Abstract
Chemerin is a chemotactic agonist recently identified as the ligand of ChemR23, a serpentine receptor expressed by mononuclear phagocytes and dendritic cells (DCs). This study shows that blood CD56lowCD16+ natural killer (NK) cells selectively express functional ChemR23 and that this receptor is coexpressed with CXCR1, the CXCL8 receptor, and the KIR receptors. In vitro culturing of NK cells with IL-2 or IL-15 induced a delayed and time-dependent down-regulation of ChemR23 that was associated with the inhibition of NK cell migration to chemerin. Biopsies obtained from patients with oral lichen planus presented an infiltration of CD94+CD3−CD56+ NK cells that coexpressed ChemR23. The same biopsies were infiltrated by myeloid, DC-SIGN+ and plasmacytoid, CD123+BDCA2+, ChemR23+ dendritic cells that were occasionally associated with NK cells. In the same histologic sections, chemerin was expressed by inflamed dermal endothelium. These findings propose a role for the ChemR23/chemerin axis in the recruitment of blood NK cells and strongly implicate chemerin as a key factor for the colocalization of NK cells and DC subsets in pathologic peripheral tissues.
Introduction
Natural killer (NK) cells represent a minor population (5%-20%) of peripheral blood lymphocytes that is also present in secondary lymphoid organs, such as spleen, and lymph nodes, as well as in liver, bone marrow, and maternal uterus.1–3 The function of NK cells in humans is regulated by a balance between opposite signals delivered by a set of HLA class I-specific inhibitory receptors and by a number of activating receptors and coreceptors responsible for NK cell triggering. By the combined use of these receptors, NK cells can discriminate between normal HLA class I+ cells and cells that have lost the expression of HLA class I molecules as a consequence of tumor transformation or viral infection.4–7 Most NK cells in peripheral blood express the CD56lowCD16+ phenotype, whereas the remainders are CD56highCD16− cells. It was proposed that CD56high NK cells represent a primary source of immunoregulatory cytokines, whereas the CD56lowC16+ subset represents the principal cytotoxic population.8
During inflammation, viral infection and tumor growth, NK cells are rapidly recruited from the blood into injured tissues.9–11 NK cell recruitment is governed by integrated signals, which include adhesion molecules and chemotactic factors. CD56lowCD16+ NK cells express both β1 and β2 integrins, as well as the ligands for E- and P-selectins. In addition to these molecules, CD56high NK cells also express high levels of L-selectin, a pivotal molecule for the interaction with lymph node high endothelial venules.12–14 With respect to chemokine receptors, CD56lowCD16+ NK cells express high levels of CXCR1 and CX3CR1.9,15 By contrast, CD56high NK cells express CCR7 as well as CCR5 and CXCR3.8,9,15 It is likely that the different expression profile of adhesion molecules and chemokine receptors between the 2 major blood NK cell subsets is responsible for the preferential migration of CD56lowCD16+ and CD56highCD16− NK cells into inflamed tissues and secondary lymphoid organs, respectively.16 In fact, the CD56highCD16− NK cell subset although poorly represented in peripheral blood constitutes the only type of NK cells present in secondary lymphoid tissues.2,3
We have recently identified a novel protein, chemerin, as the natural ligand of the previously orphan receptor ChemR23.17 ChemR23 exhibits a unique expression profile among leukocyte populations being expressed preferentially by monocyte/macrophages and by immature myeloid and plasmacytoid dendritic cells (DCs).18 Chemerin, originally isolated from inflamed biologic fluids, such as ovarian cancer ascites and rheumatoid arthritis synovial fluids, is synthesized as a secreted precursor protein. Prochemerin is poorly active but can be rapidly converted into a full ChemR23 agonist by the proteolytic removal of the last 6 amino acids by neutrophil-derived proteases (elastase and cathepsin G), mast cell products (triptase), and proteases of the coagulation cascade.19,20 Therefore, prochemerin represents a “ready to use” chemotactic factor for the rapid recruitment of leukocytes into pathologic tissues.
In this study we show that blood CD56lowCD16+, but not CD56highCD16− NK cells, express functional ChemR23. In human biopsies of lichen planus, ChemR23 is expressed by infiltrating NK cells and by both myeloid and plasmacytoid DCs. DC subsets and NK cells colocalize in areas where chemerin is detected on the surface of inflamed endothelium. These results strongly suggest that the ChemR23/chemerin axis is involved in the trafficking of CD56lowCD16+ NK cells into inflamed peripheral tissues and in their colocalization with myeloid and plasmacytoid DCs. Furthermore, these results represent one of few reports that directly support NK-DC cell cross-talk, in vivo.21
Materials and methods
The study was conducted in accordance with a protocol approved by the Spedali Civili of Brescia institutional ethical board and informed consent was obtained from all patients.
Monoclonal antibodies and cytofluorimetric analysis
The following monoclonal antibodies (mAbs), produced in our laboratories, were used in this study: JT3A (IgG2a, anti-CD3), c227 (IgG1, anti-CD69), FS24 (IgG2b, anti-HLA-DR), BAB281 and KL247 (IgG1 and IgM, respectively, anti-NKp46), Z25 and F252 (IgG1 and IgM, respectively, anti-NKp30), c127 and KD1 (IgG1 and IgG2a, respectively, anti-CD16), c218 and c280 (IgG1 and IgG2a, respectively, anti-CD56), GL183 (IgG1, anti-CD158b), AZ115 (IgG1, anti-CD158a), Z27 (IgG1, anti-CD158e), XA185 (IgG1, anti-CD94), Z270 (IgG1, anti-NKG2A), F278 (IgG1, anti-CD85j), and anti-ChemR23 (clone 1H2; IgG2a). Anti-CXCR1 (IgG1) was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). For one- or 2-color cytofluorimetric analysis, cells were stained with the appropriate mAbs followed by PE- or FITC-conjugated isotype-specific goat anti–mouse secondary antibody (Southern Biotechnology, Birmingham, AL). Cell acquisition was performed using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) and data analyzed using the CellQuest software (Becton Dickinson).
PBMC and NK cell purification and culture
Peripheral blood mononuclear cells (PBMCs) were obtained from heparinized blood by density gradient centrifugation over Ficoll (Sigma, St Louis, MO). NK cells were purified by NK Cell Separation Cocktails (Rosette Sep, StemCell Technologies, Vancouver, BC, Canada). The purity of NK cells was more than 96% as assessed by flow cytometric analysis of cells stained with a mixture of CD56-PC5 and CD3-FITC (Beckman Coulter, Immunotech, Marseille, France).15 CD3 contamination in purified NK cells was less than 1%. Freshly purified NK cells were resuspended in RPMI 1640 medium, supplemented with 2 mM glutamine, 50 μg/mL penicillin, 50 μg/mL streptomycin, and 10% heat-inactivated FCS (PAA Laboratories, Linz, Austria) and cultured as previously described22 in the presence of 100 U/mL IL-2 (Proleukin; Chiron, Emeryville, CA) or 20 ng/mL IL-15 (all purchased from PeproTech, London, United Kingdom) to obtain activated polyclonal NK cell populations. Cells were plated at 106 cells/mL in flat-bottom 24-well tissue culture plates (Costar, Corning, Cambridge, MA).
NK cell migration
Migration of fresh and cultured NK cells was evaluated using 24 well Costar Transwell chambers (5 μm pore size; Corning). Then, 100 μL NK cell suspension (4 × 106 cells/mL) was seeded in the upper chambers and 600 μL of chemoattractant or control medium was added to the lower wells. The chambers were incubated at 37°C in humidified atmosphere in the presence of 5% CO2 for 4 hours. The number of migrated cells, recovered from the lower well, was expressed as the percentage of input cells. For transendothelial migration, human umbilical vein endothelial cells (HUVECs, passage < 5) were subcultured to confluent monolayers on Transwell inserts precoated with gelatin, as previously described.18 In vivo migration of NK cells was evaluated following the intraperitoneal injection of 5 μg/mouse of murine recombinant chemerin (R&D Systems, Minneapolis, MN). Cells were recovered from the peritoneal cavity 20 hours later and NK cells were stained with anti-CD3 and anti-NK1.1 mAb (PharMingen, San Diego, CA), as previously described.23
Tissues and reagents
Mucosal specimens from 9 patients with active oral lichen planus (OLP) were analyzed. Each specimen was snap frozen in isopentane precooled to liquid nitrogen temperature and stored at −80°C. Thin (5-μm) cryostat sections were air-dried overnight at room temperature and fixed in acetone for 10 minutes before staining. Tissue expression of ChemR23, CD56, CD94, CD123, and BDCA-2 was evaluated by immunohistochemistry using anti-ChemR23 mAbs (4C7 and 1H7; mouse, IgG2b and IgG2a, respectively; dilution 1:15),18 anti-CD56 mAb (clone 280, mouse, IgG2a), anti-CD94 mAb (clone 39 BIO-CL1, mouse, IgG1, dilution 1:10, kindly provided by M. Colonna (Washington University, St Louis, MO), anti-CD123 mAb (clone 7G3, mouse, IgG2a, dilution 1:40, PharMingen), and anti-BDCA-2 mAb (clone AC144, mouse, IgG1, dilution 1:5, Miltenyi Biotec, Auburn, CA). Chemerin expression was evaluated by immunohistochemistry as previously described.18 After treatment with goat serum for 5 minutes to inhibit nonspecific binding, tissue sections were incubated with primary antibodies at room temperature for 1 hour, followed by biotinylated goat-polyvalent immunoglobulin, the streptavidin-peroxidase amplification system (StrAviGen Multilink Kit, Biogenex, San Ramon, CA) and visualized by applying 3-amino-9-ethylcarbazole (LabVision, Fremont, CA). Nuclei were counterstained with hematoxylin. MxA immunostaining was performed on paraffin sections (3 μm) using anti-MxA (clone M143, mouse, IgG2a, dilution 1:500, provided by Otto Haller, University of Freiburg, Germany) and an indirect immunoperoxidase technique (Dako LSAB+ Kit peroxidase; Dako, Carpinteria, CA). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 10 minutes. Double immunofluorescence was performed using a combination of primary antibodies, including anti-ChemR23 (mouse, IgG2b), anti-CD56 (mouse, IgG2a), anti-CD94 (mouse, IgG1), anti-CD123 (mouse, IgG2a), anti-BDCA-2 (mouse, IgG1), anti-DC-sign/CD209 (clone DCN46, mouse, IgG2b, dilution 1:200, PharMingen), anti-Langerin/CD207 (mouse, IgG1, dilution 1:500, kindly provided by Dr G. Trinchieri, National Cancer Institute, Bethesda, MD), anti-CD69 (mouse, IGg1, dilution 1:50, Dako), anti-CD3 (polyclonal rabbit, IgG, dilution 1:100, Dako). Appropriate Texas red- and FITC-conjugated isotype-specific secondary antibodies were used to reveal the primary antibodies, as previously described.18,24 Immunostained sections were analyzed and photographed using an Olympus BX60 fluorescence microscope, equipped with the Olympus DP-70 digital camera. Pictures were analyzed by Analysis 3.2 soft imaging system GMBH and Adobe Photoshop 5.0, limited edition. Lenses used were: Olympus: 20×/0.70, ∞/0.17, and 40×/0.85, ∞/0.11-0.23.
Results
Expression of ChemR23 in human peripheral blood NK cell subsets
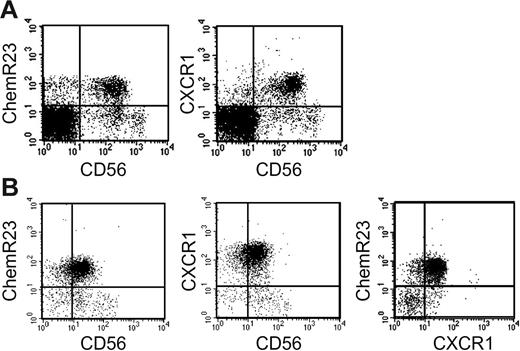
In a previous study, we reported that ChemR23 is mostly expressed by MHC class II+ cells, including monocytes, myeloid and plasmacytoid DCs, and by a minor subset of CD3+ and CD20+ cells.18 Here, the expression of ChemR23 was further investigated in unfractionated PBMCs and highly purified NK cells. This analysis revealed that the majority of CD56+ cells present in PBMCs (Table 1A) and in purified NK cells (Table 1B) express ChemR23. Remarkably, ChemR23 expression was confined to CD56low cells, whereas it was absent from the CD56high subset. CD56low NK cells are known to express high levels of CXCR1, the CXCL8 receptor,8,9,25,26 and Table 1B shows that most NK cells coexpressed ChemR23 and CXCR1, although small subsets of single-positive cells could be reproducibly detected.
Expression of ChemR23 by CD56+ NK cells. Freshly isolated peripheral blood mononuclear cells (A) and freshly purified NK cells (B) were analyzed by 2-color immunofluorescence and flow cytometry for the expression of CD56, ChemR23 or CXCR1. This experiment is representative of at least 5 independent donors.
Expression of ChemR23 by CD56+ NK cells. Freshly isolated peripheral blood mononuclear cells (A) and freshly purified NK cells (B) were analyzed by 2-color immunofluorescence and flow cytometry for the expression of CD56, ChemR23 or CXCR1. This experiment is representative of at least 5 independent donors.
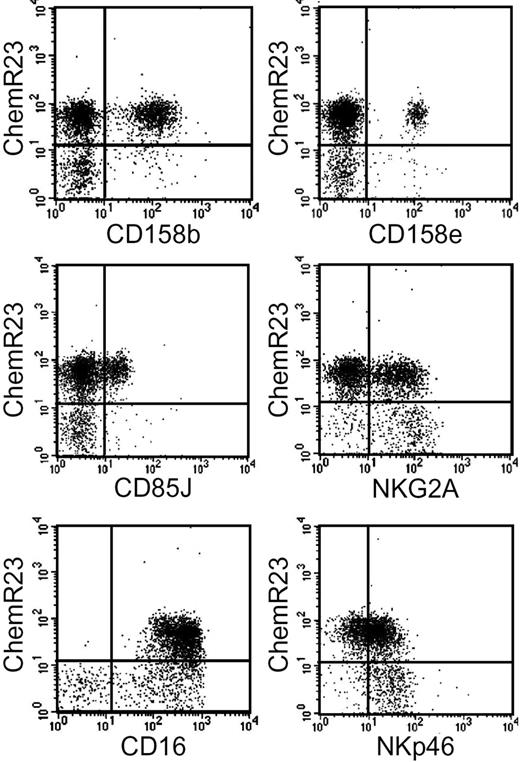
ChemR23+ NK cells were further characterized for the expression of inhibitory and activating receptors. ChemR23+ cells expressed various members of the KIR family including KIR2DL2/2DL3/2DS2 (CD158b) and KIR3DL1/3DS1 (CD158e). They also expressed LIR1/ILT2 (CD85J), NKG2A, and the activating receptors CD16 and NKp46.27 A subset of ChemR23− NK cells was characterized by the NKG2Abright NKp46bright phenotype, a result that is consistent with previous reports showing that CD56high (ChemR23−) cells express high levels of both NKG2A and NKp46.15 As expected on the basis of the lack of CD16 expression in the CD56high subset, no ChemR23+CD16− NK cells were detected (Figure 2).
Expression of inhibitory and activator receptors by ChemR23+ NK cells. Freshly isolated NK cells were analyzed by 2-color immunofluorescence and flow cytometry for the expression of ChemR23 in combination with antibodies against the indicated molecules.
Expression of inhibitory and activator receptors by ChemR23+ NK cells. Freshly isolated NK cells were analyzed by 2-color immunofluorescence and flow cytometry for the expression of ChemR23 in combination with antibodies against the indicated molecules.
Migration of NK cells in response to chemerin
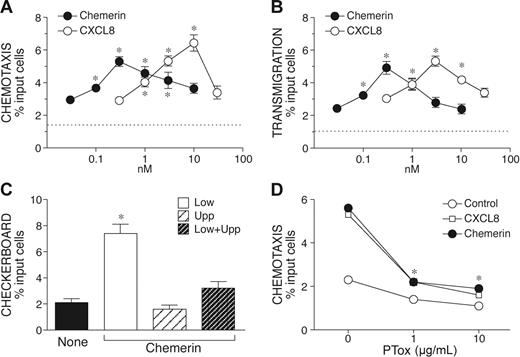
We next evaluated the ability of chemerin, the ChemR23 ligand, to induce NK cell migration using a Transwell-based chemotaxis assay. Chemerin induced a bell-shaped dose-dependent migration of NK cells, with a peak of activity observed at 300 pM chemerin and an EC50 of 0.05 nM. In the same experimental conditions, CXCL8 induced NK cell migration with a maximal response observed at the concentration of 10 nM (EC50 = 1 nM). Therefore, chemerin is characterized by a 20-fold higher potency and by a slightly reduced efficacy (percentage of migrated cells) as compared to CXCL8 (Figure 3A). Similar results were obtained when chemerin was tested in a transendothelial migration assay (Table 3B). The response of NK cells to chemerin was chemotaxis (ie, directional migration) rather then chemokinesis (ie, activated random migration), since chemerin induced NK cell migration only when added to the lower compartment of the chemotactic chamber, with no activity in the presence of a negative gradient (chemerin in the upper well) or in the absence of chemotactic gradient (chemerin in both lower and upper wells; Table 3C). As expected on the basis of previous results,18 NK cell migration to chemerin was completely blocked by the action of Bordetella pertussis toxin (Table 3D) and in the presence of an anti-ChemR23 mAb (data not shown). These results provide evidence that the activation of ChemR23 is responsible for chemerin-induced NK cell migration. Finally, the ability of chemerin to induce NK cell recruitment in vivo was investigated in vivo following intraperitoneal administration in mice. After 20 hours, the number of NK1.1+/CD3− cells recovered from the peritoneal cavity was strongly increased in chemerin-injected animal compared to the control group (0.89 × 106 and 0.38 × 106 cells, respectively; n = 3).
Chemotactic response of NK cells in response to chemerin. Freshly purified NK cells were isolated and used in Transwell-chemotactic assays (A) or in endothelial transmigration experiments (B). CXCL8 was used as reference NK cell chemotactic agonist. Checkerboard analysis was performed using 300 nM chemerin in the lower, upper, or both lower and upper wells (C). (D) NK cells were preincubated with different concentrations of Bordetella pertussis toxin (PTox) at 37°C for 3 hours and then tested in chemotaxis assays (300 nM chemerin, 10 nM CXCL8). Results are expressed as percent of migrated cells; values are the mean ± SE of 3 (A-C) or 2 (D) independent experiments. * P < .05 by paired Student t test versus control cells. Dotted lines (A-B) represent basal cell migration.
Chemotactic response of NK cells in response to chemerin. Freshly purified NK cells were isolated and used in Transwell-chemotactic assays (A) or in endothelial transmigration experiments (B). CXCL8 was used as reference NK cell chemotactic agonist. Checkerboard analysis was performed using 300 nM chemerin in the lower, upper, or both lower and upper wells (C). (D) NK cells were preincubated with different concentrations of Bordetella pertussis toxin (PTox) at 37°C for 3 hours and then tested in chemotaxis assays (300 nM chemerin, 10 nM CXCL8). Results are expressed as percent of migrated cells; values are the mean ± SE of 3 (A-C) or 2 (D) independent experiments. * P < .05 by paired Student t test versus control cells. Dotted lines (A-B) represent basal cell migration.
Regulation of ChemR23 expression and function in NK cells
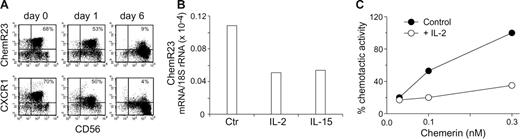
The results so far presented were generated using freshly purified blood NK cells. When cultured in vitro in the presence of cytokines, such as IL-2, NK cells acquire an activated phenotype in terms of cytokine production and cytotoxic ability and modify their repertoire of chemokine receptors.25,28 To analyze the regulation of ChemR23 expression, purified NK cells were cultured in the presence of either IL-2 or of IL-15 and analyzed for ChemR23 expression at different days of culture. As shown in Table 4A (upper panel), after 1 day of culture in the presence of IL-2, ChemR23 expression was virtually unchanged as compared to freshly purified NK cells although at this stage CD69 was already expressed by most of the cells and both CD56 and NKp46 were up-regulated (data not shown). However, a sharp down-regulation of ChemR23 expression was detected after 4 days with a complete down-regulation observed at day 6 of culture. Similar results were obtained when NK cells were cultured with IL-15 instead of IL-2 (data not shown). It is interesting to note that CXCR1 was down-regulated in parallel to ChemR23 with a similar kinetics (Table 4A bottom panels). The down-regulation of ChemR23 membrane expression was paralleled by a decrease of ChemR23 mRNA and by the inhibition of cell migration in response to chemerin (Table 4B-C). At longer time of IL-2 activation, such as after 2 to 4 weeks, NK cells were virtually ChemR23−. These results were likely due to receptor down-modulation rather than to the in vitro selection of ChemR23− cells. In fact, consistent with their derivation from CD56low NK cells, the IL-2–cultured population was mostly CD16+ and expressed KIR2DL2/3 and KIR3DL1 (36% and 22%, respectively). These data suggest that NK cells express ChemR23 only during the early phases of their recruitment to the site of infection.
Down-regulation of ChemR23 in activated NK cells. Purified peripheral blood NK cells were cultured in the absence or presence of 100 U/mL IL-2 for various time and then assessed by 2-color immunofluorescence and flow cytometry for the expression of ChemR23 (upper panel A) or CXCR1 (lower panel A) in combination with CD56. This experiment is representative of 4 independent donors. (B) Real-time polymerase chain reaction (PCR) evaluation of ChemR23 expression in NK cells cultured with IL-2 or IL-15 (20 ng/mL) for 24 hours. One experiment representative of 3 is shown. (C) NK cells were cultured with medium (control) or with IL-2 for 4 days and then tested for their ability to migrate in response to chemerin. Results are the average values of 2 experiments.
Down-regulation of ChemR23 in activated NK cells. Purified peripheral blood NK cells were cultured in the absence or presence of 100 U/mL IL-2 for various time and then assessed by 2-color immunofluorescence and flow cytometry for the expression of ChemR23 (upper panel A) or CXCR1 (lower panel A) in combination with CD56. This experiment is representative of 4 independent donors. (B) Real-time polymerase chain reaction (PCR) evaluation of ChemR23 expression in NK cells cultured with IL-2 or IL-15 (20 ng/mL) for 24 hours. One experiment representative of 3 is shown. (C) NK cells were cultured with medium (control) or with IL-2 for 4 days and then tested for their ability to migrate in response to chemerin. Results are the average values of 2 experiments.
Role of chemerin in the colocalization of NK cells and DC in lichen planus lesions
Lichen planus is a mucocutaneous inflammatory disease histologically characterized by basal keratinocyte damage and lymphocyte reaction. Analysis of the T-lymphocyte infiltrate suggests that activated cytotoxic T cells may trigger apoptosis of epithelial cells.29–31 Table 5 shows that in OLP, NK cells also infiltrate skin lesions as documented by the presence of numerous CD56+ and CD94+ cells. Of note, only a minor fraction (< 2%) of CD3+ cells coexpressed the CD94 marker, indicating their true NK cell nature (Figure S1, available online at the Blood website; see the Supplemental Figure link at the top of the online article). NK cells were mostly located in the dermis with fewer sparse cells in the epidermis; this distribution mirrors the distribution of ChemR23+ cells (Table 5). Therefore, ChemR23 expression by OLP-associated NK cells was evaluated by double immunofluorescence; most (> 70%) of the CD56+ and CD94+ cells also expressed ChemR23. A minor fraction (approximately 40%) of the CD56+ cells were also CD69+ suggesting that ChemR23 was mostly expressed by freshly recruited NK cells (Table 5). Conversely, CD3+ cells were mostly negative (> 97%) for ChemR23 expression (Table 1S).
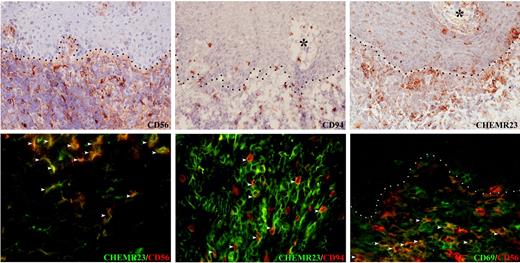
CD56+ and CD94+ NK cells coexpress ChemR23 in OLP. In OLP, CD56+, CD94+ NK cells (top series) and ChemR23+ cells (middle left) occur in the inflammatory infiltrate and within the epithelium. About 70% of CD56+ and CD94+ NK cells coexpress ChemR23 (middle right and bottom left; white arrowheads). A minor subset (about 40%) of CD56+ NK cells that infiltrate OLP lesions express the activation marker CD69 (bottom right, white arrowheads) Dot lines and asterisks indicate, respectively, stromal-epithelial interfaces and stromal papillae. Original magnification × 200 (for streptavidin-peroxidase staining) and × 400 (for immunofluorescence).
CD56+ and CD94+ NK cells coexpress ChemR23 in OLP. In OLP, CD56+, CD94+ NK cells (top series) and ChemR23+ cells (middle left) occur in the inflammatory infiltrate and within the epithelium. About 70% of CD56+ and CD94+ NK cells coexpress ChemR23 (middle right and bottom left; white arrowheads). A minor subset (about 40%) of CD56+ NK cells that infiltrate OLP lesions express the activation marker CD69 (bottom right, white arrowheads) Dot lines and asterisks indicate, respectively, stromal-epithelial interfaces and stromal papillae. Original magnification × 200 (for streptavidin-peroxidase staining) and × 400 (for immunofluorescence).
Previous work documented a high degree of accumulation of both myeloid and plasmacytoid DCs in OLP lesions.24 Since the number of ChemR23+ cells regularly outnumbered CD56+ and CD94+ cells, the expression of ChemR23 by DCs was also investigated. Table 6 shows that OLP lesions presented an elevated number of CD123+, BDCA2+ plasmacytoid DCs, and DC-SIGN+ myeloid DCs. A proportion of DC-SIGN+ mononuclear cells, thus corresponding to stromal DCs, as well as plasmacytoid DCs (CD123+) coexpressed ChemR23 (Table 6 central panels), whereas Langerhans cells were negative (data not shown). Therefore, the majority of the ChemR23+ cells coexpressed a DC phenotype, whereas approximately 25% of them coexpressed CD56 or CD94. It is interesting to note that NK cells and DCs not only are colocalized in OLP sections but also that close contacts can occasionally be observed between CD56+ cells and BDCA2+ or DC-SIGN+ cells (Table 6 bottom left panels; see arrowheads). To investigate the possible consequences of this NK cell/DC colocalization, the secretion of type I interferon, the major cytokine released by plasmacytoid DCs,32 was investigated. For this purpose it was evaluated by immunohistochemistry the expression of MxA, an IFN-α–inducible protein.33 MxA was strongly expressed on epithelial and inflammatory cells in all OLP lesions (Table 6). In particular, epithelial MxA expression was significantly enhanced when compared with contiguous unaffected mucosa. Conversely, no MxA expression was noticed in oral mucosal biopsies from 3 patients showing reactive acanthosis.
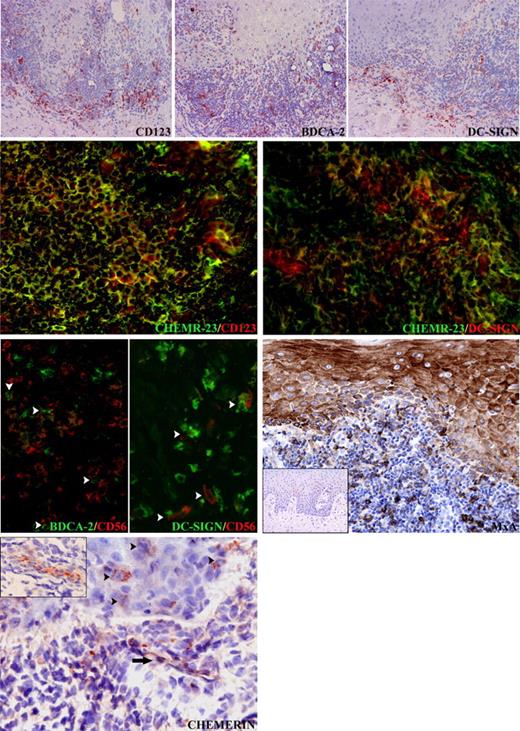
Chemerin as a colocalization signal for CD123+/BDCA-2+ plasmacytoid DCs, DC-SIGN+ myeloid DCs, and NK cells in OLP. In OLP, CD123+/BDCA-2+ plasmacytoid DCs and DC-SIGN+ DCs are present in the inflammatory infiltrate and within the epithelium. The vast majority of CD123+ plasmacytoid DCs and a proportion of DC-SIGN+ DCs coexpress ChemR23+ cells. Close cell-to-cell contacts (white arrowheads) can be observed between BDCA-2+ plasmacytoid DCs and CD56+ NK cells as well as DC-SIGN+ DCs and CD56+ NK cells. Epithelial and inflammatory cells in oral lichen planus express MxA, as opposed to oral mucosal biopsies from patients showing reactive acanthosis (inset), which is completely negative. In the same sections, chemerin is expressed on the luminal side of the endothelial cells (black arrows and inset). In addition scattered chemerin-positive cells with epithelial morphology are detectable in the epithelium (black arrowheads). Original magnifications are all × 400, except top row (× 100) and MxA (× 200) and insets: MxA (× 50) and chemerin (× 300).
Chemerin as a colocalization signal for CD123+/BDCA-2+ plasmacytoid DCs, DC-SIGN+ myeloid DCs, and NK cells in OLP. In OLP, CD123+/BDCA-2+ plasmacytoid DCs and DC-SIGN+ DCs are present in the inflammatory infiltrate and within the epithelium. The vast majority of CD123+ plasmacytoid DCs and a proportion of DC-SIGN+ DCs coexpress ChemR23+ cells. Close cell-to-cell contacts (white arrowheads) can be observed between BDCA-2+ plasmacytoid DCs and CD56+ NK cells as well as DC-SIGN+ DCs and CD56+ NK cells. Epithelial and inflammatory cells in oral lichen planus express MxA, as opposed to oral mucosal biopsies from patients showing reactive acanthosis (inset), which is completely negative. In the same sections, chemerin is expressed on the luminal side of the endothelial cells (black arrows and inset). In addition scattered chemerin-positive cells with epithelial morphology are detectable in the epithelium (black arrowheads). Original magnifications are all × 400, except top row (× 100) and MxA (× 200) and insets: MxA (× 50) and chemerin (× 300).
To gain further information about the possible role of ChemR23 in the colocalization of DC subsets and NK cells, the expression of chemerin, the ChemR23 ligand, was examined by immunohistochemistry. Chemerin reactivity was observed on the endothelial cells lining stromal vessels in 7 of the 9 cases of OLP investigated (Table 6). In addition, granular cytoplasmic positivity for chemerin was observed in rare mononuclear cells with epithelial morphology located in the epithelium. Altogether, these data strongly support the hypothesis that chemerin may orchestrate the colocalization of DCs and NK cells in OLP lesions.
Discussion
This study shows that ChemR23, a chemotactic receptor mainly expressed by antigen-presenting cells, such as monocytes/macrophages and DCs,18 is also a functional receptor for human NK cells. ChemR23 expression marks the major CD56lowCD16+ peripheral blood NK cell subset, with no expression by CD56high NK cells. Consistent with their CD56low phenotype, ChemR23+ cells express KIRs as well as CD16 molecules. Chemerin, the ChemR23 ligand, promotes the migration of NK cells at picomolar concentrations in a dose-dependent manner, and this migration is dependent on ChemR23, as based on the on the inhibitory effects of ChemR23-blocking antibodies and Bordetella pertussis toxin. Cell activation induced by IL-2 or IL-15 down-regulates ChemR23 expression both at the mRNA and membrane protein levels with maximal inhibition observed at the 6-day activation. Therefore, chemerin represents a new chemotactic factor for circulating CD56low NK cells.
It was previously shown that the CD56low and CD56high NK subsets display important differences in terms of functional capabilities and tissue distribution. CD56low cells are characterized by high cytotoxic activity, which correlates with their content in cytoplasmic perforin. On the other hand, CD56high cells produce higher levels of cytokines, including IFN-γ and TNF-α.8 These 2 subsets are also differently represented in peripheral blood and in secondary lymphoid compartments. Indeed, approximately 90% of peripheral NK cells are CD56low, whereas only CD56high NK cells populate lymph nodes.2,3,9 In line with this evidence, the pattern of chemokine receptor expression by the 2 NK subsets is markedly different. CD56high cells express CCR7,8,9,15 the lymph node homing receptor that binds CCL21 and CCL19, 2 chemokines expressed, respectively, at the luminal side of high endothelial venules and in the T cell-rich areas of secondary lymphoid organs (for a review, see Sozzani34 ). CD56high cells also express the lymph node addressin CD62L and CXCR3,8,9 2 molecules recently implicated in the migration of murine NK cells to peripheral lymph nodes.35 Conversely, CD56low cells do not express these receptors but are equipped with CXCR1, the CXCL8 receptor, and CX3CR1, the fractalkine receptor, that allow their migration from the blood into inflamed peripheral tissues.8,9,15 The distribution and regulation of ChemR23 in peripheral blood NK cells closely parallel the expression of CXCR1 and the 2 receptors were found to be coexpressed by circulating NK cells. This finding strongly suggests a role for the ChemR23/chemerin axis in the recruitment of CD56low NK cells into inflamed peripheral pathologic tissues.
Chemerin belongs to the cathelicidin/cystatin family of proteins, which includes precursors of bactericidal peptides (cathelicidins), precursors of mediators active on leukocytes through G protein-coupled receptors (prokininogen, cathelicidin precursors), as well as cysteine protease inhibitors (cystatins).36 Elevated chemerin production was found in ovarian cancer ascites and in the synovial fluids of patients with rheumatoid arthritis.17 Furthermore, prochemerin was found expressed in skin biopsies obtained from nonlesional psoriatic skin and in patients with systemic lupus erythematosus.18,37 Therefore, it is likely that chemerin represents an important signal involved in leukocyte recruitment during inflammation and autoimmune diseases and at tumor sites. Chemerin was shown to serve as a chemoattractant for plasmacytoid and myeloid DCs that populate secondary lymphoid compartments as well as for DCs that are recruited to skin lesions in lupus erythematosus.18 Thus, chemerin local production may promote the colocalization of DCs and CD56low NK cells in pathologic tissues.
Recent studies have highlighted the role of NK cells during the early phases of innate immune responses. In particular, it was suggested that NK cells can interact with myeloid DCs in inflamed peripheral tissues, where a potent cross-talk would occur between these 2 cell types.9,10,16,38–40 During this interaction, CD56lowCD16+ NK cells acquire the ability to kill immature DCs and to release high levels of cytokines including IFN-γ and TNF-α, which promote DC maturation.22,41–45 These functions may be facilitated by the simultaneous activation of both NK cells and DCs by pattern recognition receptors. Indeed, CD56low NK cells, in the presence of IL-12 and of the concomitant activation of Toll-like receptors, become rapidly activated and acquire cytolytic activity against tumor target cells as well as against immature DCs.16,46 Thus, the recruitment of the CD56low NK cell subset in response to chemerin, will regulate the number and the maturation process of DCs before their migration to lymph nodes, whereas the CD56high subset, recruited to lymph nodes via chemerin-independent mechanisms, would produce IFN-γ necessary for DC-dependent priming of Th1 responses.35
Despite the large body of evidence about the role of NK cell/DC interactions, little evidence supports the concept that this interaction indeed occurs in peripheral tissues, in vivo.21 In an effort to provide evidence for this model, we have investigated the expression of ChemR23 by NK cells in a pathologic condition known to be characterized by elevated tissue accumulation of DCs. Biopsies obtained from patients with OLP showed, in addition to numerous CD123+/BDCA2+ and DC-SIGN+ DCs, the presence of CD3−CD56+ CD94+ NK cells. Both DC subsets and NK cells expressed ChemR23 with NK cells accounting for about 25% of the total ChemR23+ cells present in each lesion. Only a minority of ChemR23+ NK cells expressed CD69, an early activation marker, suggesting that ChemR23 was mostly expressed by recently recruited NK cell.
Because NK cells were found in close proximity with both plasmacytoid and myeloid DCs, it is likely that this interaction will result in reciprocal cell activation. Recent work has described the effect of NK/plasmacytoid DC interaction in vitro.47,48 OLP sections characterized by plasmacytoid DC and NK cell accumulation strongly expressed MxA, an IFN-α–inducible protein. Furthermore, it is worth considering that plasmacytoid DCs present in OLP lesions were found to produce high levels of type I IFN24 and that CD69 is known to be induced by this cytokine.49 In normal conditions, chemerin is absent in nonlymphoid peripheral tissues, including dermis and epidermis18 ; on the other hand, in OLP lesions, endothelial cells lining blood vessels express chemerin. This finding further supports the role of chemerin in the recruitment and colocalization of NK cells and DC subsets in OLP lesions.
In summary, this study shows that CD56low circulating NK cells express functional ChemR23 and that chemerin is expressed by inflamed endothelial cells in lichen planus lesions, a pathologic condition characterized by the accumulation of NK cells and DCs. These results strongly support the idea that the ChemR23/chemerin axis may play a crucial role in the colocalization of these 2 cells subsets in pathologic peripheral tissues and provide one of the few pieces of direct evidence that this interaction might indeed occur in vivo.
Authorship
Contributions: S.P. performed research and analyzed data; A.S. performed research and analyzed data; E.M. performed research and analyzed data; W.L. performed research; L.M. performed research; F.F. analyzed data; D.C. contributed new reagents; M.P. contributed new reagents; A. Majorana contributed tissues; M.S. performed research; G.T. performed research; A. Moretta analyzed data and wrote the paper; and S.S. planned the experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
S.P., A.S., E.M. contributed equally to the study.
Correspondence: Silvano Sozzani, Section of General Pathology and Immunology, University of Brescia, viale Europa 11, 25123 Brescia, Italy; e-mail: sozzani@med.unibs.it.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the Associazione Italiana per la Ricerca sul Cancro; (A.M and S.S.); the Association for International Cancer Research (grant no. 04-223); Istituto Superiore di Sanità (I.S.S.); Ministero della Salute (RF 2002/149); Ministero dell'Istruzione dell'Università e della Ricerca (M.I.U.R.); FIRB-MIUR progetto–cod.RBNE017B4C; Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST); European Union FP6; Compagnia di San Paolo; and PNR Biotecnologie Avanzate Tema 2. This work was also supported by the Belgian program on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, the LifeSciHealth (grant LSHB-CT-2003-503337) program of the European Community, the Fonds de la Recherche Scientifique Médicale of Belgium, Télévie and the Fondation Médicale Reine Elisabeth (M.P.). D.C. is Research Associate of the Belgian Fonds National de la Recherche Scientifique.
We are grateful to F. Gentili and to D. Bosisio for their help in immunohistochemistry and RT-PCR; C. Buracchi for FACS analysis, and A. Vecchi for helpful discussion of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal