thymidine uptake (Figure 3C). To more definitively show that an increase in SOD2 expression leads to decreased proliferation in MM cells, we tested proliferation rates of our SOD2-engineered cell lines. When overexpressed, SOD2 leads to an approximate 50% reduction in proliferation rates in both LP-1 and KMS-11 (Figure 3D). This decrease in proliferation is consistent with several other reports that demonstrate slowed proliferation in response to enforced SOD2 expression. Knock-down of SOD2 in OPM-2 did not significantly increase proliferation of these cells (Figure 3D).
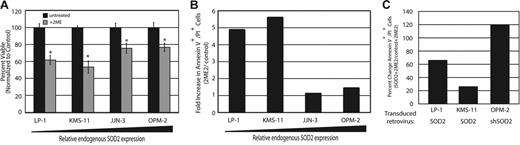
Treatment with 2ME2 induces DNA damage and apoptosis in SOD2-deficient myeloma cells. Since it has been shown that 2ME2 induces apoptosis through the accumulation of ROS,22 we sought to determine if SOD2 expression itself was predictive of response to 2ME2. Treatment of MM cell lines with 2ME2 resulted in a greater decrease in survival and/or proliferation of MM cell lines with low endogenous SOD2 expression as measured by MTT assay (Figure 4A). Inclusion of the free radical scavenger N-acetyl-cysteine (NAC) was able to reverse the effects of 2ME2, indicating that 2ME2 exerted its effect, at least in part, through free radical generation (data not shown). Annexin V and propidium iodide staining of 2ME2-treated cells showed that these cells were undergoing apoptosis (Figure 4B). Furthermore, overexpression of SOD2 protected both LP-1 and KMS-11 cells, whereas knock-down of SOD2 sensitized OPM-2 cells to 2ME2-induced apoptosis, as measured by Annexin V and propidium iodide staining (Figure 4C).
Treatment with 2ME2 leads to growth arrest and apoptosis in myeloma cell lines with low endogenous SOD2 that can be reversed by enforced expression of SOD2. (A) An MTT assay was preformed to determine proliferation of cell lines either untreated (▪) or treated (⊡) with 30 μM 2ME2 for 72 hours. The assay was performed in triplicate, and standard error is shown. *P < .005. This is a representative experiment from 2 experiments performed. (B) The amount of apoptosis in the cell lines following treatment with 2ME2 was determined by flow cytometry. The fold increase in apoptotic cells (treated cells vs untreated cells) is shown. (C) Percentage of apoptosis in cell lines with altered SOD2 expression following treatement with 2ME2 was determined by flow cytometric measurement of Annexin V+/PI+ cells. The percentage change in apoptotic cells relative to control cells treated with 2ME2 is shown.
Treatment with 2ME2 leads to growth arrest and apoptosis in myeloma cell lines with low endogenous SOD2 that can be reversed by enforced expression of SOD2. (A) An MTT assay was preformed to determine proliferation of cell lines either untreated (▪) or treated (⊡) with 30 μM 2ME2 for 72 hours. The assay was performed in triplicate, and standard error is shown. *P < .005. This is a representative experiment from 2 experiments performed. (B) The amount of apoptosis in the cell lines following treatment with 2ME2 was determined by flow cytometry. The fold increase in apoptotic cells (treated cells vs untreated cells) is shown. (C) Percentage of apoptosis in cell lines with altered SOD2 expression following treatement with 2ME2 was determined by flow cytometric measurement of Annexin V+/PI+ cells. The percentage change in apoptotic cells relative to control cells treated with 2ME2 is shown.
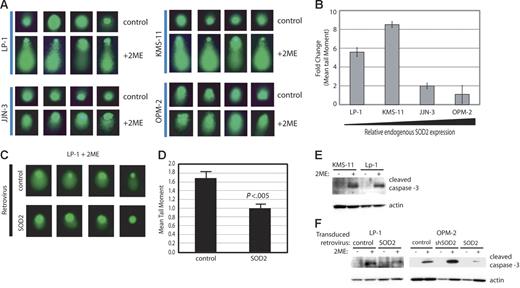
While ROS can lead to DNA damage and 2ME2 leads to ROS formation, the effect of 2ME2 on inducing DNA damage has not been established. To determine if the mechanism of 2ME2 includes DNA damage, we performed comet assays on cell lines with differing levels of SOD2 expression and responses to 2ME2 (Figure 5A-B). There is extensive tailing in both LP-1 and KMS-11, the 2 cell lines with the lowest levels of SOD2 (Figure 5A). The tailing in both JJN-3 and OPM-2 were dramatically less than in LP-1 and KMS-11. Furthermore, the extent of DNA damage induced by 2ME2 was inversely correlated with SOD2 expression levels (Figure 5B). To directly determine if SOD2 expression alone could ameliorate the damage induced by 2ME2, we performed the comet assay on LP-1 cells overexpressing SOD2. In LP-1, the overexpression of SOD2 significantly reduced the length of the comet tail (Figure 5C-D). Since ROS can also result in activation of the caspase pathway, we also determined the levels of cleaved caspase-3 following treatment with 2ME2. Both KMS-11 and LP-1 showed a large increase in the level of cleaved caspase-3 in response to treatment with 2ME2 (Figure 5E). Overexpression of SOD2 in both LP-1 and OPM-2 showed a significant decrease in the levels of cleaved caspase-3 following treatment with 2ME2, whereas knock-down of SOD2 in OPM-2 showed a corresponding increase (Figure 5F).
Treatment with 2ME2 results in DNA damage and cleavage of caspase-3, the extent of which is indirectly proportional to SOD2 expression. (A) DNA damage following treatment with 2ME2 was determined by comet assay. Four representative nuclei are shown. (B) Quantitation of the tail moments from the comet assay. The fold change in mean tail moments from 2ME-treated versus untreated cells is shown. For each treatment, 50 tails were measured. Error bars depict standard deviation. (C) DNA damage in cell lines with altered SOD2 was determined by comet assay. Four representative nuclei are shown. (D) Quantitation of the tail moments of the comet assay. A total of 50 tails were measured, and the mean tail moment is shown. Error bars depict standard deviation. (E) Western blot for cleaved caspase-3 in myeloma cell lines following treatment with 2ME2. Actin serves as the loading control. (F) Western blot for cleaved caspase-3 in myeloma cell lines with altered SOD2 following treatment with 2ME2. Actin serves as the loading control.
Treatment with 2ME2 results in DNA damage and cleavage of caspase-3, the extent of which is indirectly proportional to SOD2 expression. (A) DNA damage following treatment with 2ME2 was determined by comet assay. Four representative nuclei are shown. (B) Quantitation of the tail moments from the comet assay. The fold change in mean tail moments from 2ME-treated versus untreated cells is shown. For each treatment, 50 tails were measured. Error bars depict standard deviation. (C) DNA damage in cell lines with altered SOD2 was determined by comet assay. Four representative nuclei are shown. (D) Quantitation of the tail moments of the comet assay. A total of 50 tails were measured, and the mean tail moment is shown. Error bars depict standard deviation. (E) Western blot for cleaved caspase-3 in myeloma cell lines following treatment with 2ME2. Actin serves as the loading control. (F) Western blot for cleaved caspase-3 in myeloma cell lines with altered SOD2 following treatment with 2ME2. Actin serves as the loading control.
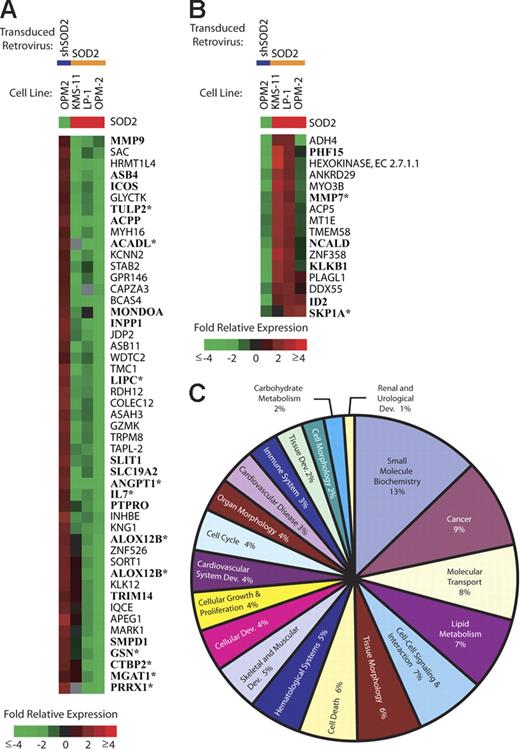
SOD2 expression leads to gene expression changes. In order to determine if SOD2 expression and enzymatic activity lead to alterations in gene expression, we preformed microarray analysis on our panel of MM cell lines with altered SOD2 levels. Figure 6A shows all named genes (65 genes) that have decreased expression levels (≥ 2-fold) in at least 2 of 3 overexpression experiments and a reciprocal increase in expression in the knock-down experiment. Among the genes decreased by SOD2 overexpression are genes involved in proliferation (ICOS, IL-7, and ID2), cell-cycle regulation (ANGPT1 and PTPRO), lipid metabolism (ASAH3, KNG1, SMPD1, and LIPC), and cell-cell interaction/signaling (GSN, KNG1, MMP9, and MMP7). Figure 6B shows all genes with increased expression levels (same criteria as in Figure 6A) with SOD2 overexpression. The up-regulated genes include several genes of unknown function as well as genes involved in diverse cellular processes. PLAGL1 has been shown to be involved in cell-cycle arrest and apoptosis. KLK1B and MMP7 are involved in adhesion and aggregation. We performed a meta-analysis of publicly available MM patient microarray data (GSE755; Gene Expression Omnibus database21 ). There were 28 genes present on the analyzed arrays, and 13 (46%) showed a correlative expression pattern (Figure 6A-B; asterisks).
Gene expression changes in cell lines with manipulated SOD2 levels. SOD2 levels in myeloma cell lines were manipulated by a retroviral transduction of either SOD2 or a shRNA directed against SOD2 (shSOD2). Gene expression changes are shown by the color bar where red represents an increase, green represents a decrease, and black represents no change. Gray indicates missing data. (A) All named genes showing a decrease (≤ 2-fold in 2 of 3 experiments) in expression following transduction with SOD2 and a corresponding increase in OPM-2 transduced with shSOD2 are shown. Boldfaced gene names were identified in publicly available MM patient gene expression databases (GSE755; Gene Expression Omnibus database21 ). *Genes that have a similar pattern of expression in the MM patient databases. (B) All named genes showing an increase (≥ 2-fold in 2 of 3 experiments) in expression following transduction with SOD2 and a corresponding decrease in OPM-2 transduced with shSOD2. Boldfaced gene names were identified in publicly available MM patient gene expression databases (GSE755; Gene Expression Omnibus database21 ). *Genes that have a similar pattern of expression in the MM patient databases. (C) The top 20 functional categories of SOD2-regulated genes identified using Ingenuity's Pathway Assist software.
Gene expression changes in cell lines with manipulated SOD2 levels. SOD2 levels in myeloma cell lines were manipulated by a retroviral transduction of either SOD2 or a shRNA directed against SOD2 (shSOD2). Gene expression changes are shown by the color bar where red represents an increase, green represents a decrease, and black represents no change. Gray indicates missing data. (A) All named genes showing a decrease (≤ 2-fold in 2 of 3 experiments) in expression following transduction with SOD2 and a corresponding increase in OPM-2 transduced with shSOD2 are shown. Boldfaced gene names were identified in publicly available MM patient gene expression databases (GSE755; Gene Expression Omnibus database21 ). *Genes that have a similar pattern of expression in the MM patient databases. (B) All named genes showing an increase (≥ 2-fold in 2 of 3 experiments) in expression following transduction with SOD2 and a corresponding decrease in OPM-2 transduced with shSOD2. Boldfaced gene names were identified in publicly available MM patient gene expression databases (GSE755; Gene Expression Omnibus database21 ). *Genes that have a similar pattern of expression in the MM patient databases. (C) The top 20 functional categories of SOD2-regulated genes identified using Ingenuity's Pathway Assist software.
There were 242 genes with at least 2-fold regulation in the SOD2 overexpression experiments, but showed a lower level of regulation in the SOD2 shRNA experiment. In order to investigate the functions of these genes, we included all genes that showed a 2-fold or greater change in 2 of the 3 overexpression experiments in an analysis using Ingenuity's Pathway Assist software (Ingenuity, Redwood City, CA). Of the 242 genes identified on the microarrays, 101 genes where identified in the database (called focus genes). Each of these genes is assigned to a functional category. The top 20 categories, consisting of 47 genes, are represented as a pie chart (Figure 6C). Among the top categories are small-molecule biochemistry, cancer, cell-cell signaling, and interaction and cell death. Further analysis defined the networks connecting these 101 genes. They mapped to 18 different networks, which is defined by Ingenuity as the sum of the interactions a given protein has with other proteins based upon literature results (Table S1). There were 7 networks that contained 10 or more focus genes, whereas the remaining groups contained only 1 focus gene. Of these 7 networks, cancer was identified as 1 of the top cellular function in 3 networks, cell cycle and cellular growth and proliferation were each identified in 2 networks, and lipid metabolism was identified in 2 networks. Hematologic system development and function was also identified in 1 of the networks.
Identification of signaling molecules regulated by SOD2 expression using RPAs
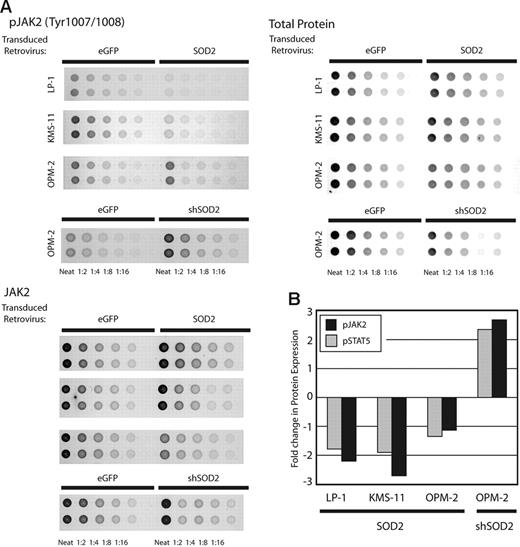
To determine signaling pathways that might be affected by SOD2 expression, we examined phosphoprotein levels using RPAs.23 We prepared lysates from the myeloma cell lines with altered SOD2 expression levels, made serial 2-fold dilutions, and printed them on slides containing a nitrocellulose pad. Following printing, each slide was incubated with a specific antibody and normalized to total protein printed. Figure 7A shows results obtained from 3 arrays using an anti-JAK2 antibody, an anti–phospho-JAK2 antibody, and colloidal gold staining for total protein. Figure 7B shows the fold change in both JAK2 and STAT5 phosphorylation normalized to total protein and total JAK2 and STAT5 expression, respectively. There is an approximately 2-fold reduction in phosphorylation of both JAK2 and STAT5 in the LP-1 and KMS-11 cell lines overexpressing SOD2 and a similar increase in JAK2 and STAT5 phosphorylation in the OPM-2 cell line with SOD2 knock-down. There is only a minimal change in JAK2 and STAT5 phoshporylation of these cell lines in OPM-2 overexpressing SOD2, which may be due to the higher endogenous expression of SOD2 in this cell line. Madamanchi et al observed a similar regulation of JAK2 and STAT3 in aortic smooth muscle cells from SOD2 heterozygous mice.4 Furthermore, there are several reports of ROS mediating the JAK-STAT pathway through increased phosphorylation in JAK2.24,25 We did not observe consistently altered levels of phosphorylated STAT3, IκB, VEGF, or src using our RPAs (data not shown).
RPAs using cell lines with manipulated SOD2 levels. The levels of phosphorylated JAK2 and STAT5 were determined using RPAs and cell lines with manipulated SOD2 expression. (A) Results of 3 arrays for the detection of phosphorylated JAK2, total JAK2, and colloidal gold staining for total protein are shown. (B) Quantitation of JAK2 and STAT5 phosphorylation. The values obtained using linear regression for phosphorylated JAK2 and STAT5 were normalized to total protein printed, which was then normalized to total JAK2 and STAT5 expression, respectively. The fold change in phosphorylated values for SOD2-manipulated lines versus the corresponding control is presented.
RPAs using cell lines with manipulated SOD2 levels. The levels of phosphorylated JAK2 and STAT5 were determined using RPAs and cell lines with manipulated SOD2 expression. (A) Results of 3 arrays for the detection of phosphorylated JAK2, total JAK2, and colloidal gold staining for total protein are shown. (B) Quantitation of JAK2 and STAT5 phosphorylation. The values obtained using linear regression for phosphorylated JAK2 and STAT5 were normalized to total protein printed, which was then normalized to total JAK2 and STAT5 expression, respectively. The fold change in phosphorylated values for SOD2-manipulated lines versus the corresponding control is presented.
SOD2 expression alters binding activity of transcription factors
Changes in redox status of cells can lead to changes in the activities of several transcription factors. Using Panomic's Protein/DNA array that measures the binding activites of 354 transcription factors, we analyzed OPM-2 with and without SOD2 overexpression and knock-down. Table 1 shows the 31 transcription factors that showed a reciprocal relationship in the overexpression and shRNA cell lines. There were 18 transcription factors identified that showed decreased DNA binding activity by SOD2 overexpression, and 13 transcription factors identified with increased DNA-binding activity by SOD2 overexpression. The transcription factors showing the highest decrease in activity with SOD2 overexpression are EGR2BP, transcription factors including STATs that bind the gamma interferon activation site (GAS), EKLF, EGFBP, c-ets, and XBP-1. The transcription factors showing the highest increase in binding with SOD2 overexpression are KPF1, Tf-LF, FoxF1, FoxL1, IRF-1, and HiNF-A.
Transcription factors with altered DNA binding in cell lines with manipulated SOD2 expression levels
| Transcription factor . | Fold change SOD2 . | Fold change shSOD2 . |
|---|---|---|
| With decreased DNA binding upon infection with SOD2 retrovirus . | ||
| EKLF | −174.13 | 1.87 |
| EGFBP | −33.25 | 2.61 |
| NF-Atp | −10.67 | 1.98 |
| c-ets-1 | −10.09 | 1.13 |
| XBP1 | −8.73 | 1.10 |
| RSRFC4 (Ras-responsive element) | −7.25 | 1.42 |
| NFIL-2 (IL-2-binding element) | −6.63 | 1.31 |
| NFATc | −4.55 | 4.66 |
| ACPBP | −4.23 | 1.68 |
| KTP1 | −3.07 | 5.50 |
| TCE | −2.79 | 1.47 |
| HiNF-B/H1 | −2.54 | 4.95 |
| WT1 | −2.38 | 1.08 |
| Isl-1 | −1.73 | 1.11 |
| PYR | −1.62 | 1.45 |
| NRF-1 | −0.28 | 8.20 |
| EGR2BP | Undetectable | 273.75 |
| GAS | Undetectable | 21.66 |
| With increased DNA-binding upon infection with SOD2 retrovirus | ||
| KPF1 | 31.28 | Undetectable |
| Tf-LF | 12.62 | −44.28 |
| Freac-2 (FoxF2) | 9.01 | Undetectable |
| Freac-7 (FoxL1) | 8.02 | −2.02 |
| IRF-1 | 4.57 | −2.33 |
| DEI (rat albumin-binding element) | 3.35 | −8.43 |
| HiNF-A | 2.50 | −10.06 |
| Ikaros | 2.32 | −1.16 |
| COUP-TF | 1.92 | −1.14 |
| NPAS2 | 1.67 | −1.01 |
| CREB2 | 1.66 | −1.36 |
| cyp1a1 | 1.49 | −1.26 |
| CdxA/NKX | 1.27 | −4.86 |
| Transcription factor . | Fold change SOD2 . | Fold change shSOD2 . |
|---|---|---|
| With decreased DNA binding upon infection with SOD2 retrovirus . | ||
| EKLF | −174.13 | 1.87 |
| EGFBP | −33.25 | 2.61 |
| NF-Atp | −10.67 | 1.98 |
| c-ets-1 | −10.09 | 1.13 |
| XBP1 | −8.73 | 1.10 |
| RSRFC4 (Ras-responsive element) | −7.25 | 1.42 |
| NFIL-2 (IL-2-binding element) | −6.63 | 1.31 |
| NFATc | −4.55 | 4.66 |
| ACPBP | −4.23 | 1.68 |
| KTP1 | −3.07 | 5.50 |
| TCE | −2.79 | 1.47 |
| HiNF-B/H1 | −2.54 | 4.95 |
| WT1 | −2.38 | 1.08 |
| Isl-1 | −1.73 | 1.11 |
| PYR | −1.62 | 1.45 |
| NRF-1 | −0.28 | 8.20 |
| EGR2BP | Undetectable | 273.75 |
| GAS | Undetectable | 21.66 |
| With increased DNA-binding upon infection with SOD2 retrovirus | ||
| KPF1 | 31.28 | Undetectable |
| Tf-LF | 12.62 | −44.28 |
| Freac-2 (FoxF2) | 9.01 | Undetectable |
| Freac-7 (FoxL1) | 8.02 | −2.02 |
| IRF-1 | 4.57 | −2.33 |
| DEI (rat albumin-binding element) | 3.35 | −8.43 |
| HiNF-A | 2.50 | −10.06 |
| Ikaros | 2.32 | −1.16 |
| COUP-TF | 1.92 | −1.14 |
| NPAS2 | 1.67 | −1.01 |
| CREB2 | 1.66 | −1.36 |
| cyp1a1 | 1.49 | −1.26 |
| CdxA/NKX | 1.27 | −4.86 |
Transcriptional binding activity of 354 transcription factors were determined by Panomic's DNA/protein array in OPM-2 transduced with either a control retrovirus, a SOD2 retrovirus or an shSOD2 retrovirus. Changes in intensity of spots were determined by densitometry. All transcription factors showing a reciprocal modulation in binding activity with the SOD2 overexpression and the shSOD2 array are shown, and their fold change respective to the control retrovirus is shown. The experiment was performed twice, and a representative experiment is shown.
Determination of binding sites for SOD2-regulated transcription factors in SOD2-regulated genes
Given the identification of both SOD2-regulated transcription factors and SOD2-regulated genes, it is possible to determine what promoters have binding sites for the regulated transcription factors. To this end, we determined which genes identified in Figure 6 have binding sites for the regulated transcription factors identified in Table 1 in their promoter regions using the Genomatix software suite (Genomatix, München, Germany). Using the Gene2Promoter database,26 we identified the promoter regions of the SOD2-regulated genes. Once the promoter regions were obtained, we analyzed these regions for promoter modules, defined as 2 or more transcription factor binding sites in a functionally defined distance range, using MatInspector (Genomatix).27 We searched for promoter modules rather than individual binding sites, since modules have increased specificity (promoter modules have less than 1 match in 10 000 base pairs) and validity (each promoter module is experimentally validated).28 Tables 2 and 3 summarize the promoter modules containing at least 1 of the transcription factors showing altered DNA binding as a result of SOD2 expression in the promoter regions of the genes down-regulated and up-regulated by SOD2 expression, respectively. Of the 49 genes, 42 are down-regulated by SOD2 (Figure 5A), and 11 of the 16 genes up-regulated by SOD2 had binding sites for the transcription factors identified in Table 1. Interestingly, the binding sites identified in SOD2 down-regulated genes are predominately for transcription factors showing a decrease in binding activity with SOD2 overexpression, such as c-ets family, GAS binding transcription factors, and NFAT. Likewise, the binding sites identified in up-regulated genes are predominately for transcription factors showing an increase in binding activity with SOD2 overexpression, such as forkhead proteins and Ikaros (Table 1). There is an almost exclusive relationship observed between down-regulated genes and transcription factors with down-regulated binding activities and between up-regulated genes and transcription factors with up-regulated binding activities.
Binding sites for transcription factors with altered binding activity in the promoters of SOD2-down-regulated genes
| Transcription factor module . | Genes containing modules . |
|---|---|
| ETS_AP1 | ALOX12B, APEG1, CTBP2, GLYCTK, INHBE, JDP2, MARK1, MONDOA, MYH16, NXF2, PRRX1, RDH12, SAC, SLC19A2, SORT1, SLIT1, STAB2, TMC, TULP2, WDTC2, ZNF526 |
| ETS_sp1 | ALOX12b, APEG1, BCAS4, CTBP2, GLCTK, SPR146, GSN, HRMT1L4, INPP1, IQCE, JDP2, KCNN2, KLK12, MOGAT1, MONDOA, NXF2, PRRX1, SLC19A2, SMPD1, TMC1, TRIM14, TPRM8, TULP2, WDTC2 |
| STAT_ETS | ACPP, GSN, KLK12, MARK1, NXF2, SMPD1, SORT1, STAB2, TMC1, TRIM14, WDTC2 |
| ETS_CEBP | APEG1, CTBP2, HRMT1L4 |
| AP1_ETS_EGRF | ALOX12B, APEG1, ASB4, GSN, JDP2, KLK12, MARK1, MOGAT1, SAC, SMPD1, WDTC2 |
| EGR_SP1 | ALOX12B, APEG1, BCAS4, COLEC12, CTBP2, GYCTK, GSN, HRMT1L4, INHBE, ANPP1, KCNN2, MONDOA, SLIT1, SMPD1, TRIM14, TRPM8, WDTC2, ZNF526 |
| EGR_NFAT | ASAH3, GSN, GZMK, HRMT1L4, ICOS, JDP2, KNG1, SAC, SLIT1, TRPM8, WDTC2 |
| CREB_NFAT_NFAT | CAPZA3, CZMK, MONDOA, TMC1 |
| EKLF_SP1 | CTBP2, JDP2 |
| Transcription factor module . | Genes containing modules . |
|---|---|
| ETS_AP1 | ALOX12B, APEG1, CTBP2, GLYCTK, INHBE, JDP2, MARK1, MONDOA, MYH16, NXF2, PRRX1, RDH12, SAC, SLC19A2, SORT1, SLIT1, STAB2, TMC, TULP2, WDTC2, ZNF526 |
| ETS_sp1 | ALOX12b, APEG1, BCAS4, CTBP2, GLCTK, SPR146, GSN, HRMT1L4, INPP1, IQCE, JDP2, KCNN2, KLK12, MOGAT1, MONDOA, NXF2, PRRX1, SLC19A2, SMPD1, TMC1, TRIM14, TPRM8, TULP2, WDTC2 |
| STAT_ETS | ACPP, GSN, KLK12, MARK1, NXF2, SMPD1, SORT1, STAB2, TMC1, TRIM14, WDTC2 |
| ETS_CEBP | APEG1, CTBP2, HRMT1L4 |
| AP1_ETS_EGRF | ALOX12B, APEG1, ASB4, GSN, JDP2, KLK12, MARK1, MOGAT1, SAC, SMPD1, WDTC2 |
| EGR_SP1 | ALOX12B, APEG1, BCAS4, COLEC12, CTBP2, GYCTK, GSN, HRMT1L4, INHBE, ANPP1, KCNN2, MONDOA, SLIT1, SMPD1, TRIM14, TRPM8, WDTC2, ZNF526 |
| EGR_NFAT | ASAH3, GSN, GZMK, HRMT1L4, ICOS, JDP2, KNG1, SAC, SLIT1, TRPM8, WDTC2 |
| CREB_NFAT_NFAT | CAPZA3, CZMK, MONDOA, TMC1 |
| EKLF_SP1 | CTBP2, JDP2 |
Using Genomatix's Gene2Promoter software, we identifed the known and predicted promoters of 42 of 49 SOD2 down-regulated genes identified in Figure 5A. Using Genomatix's ModelInspector, we searched for promoter modules contained within the promoters. This is a summarized list of the promoter modules identified. A detailed listing of the identified modules and the locations is provided in Table S2.
Transcription factor-binding modules in SOD2-up-regulated gene promoters
| Transcription factor module . | Genes containing module . |
|---|---|
| STAT_FKHD | ADH4, ANKRD29, KLKB1, MYO3B |
| IKAROS_AP2 | ANKRD29, HK1, MYO3B, NCALD, PHF15, ZNF358 |
| LEFF_SMAD | ANKRD29, DDX55, MYO3B, NCALD, PHF15, PLAGL1 |
| STAT_ETS | ANKRD29, DDX55, HK1, PLAGL1 |
| CREB_CEBP | MYO3B, NCALD, PHF15 |
| AP1_ETS | HK1, KLKB1, MYO3B |
| CREB_EBOX | HK1, MYO3B, PHF15 |
| ETS_IRF | KLKB1, MYO3B, NCALD |
| NFkB_CREB | PHF15, PLAGL1 |
| LEFF_LEFF_LEFF | HK1, MMP7 |
| STAT_IRF | ANKRD29, NCALD |
| FKHD_CEBP | ADH4, NCALD |
| STAT_GRE | DDX55, KLKB1 |
| Transcription factor module . | Genes containing module . |
|---|---|
| STAT_FKHD | ADH4, ANKRD29, KLKB1, MYO3B |
| IKAROS_AP2 | ANKRD29, HK1, MYO3B, NCALD, PHF15, ZNF358 |
| LEFF_SMAD | ANKRD29, DDX55, MYO3B, NCALD, PHF15, PLAGL1 |
| STAT_ETS | ANKRD29, DDX55, HK1, PLAGL1 |
| CREB_CEBP | MYO3B, NCALD, PHF15 |
| AP1_ETS | HK1, KLKB1, MYO3B |
| CREB_EBOX | HK1, MYO3B, PHF15 |
| ETS_IRF | KLKB1, MYO3B, NCALD |
| NFkB_CREB | PHF15, PLAGL1 |
| LEFF_LEFF_LEFF | HK1, MMP7 |
| STAT_IRF | ANKRD29, NCALD |
| FKHD_CEBP | ADH4, NCALD |
| STAT_GRE | DDX55, KLKB1 |
Using Genomatix's Gene2Promoter software, we identifed the known and predicted promoters of 11 of 16 SOD2 up-regulated genes identified in Figure 5B. Using Genomatix's ModelInspector, we searched for promoter modules contained within the promtoers. This is a summarized list of the promoter modules identified. A detailed listing of the identified modules and the locations is provided in Table S3.
Discussion
The induction of ROS induces DNA damage and has been clearly shown to be involved in the etiology of several disease states, including cancer.2,7 SOD2 is an important mediator in the clearance of potentially detrimental ROS. SOD2 itself has been implicated in the induction of many carcinomas, including increase incidences of lymphoma in heterozygous mice29 and the etiology of matrix metalloproteinase–induced breast carcinoma.13 While we show the molecular consequences of altered SOD2 expression, it is important to keep in mind that these are the changes observed in the cell as a result of the altered redox status of the cell that results from SOD2 expression.
While the expression of SOD2 has been reported to decrease in several cancers (reviewed in Oberley and Buettner30 ) and hypermethylation of the SOD2 promoter has been shown to occur in SV40-transformed cells,31 methylation of the SOD2 promoter in carcinomas has not been extensively studied. We previously demonstrated that SOD2 is methylated in the myeloma cell line KAS-6/1,17 and we show here that SOD2 is methylated in several other myeloma cell lines, which is correlated with decreased expression of SOD2 (Figure 1). Moreover, there is decreased expression of SOD2 in patients myeloma (Figure 2), but the mechanism(s) of this decrease in patient samples remains to be determined.
SOD2 has been implicated as a tumor suppressor since heterozygous mice have increased incidences of tumors.29 Furthermore, a decrease in proliferation in response to SOD2 expression has been reported for many model systems, including breast cancer, prostate cancer, and pancreatic carcinoma cell lines (reviewed in Oberley32 ). We demonstrate here, that there is an inverse correlation in the rates of myleoma proliferation and SOD2 expression (Figure 3C). Furthermore, infection with a SOD2 retrovirus decreases proliferation rates of LP-1 and KMS-11 (Figure 3D). This continues to support the Oberley-Buettner hypothesis that SOD2 expression should reverse at least some of the cancer phenotype.30
The decreased expression of SOD2 in cancer cells makes them vulnerable to antitumor agents that invoke ROS as their mode of action, constituting an Achilles heel for tumors with low SOD2. We demonstrated that cell lines with decreased SOD2 were in fact more sensitive to 2ME2 (Figure 4), which increases ROS generation.33 Apoptosis involved activation of the caspases (Figure 5E-F). The 2ME2-induced ROS lead to increased DNA damage in cell lines with low endogenous SOD2 (Figure 5A-B) and overexpression of SOD2 lead to a decrease in 2ME2-induced DNA damage in the LP-1 cell line (Figure 5C-D). Therefore, decreased SOD2 levels may be exploited, and therapies including agents that induce ROS may prove to be the most useful. In the case of myeloma, 2ME2 has shown promise in a preclinical setting34 and was shown to overcome dexamethasone resistance.35
The effects of ROS on cellular processes are arguably global; significant genomic and nongenomic observations have been made. With that in mind, we explored an integrated approach to examine the consequences of SOD2 expression. Genes induced by SOD2 overexpression that lead to radioresistance have been identified in breast carcinoma.36 However, gene expression changes due to the altered expression of SOD2 have not been comprehensively identified. Using our cell lines with overexpressed and knocked-down SOD2, we identified 65 genes regulated by altered levels of SOD2 expression (Figure 6A-B). The expression patterns of 13 of these genes were similar in analyzed patient samples (Figure 6A-B). Among the identified genes are genes involved in growth and proliferation and cancer, as identified by Ingenuity's Pathway software (Table S1). The increased number of genes identified in these pathways again lends further support to the role of SOD2 as tumor suppressor. Also interesting to note is the number of genes, including MMP9, ALDOC, and ID2, that were identified as functioning in the development of the cardiovascular system, since SOD2 knock-out mice die neonatally of cardiomyopathy.37 It is also interesting to note that MMP-9 was determined to be increased in SOD2-deficient mice.38
Further nongenomic information regarding the functional consequences of altered SOD2 expression levels was garnered from RPAs (Figure 7). ROS has been shown to regulate a host of signaling pathways,5 including the JAK/STAT pathway.24,39,40 Here we show specifically that overexpression of SOD2 specifically leads to decreased phosphorylation of JAK2 and STAT5. Both the JAK/STAT pathways41,42 and EGFR43,44 have well-established roles in the etiology of several tumor types, including hematologic malignancies. The decrease in either 1 or both of these pathways could explain the decreased proliferation of SOD2-overexpressing cells, but the exact mechanism of decreased proliferation remains to be determined. While others have demonstrated that ROS is involved in activation of NFκB (reviewed in Martindale and Holbrook45 ), we did not find evidence for the activation of NFκB in SOD2-manipulated cell lines. This is likely due to the different cell types used in these studies as well as the fact that myelomas are often characterized by constitutively active NFκB pathways.46
To further identify the signaling cascades altered by SOD2 expression, we profiled activated transcription factors (Table 1). The STAT-binding site, GAS, was identified as decreased with SOD2 overexpression, consistent with the finding of decrease phosphorylation of STAT5. A decrease of XBP1-binding activity was also identified. Interestingly, there have been recent reports demonstrating that 2ME2 can lead to the differentiation of myeloma cells,47 including the up-regulation of XBP-1,48 a transcription factor vital for plasma-cell differentiation.49 The mechanism behind up-regulation of XBP-1 remains to be elucidated. In our study, the transcriptional binding activity of XBP-1 was regulated by SOD2 expression (Table 1). The combination of these 2 lines of evidence suggests that XBP-1 may, in fact, be regulated at several levels by ROS.
In an attempt to integrate the gene expression data with the identification of regulated transcription factors, we identified binding sites for the identified transcription factors in the promoters of the regulated genes (Tables 2–3). It is interesting to note that the down-regulated genes showed an increase in the number of binding sites identified for the down-regulated transcription factors. This was also true for the up-regulated genes. This strengthens the use of in silica analysis and provides a possible framework for the identification of transcriptional networks.
The ability to profile cellular functions through genomic and proteomic analysis has lead to great advancements in our understanding of normal cell functions as well as the altered functions of disease states. While both approaches of genomic and proteomic analyses lead to a plethora of knowledge, they inherently miss other aspects of cellular regulation. Therefore, the combinatorial approaches yield more complete and expectedly complex understanding of molecular events occurring in cells. Our cell lines with altered SOD2 activity afforded us the opportunity to determine, more completely to date, the molecular events altered by the loss of this enzymatic activity. We preformed analysis for alterations in (1) phosphorylated proteins, (2) transcription factor–binding activities, and (3) gene expression. Thereby, we have a gained an understanding of both the signaling networks altered by SOD2 activity and the gene expression changes that result. As many fields advance toward a systems biology approach, integrating the immense changes a single perturbation or extrinsic signal has on a cell offers many informational challenges. While the data gained from this type of analysis is vast and the full understanding of it will, most likely, not come for many years, we have laid the foundation for a greater perspective of altered SOD2 activity.
Authorship
Author contributions: B.P. was responsible for design, performance, and analysis of sodium bisulfite sequencing and comet assays on the LP-1 retrovirally transfected cell lines. E.M.H. was responsible for the design, performance with assistance from S.B.T., and analysis of all other experiments, and wrote the paper. W.L.F. was responsible for design of experiments.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Elaine M. Hurt, Laboratory of Cancer Prevention, NCI-Frederick, 1050 Boyles St, Bldg 560, Rm 21-81, Frederick, MD 21702; e-mail: hurte@ncifcrf.gov; or William L. Farrar, Laboratory of Cancer Prevention, NCI-Frederick, 1050 Boyles St, Bldg 560, Rm 21-78, Frederick, MD 21702; e-mail: farrar@mail.ncifcrf.gov.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supported in part with Federal funds from the National Cancer Institute, National Institutes of Health (NIH), under contract no. N01-CO-12400. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
Supplemental data
Promoter modules identified using Genomatix’s ModelInspector (www.genomatix.de) in the 11 genes up-regulated by SOD2 are listed along with the location of the module and the strand orientation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal