Graft-versus-host disease is associated with poor thymic function and immune reconstitution after hematopoietic stem cell transplantation. Hauri-Hohl and colleagues have elucidated the mechanism by which GVHD damages the thymus.
In this issue of Blood, Hauri-Hohl and colleagues resolve a key issue in understanding how graft-versus-host disease (GVHD) leads to prolonged immune deficiency after hematopoietic stem cell transplantation (HSCT). Clinical studies have established that the occurrence of acute GVHD is a major predictor of the likelihood of opportunistic infection in hematopoietic stem cell (HSC) transplant recipients.1 Of interest, recipients of an unrelated donor (URD) HSC transplant, including those who do not develop clinical GVHD of the skin, gut, or liver, have a rate of infection that is comparable with those of matched related donor (MRD) recipients with GVHD. While there is no doubt a role for the effects of intensive immunosuppressive medications in the increased clinical infection rate, GVHD itself has profound effects on immune reconstitution after transplantation. For example, critical for the development of a broad and durable immunologic repertoire after HSCT is the generation of new T lymphocytes in the thymus from donor-derived progenitors.2 The occurrence of GVHD is associated with the decreased production of new T lymphocytes.2,3
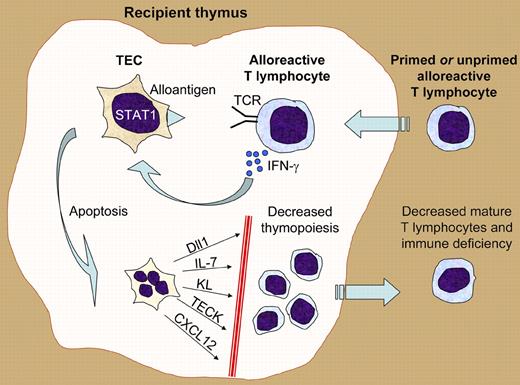
Hauri-Hohl and colleagues have advanced the field significantly by establishing the immunologic mechanisms by which alloreactive T cells recognize and attack the thymus (see figure). Thirty years ago, Seemayer et al first proposed that the thymus may be a target of GVHD after demonstrating infiltration of the thymus by donor T lymphocytes in experimental murine models.4 Key to the present studies were analyses of both in vivo and in vitro murine models of thymopoiesis and allogeneic transplant that demonstrate that even unprimed T cells can recognize allogeneic antigens presented by thymic epithelial cells (TECs). After allogeneic recognition of the TECs, the donor T cells secrete interferon-gamma (IFN-γ), which activates a STAT-1–induced apoptotic pathway in the TECs. The authors propose that the unique biology of TECs as intrathymic presenters of self-antigen to developing thymocytes allows them to present antigen and prime allogeneic immune responses, even in the absence of typical hematopoietically derived professional antigen-presenting cells (APCs). Although APC depletion has been proposed as a strategy to decrease acute GVHD, the authors' results indicate that this would not prevent the development of thymic injury.
Illustration of how killing of thymic epithelial cells (TECs) by alloreactive T lymphocytes can lead to posttransplantation immune deficiency. Alloreactive primed or unprimed naive T lymphocytes enter the recipient thymus after HSCT. As a result of T-cell receptor–mediated recognition of alloantigens presented by the TECs, the alloreactive T cells secrete interferon-gamma (IFN-γ). The TEC response to interferon signaling includes activation of the STAT1 transcription factor, leading to an apoptotic program and TEC death. The loss of TECs results in defective microenvironmental support of thymopoiesis via numerous molecules such as the Notch ligand Dll1, the cytokines IL-7 and Kit ligand (KL), and the chemokines TECK and CXCL12. The resultant decrease in T-cell production ultimately leads to immune deficiency.
Illustration of how killing of thymic epithelial cells (TECs) by alloreactive T lymphocytes can lead to posttransplantation immune deficiency. Alloreactive primed or unprimed naive T lymphocytes enter the recipient thymus after HSCT. As a result of T-cell receptor–mediated recognition of alloantigens presented by the TECs, the alloreactive T cells secrete interferon-gamma (IFN-γ). The TEC response to interferon signaling includes activation of the STAT1 transcription factor, leading to an apoptotic program and TEC death. The loss of TECs results in defective microenvironmental support of thymopoiesis via numerous molecules such as the Notch ligand Dll1, the cytokines IL-7 and Kit ligand (KL), and the chemokines TECK and CXCL12. The resultant decrease in T-cell production ultimately leads to immune deficiency.
The destruction of TECs by alloreactive T lymphocytes may have implications for allogeneic HSCT beyond leading to immune deficiency from prolonged defects in T lymphopoiesis. A recently recognized function of the medullary TEC subset in the development of central tolerance is the negative selection of thymocytes that might develop into autoreactive T lymphocytes. A primary disease of immune development and regulation, the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) syndrome, is due to loss-of-function mutations of the autoimmune regulator (Aire) gene that controls expression and presentation of self-antigens by medullary TECs.5 While it has been generally assumed that donor-derived T cells that develop in the host thymus after HSCT are host tolerant, the destruction of medullary TECs could lead to defects in central tolerance and consequent autoimmunity. Thus, the destruction of TECs as a result of acute GVHD may provide an etiologic link between the alloreactivity of acute GVHD and the autoimmunity typically seen in chronic GVHD.
GVHD has traditionally been assessed and graded in the skin, gut, and liver. As shown by Hauri-Hohl et al, the ability of TECs to present antigen is a mechanism by which GVHD in the thymus could occur without any overt clinical acute GVHD of the typical sites. The occurrence of thymic GVHD without concomitant skin, gut, or liver disease could be a confounder of clinical studies aimed at preventing either GVHD or posttransplantation immune deficiency. For example, is the increased risk of infection and poorer immune reconstitution in URD HSC transplant recipients without overt GVHD due to undiagnosed thymic GVHD?1 In contrast to the tools available for the diagnosis of skin, gut, or liver acute GVHD (physical examination, biomarkers of inflammation, biopsy, endoscopy), routine diagnosis of acute GVHD of the thymus is not readily available. The development of minimally invasive tools for diagnosis of thymic GVHD (eg, imaging or biomarker based) may be a methodologic necessity to assess thymic GVHD. Interventions to improve immune reconstitution via specific protection of TECs may be both necessary and feasible. One candidate strategy would use keratinocyte growth factor (KGF), which has been shown to protect TECs from chemotherapeutic, radiotherapeutic, and allogeneic injury after HSCT, and to stimulate TEC regeneration in aging mice.6–8 The elegant mechanistic studies by Hauri-Hohl et al should stimulate the identification of other potential targets, such as T-cell entry into the thymus or interferon-induced Stat-1 activation in TECs.
Conflict-of-interest disclosure: The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal