Abstract
Using high-performance liquid chromatography–tandem mass spectrometry, we assessed trough imatinib plasma levels in 68 patients with chronic myeloid leukemia (CML) who responded or not to standard-dose imatinib, after at least 12 months' treatment. Mean trough imatinib plasma levels were significantly higher in the group with complete cytogenetic response (56 patients) than in the group without (12 patients; P = .03) and higher in the group with major molecular response (MMR) than in the group without (34 patients [1452 ± 649 ng/mL] versus 34 patients [869 ± 427 ng/mL]; P < .001). Regarding trough imatinib plasma levels and their discrimination potential for MMR, the area under receiver operating characteristic curve was 0.775, with best sensitivity (77%) and specificity (71%) at a plasma threshold of 1002 ng/mL. Therefore, monitoring of imatinib plasma levels could be very useful for the management of patients with CML or should at least be checked in the case of treatment failure or suboptimal response.
Introduction
Continuing progress in the treatment of chronic myeloid leukemia (CML) lies in a better understanding of the disease pathobiology and the mechanisms of treatment failures. Imatinib (Gleevec or Glivec) is a competitive inhibitor of the Bcr-Abl tyrosine kinase, a constitutively active oncoprotein involved in CML pathogenesis. Bcr-Abl is the product of the BCR-ABL fusion gene that results from the reciprocal translocation between chromosomes 9 and 22, which is cytogenetically visible as the Philadelphia chromosome.1,2
Despite the excellent efficacy of imatinib, cases of treatment failure or suboptimal response have been reported. Previous studies identified cellular mechanisms of resistance to imatinib such as gene mutations in the kinase domain of Bcr-Abl, BCR-ABL gene amplification, overexpression of Src-related kinases, drug efflux mediated by the P-glycoprotein which is encoded by the MDR1 gene.3-7 Another hypothesis to explain variable responses to imatinib therapy lies in pharmacokinetic variability.8-10 Too low trough imatinib plasma levels could indicate an ineffective drug regimen, insufficient to achieve complete cytogenetic response (CCR) or major molecular response (MMR). To test this hypothesis, we measured trough imatinib plasma levels in patients with CML and compared them with the likelihood of achieving CCR or MMR to standard-dose imatinib.
Patients and methods
All patients provided informed consent to participate in this study, which was performed parallel to the molecular evaluation. The study was approved by the local Ethics Committee. Pharmacologists who performed the analysis for this study had no direct contact with humans.
Patients with chronic-phase or accelerated-phase CML were considered for our study. Patients were treated orally, for at least 12 months, with standard-dose imatinib (ie, 400 mg or 600 mg once daily for chronic-phase or accelerated-phase CML, respectively), according to the phase 2 expanded access protocols (109, 110, 113, 114) and a phase 3 trial (106) supported by Novartis Pharma and the French SPIRIT study. Exclusion criteria were initiation of imatinib therapy less than 12 months before, blast crisis before or during imatinib therapy, blood collection performed out of the trough concentration time limits, poor compliance to treatment, identification of gene mutation(s) in the kinase domain of Bcr-Abl.
Cytogenetic responses to imatinib were assessed using a conventional cytogenetic analysis of bone marrow metaphases. CCR was defined as 0% of Philadelphia chromosome–positive cells in the bone marrow aspirate.11 To assess molecular responses, total RNA was extracted from peripheral blood cells, and BCR-ABL transcript levels were quantified using real-time quantitative reverse-transcriptase polymerase chain reaction,11 according to recommendations recently proposed for harmonization of results.12 MMR was defined as a reduction in BCR-ABL transcript levels of at least 3 log after 12 months of imatinib therapy, from a standardized baseline.13 For imatinib plasma quantification, blood samples were collected between 21 and 27 hours after last drug administration. Trough imatinib plasma levels were determined using high-performance liquid chromatography coupled to electrospray-ionization tandem mass spectrometry.14
Student t test or Wilcoxon rank test was used to compare means between 2 groups. Comparisons of proportions were performed using a chi-square test or exact Fisher test. Receiver operating characteristic (ROC) curve analysis was performed to assess a discrimination potential of trough imatinib plasma levels for MMR.
Results and discussion
Ninety-five patients with CML were considered for participation in the study. One patient was excluded because he was found to be in blast crisis. Twenty-four patients were excluded because of inadequate blood collection (ie, performed out of time limits). One patient was excluded because of recognized poor compliance to therapy. One patient was excluded because a G250E mutation was identified in the kinase domain of Bcr-Abl.
Finally, 68 patients with CML were included for investigation: 50 patients and 18 patients were treated with imatinib 400 mg and 600 mg daily, respectively. Trough imatinib plasma levels were highly variable, ranging from 181 to 2947 ng/mL; means and SDs were 1058 ± 557 ng/mL and 1444 ± 710 ng/mL for the 400-mg and 600-mg daily dose regimen, respectively. These data confirm the high variability previously described between subjects in trough imatinib plasma levels.8 Such intersubject variability may be multifactorial, including diverse determinants such as genetic polymorphisms, environmental factors, concomitant diseases, or coadministered drugs.10 These determinants may be involved in the pharmacokinetic of imatinib: absorption, distribution, metabolism (involvement of cytochrome P-450 enzymes9 CYP3A4 and CYP3A5), or excretion. The role of efflux transporters such as ABCB1 (P-glycoprotein) and ABCG2 or organic cation transporter OCT1 cannot be excluded, but these transporters modify mainly the intracellular concentration of the drug.
Main characteristics of the 68 patients with CML, classified as those with (n = 34) or without (n = 34) MMR, are summarized in Table 1. Mean (± SD) trough imatinib plasma levels were significantly higher in the group with MMR than in the group without (P < .001). No significant difference was found in the imatinib daily dose between patients with or without MMR. Mean (± SD) trough imatinib plasma levels were significantly higher in the group with CCR than in the group without (56 patients [1123 ± 617 ng/mL] versus 12 patients [694 ± 556 ng/mL]; P =.03). Moreover, MMR to treatment was not significantly associated with the time elapsed between initiation of imatinib therapy and molecular analysis: mean (± SD) values were 986 ± 427 days and 966 ± 560 days in groups with and without MMR, respectively (P =.87). Our results show that trough imatinib plasma levels are associated with the likelihood of achieving CCR or MMR to standard-dose imatinib.
Patients' characteristics according to molecular response to standard-dose imatinib therapy
| Characteristics . | Without MMR* (no. patients) . | With MMR (no. patients) . | P . |
|---|---|---|---|
| Quantitative features | |||
| Trough imatinib plasma levels† | 869.3 ± 427.5 (34) | 1452.1 ± 649.1 (34) | < .001 |
| Age, y | 50.7 ± 13.6 (34) | 51.7 ± 13.7 (34) | .76 |
| Sokal score | 0.9 ± 0.4 (32) | 1.0 ± 0.4 (33) | .33 |
| Qualitative features | |||
| Sex | .09 | ||
| Male | 70.6 (24) | 50.0 (17) | |
| Female | 29.4 (10) | 50.0 (17) | |
| Sokal risk group | .69 | ||
| Less than 0.8 | 44.1 (15) | 41.2 (14) | |
| 0.8-1.2 | 35.3 (12) | 32.4 (11) | |
| Greater than 1.2 | 14.7 (5) | 23.5 (8) | |
| Accelerated-phase CML | .58 | ||
| No | 76.5 (26) | 70.6 (24) | |
| Yes | 23.5 (8) | 29.4 (10) | |
| Interferon before imatinib | .62 | ||
| No | 44.1 (15) | 38.2 (13) | |
| Yes | 55.9 (19) | 61.8 (21) | |
| Daily imatinib dose | > .999 | ||
| 400 mg | 73.5 (25) | 73.5 (25) | |
| 600 mg | 26.5 (9) | 26.5 (9) |
| Characteristics . | Without MMR* (no. patients) . | With MMR (no. patients) . | P . |
|---|---|---|---|
| Quantitative features | |||
| Trough imatinib plasma levels† | 869.3 ± 427.5 (34) | 1452.1 ± 649.1 (34) | < .001 |
| Age, y | 50.7 ± 13.6 (34) | 51.7 ± 13.7 (34) | .76 |
| Sokal score | 0.9 ± 0.4 (32) | 1.0 ± 0.4 (33) | .33 |
| Qualitative features | |||
| Sex | .09 | ||
| Male | 70.6 (24) | 50.0 (17) | |
| Female | 29.4 (10) | 50.0 (17) | |
| Sokal risk group | .69 | ||
| Less than 0.8 | 44.1 (15) | 41.2 (14) | |
| 0.8-1.2 | 35.3 (12) | 32.4 (11) | |
| Greater than 1.2 | 14.7 (5) | 23.5 (8) | |
| Accelerated-phase CML | .58 | ||
| No | 76.5 (26) | 70.6 (24) | |
| Yes | 23.5 (8) | 29.4 (10) | |
| Interferon before imatinib | .62 | ||
| No | 44.1 (15) | 38.2 (13) | |
| Yes | 55.9 (19) | 61.8 (21) | |
| Daily imatinib dose | > .999 | ||
| 400 mg | 73.5 (25) | 73.5 (25) | |
| 600 mg | 26.5 (9) | 26.5 (9) |
Data are mean values (± SD) for quantitative features. Data are proportions (%) for qualitative features. P value was assessed using Student t test for quantitative variables and the chi-square test for qualitative variables
MMR indicates major molecular response (≥ 3 log reduction in BCR-ABL transcript levels). The main characteristics of the 68 patients with CML are classified into those with or without MMR.
Trough imatinib plasma levels are expressed in nanograms per milliliter (ng/mL).
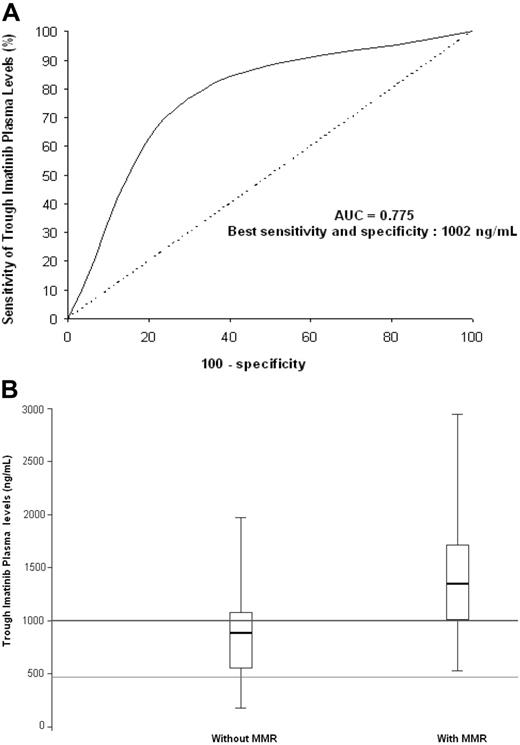
The concentration-effect ROC curve analysis shows the discrimination potential of trough imatinib plasma levels for MMR (Figure 1A). A plasma threshold of 1002 ng/mL was significantly associated with the presence of MMR (odds ratio = 7.80; 95% confidence interval, 2.64-23.03; P < .001). Box plots of trough imatinib plasma levels (Figure 1B) show the dispersion around the median for patients with or without MMR. Regarding the box plot graph, the initially described target concentration (493.6 ng/mL, ie, 1 μmol/L) required to result in BCR-ABL–positive cell death in vitro8,15,16 is not always sufficient to achieve MMR in vivo. Our results suggest that the efficient plasma threshold for trough imatinib levels should be set above 1002 ng/mL in vivo.
Trough plasma imatinib threshold for major molecular response (MMR).(A) Receiver operating characteristic (ROC) curve analysis. Regarding trough imatinib plasma levels and their discrimination potential for MMR, the area under the ROC curve (AUC) was 0.775, with best sensitivity (77%) and specificity (71%) at a plasma threshold of 1002 ng imatinib/mL. (B) Box plots of trough imatinib plasma levels. The graph shows the dispersion around the median for patients with MMR (n = 34; median = 1350 ng/mL) and those without (n = 34; median = 885 ng/mL). The line across each box is the median; the bottom edge is the first quartile and the top edge is the third quartile; the error bars represent minimal and maximal values; the bottom line shows the 493.6 ng/mL (ie, 1 μmol/L) target concentration required to result in BCR-ABL–positive cell death in vitro; the top line shows the 1002 ng/mL efficient plasma threshold for trough imatinib levels in vivo. Of the 34 patients with MMR, 26 (76%) had trough imatinib plasma levels exceeding the 1002 ng/mL threshold. Of the 34 patients without MMR, 24 (71%) had trough imatinib plasma levels below the 1002 ng/mL threshold, whereas 27 patients (79%) had trough imatinib plasma levels exceeding the initially described target concentration (493.6 ng/mL) required to result in BCR-ABL–positive cell death in vitro.8,15,16 This initial target was validated in phase 1 to 2 studies on the basis of cytogenetic criteria, but it is not always sufficient to achieve MMR in vivo. Indeed, using a sharper criterion than CCR such as a 3 log reduction of BCR-ABL transcript levels, the efficient plasma threshold for trough imatinib levels should be set above 1002 ng/mL in vivo.
Trough plasma imatinib threshold for major molecular response (MMR).(A) Receiver operating characteristic (ROC) curve analysis. Regarding trough imatinib plasma levels and their discrimination potential for MMR, the area under the ROC curve (AUC) was 0.775, with best sensitivity (77%) and specificity (71%) at a plasma threshold of 1002 ng imatinib/mL. (B) Box plots of trough imatinib plasma levels. The graph shows the dispersion around the median for patients with MMR (n = 34; median = 1350 ng/mL) and those without (n = 34; median = 885 ng/mL). The line across each box is the median; the bottom edge is the first quartile and the top edge is the third quartile; the error bars represent minimal and maximal values; the bottom line shows the 493.6 ng/mL (ie, 1 μmol/L) target concentration required to result in BCR-ABL–positive cell death in vitro; the top line shows the 1002 ng/mL efficient plasma threshold for trough imatinib levels in vivo. Of the 34 patients with MMR, 26 (76%) had trough imatinib plasma levels exceeding the 1002 ng/mL threshold. Of the 34 patients without MMR, 24 (71%) had trough imatinib plasma levels below the 1002 ng/mL threshold, whereas 27 patients (79%) had trough imatinib plasma levels exceeding the initially described target concentration (493.6 ng/mL) required to result in BCR-ABL–positive cell death in vitro.8,15,16 This initial target was validated in phase 1 to 2 studies on the basis of cytogenetic criteria, but it is not always sufficient to achieve MMR in vivo. Indeed, using a sharper criterion than CCR such as a 3 log reduction of BCR-ABL transcript levels, the efficient plasma threshold for trough imatinib levels should be set above 1002 ng/mL in vivo.
Too low trough imatinib plasma levels could lead to an ineffective drug regimen, imatinib levels being insufficient to achieve CCR or MMR. The reasons for low plasma levels of imatinib need to be identified: poor compliance to daily oral therapy, drug-drug interactions, food interaction, concomitant disease, or genetic polymorphism. Kantarjian et al17 have suggested that patients treated with high-dose imatinib had significantly higher rates of CCR and MMR than those receiving standard dose. The same group demonstrated that dose escalation of imatinib could overcome resistance in some patients with CML initially treated with standard-dose therapy.18 Dose escalation can be associated with an increased rate of side effects. In patients failing to achieve cytogenetic or molecular response, the quantification of trough imatinib plasma levels could become of interest to optimize treatment. Dose escalation may be considered for patients with low plasma levels of imatinib. In patients with trough imatinib plasma levels exceeding 1002 ng/mL, cellular resistance to imatinib and subsequent alternative therapy, such as new kinase-inhibitor nilotinib or dasatinib,19,20 should be considered.
Trough imatinib plasma levels are associated with both CCR and MMR to standard-dose imatinib in CML, with a plasma threshold of 1002 ng imatinib/mL in vivo. Our results suggest monitoring imatinib plasma levels to adjust treatment strategies in patients with CML. However, a study in patients with de novo CML with analyses at different time points (1 or 2 per year) would be useful to validate this plasma threshold and confirm our study. Leading to dosage optimization on a patient-by-patient basis, the rational quantification of imatinib blood levels could become a key feature of clinical practice in CML, with the goal of a better understanding of treatment failure or suboptimal response in patients receiving standard-dose imatinib. A prospective study is in progress to validate the impact of dose escalation in taking into account trough imatinib plasma levels on the response.
Authorship
Contribution: S.P., M.M., and F.-X.M. collaborated in the conception and design of the study. S.P., K.T., G.E., E.T., D.D., M.-A.B., and F.-X.M. did the experiments and performed data analysis. All authors contributed to data interpretation. S.P. wrote the article. All authors revised it critically for the intellectual content. All authors collaborated in the final approval of the version to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: François-Xavier Mahon, Laboratoire Hématopoïèse normale et pathologique, Université Victor Ségalen Bordeaux 2, 146 rue Léo Saignat, INSERM E217, 33076 Bordeaux, France; e-mail: francois-xavier.mahon@umr5540.u-bordeaux2.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal