Abstract
Bacterial toxins including staphylococcal enterotoxins (SEs) have been implicated in the pathogenesis of cutaneous T-cell lymphomas (CTCLs). Here, we investigate SE-mediated interactions between nonmalignant T cells and malignant T-cell lines established from skin and blood of CTCL patients. The malignant CTCL cells express MHC class II molecules that are high-affinity receptors for SE. Although treatment with SE has no direct effect on the growth of the malignant CTCL cells, the SE-treated CTCL cells induce vigorous proliferation of the SE-responsive nonmalignant T cells. In turn, the nonmalignant T cells enhance proliferation of the malignant cells in an SE- and MHC class II–dependent manner. Furthermore, SE and, in addition, alloantigen presentation by malignant CTCL cells to irradiated nonmalignant CD4+ T-cell lines also enhance proliferation of the malignant cells. The growth-promoting effect depends on direct cell-cell contact and soluble factors such as interleukin-2. In conclusion, we demonstrate that SE triggers a bidirectional cross talk between nonmalignant T cells and malignant CTCL cells that promotes growth of the malignant cells. This represents a novel mechanism by which infections with SE-producing bacteria may contribute to pathogenesis of CTCL.
Introduction
The etiology of cutaneous T-cell lymphoma (CTCL), the most common type of T-cell lymphoma, remains poorly understood, but occupational exposures, infectious agents, and genetic mutations have been proposed as etiologic factors (see reviews1-5 ). Mycosis fungoides (MF) and the leukemic variant Sezary syndrome (SS) are the 2 major clinical variants of CTCL. In the initial phase, which can last a number of years, MF presents as flat erythromatous skin patches, resembling chronic inflammation seen in chronic skin infections, psoriasis, and eczema. In the later stages, MF lesions gradually form plaques and overt tumors and eventually involve lymph nodes and internal organs. The early skin lesions in CTCL contain a heterogeneous cell population, including malignant cells that phenotypically resemble normal activated CD4+ T lymphocytes and a mixture of nonmalignant tumor-infiltrating T cells, dendritic cells, macrophages, and other inflammatory cells.1-5 SS, a leukemic and erythrodermic variant of CTCL, is characterized by the presence of circulating lymphocytes with atypical cerebriform nuclei (Sezary cells) in the skin, lymph nodes, and peripheral blood.1 It is a more aggressive form of CTCL, with a mean survival of 3 years from the time of diagnosis.
The role of chronic inflammation as a critical component in tumor progression has recently attracted much attention. It has become clear that the tumor microenvironment, which is largely made up of inflammatory cells, is a component of the malignant tumors (reviewed in de Visser et al6 ). For instance, viral-antigen–specific, tumor-infiltrating CD4+ T cells enhanced the neoplastic progression into invasive cancer in a model of squamous epithelial cell carcinogenesis induced by human papilloma virus oncogenes.7 Furthermore, gene-expression profiling in follicular lymphomas indicated that the patient survival correlated with the molecular features of nonmalignant immune cells present in the tumor at diagnosis.8 Several lines of evidence suggest that in CTCL there is a cross talk between malignant and nonmalignant T lymphocytes, keratinocytes, and dendritic cells.9,10 During the course of the disease, a shift in the composition of infiltrating T cells appears to take place and the interactions between malignant and nonmalignant cells change. In the early stages of the disease, the infiltrate consists primarily of nonmalignant T-helper 1 (TH1) cells and cytotoxic CD8+ T cells that appear to control the malignant cells via inhibitory cytokines such as interferon gamma and, possibly, direct cytotoxicity.11-13 At the later stages of the disease, TH2 cells and cytokines seem to predominate.11,12 Because malignant cells derived from MF tumors produce large amounts of IL-5, IL-6, and IL-13, it is likely that the malignant T cells orchestrate the TH1/TH2 shift in the microenvironment.14 In addition, the malignant T cells may directly suppress antitumor responses. Thus, malignant T lymphocytes may function as regulatory cells with the capacity to down-regulate cellular immunity.15 Moreover, malignant T cells secrete such factors as TGFβ and soluble interleukin-2 (IL-2) receptors (IL-2Rs) that are thought to inhibit inflammatory T cells.10,16,17
The mechanisms of CTCL development and progression are still relatively poorly understood.
Microbial superantigens have been implicated in the pathogenesis of CTCL, and many patients die from complicating bacterial infections.18 Jackow et al19 reported on a positive staphylococcal culture from skin or blood in about 75% of CTCL patients. One half of these patients grew superantigen-producing Staphylococcus aureus, especially patients with erythroderma.19 Other sources of superantigens might derive from Borrelia burgdorferi, cytomegalovirus, and Chlamydia pneumoniae, which have also been associated with CTCL.20,21 Bacterial superantigens bind TCR-Vβ independently of the antigen specificity of the T cell in question, and bacterial superantigens such as staphylococcal enterotoxins (SEs) are among the most potent activators of T cells.22 However, malignant T cells from tumor lesions often respond poorly to TCR/CD3 ligation—due to either decreased surface expression (which is seen in approximately 25% of patients) or a functional deficiency of the TCR/CD3 complex, suggesting that bacterial superantigens may not be involved in a direct stimulation of the CTCL cells.9,23,24 However, the malignant T cells generally express major histocompatibility complex (MHC) class II molecules, which are high-affinity receptors for bacterial superantigens.22,25,26 In the present study, we hypothesized that tumor cells bind bacterial superantigens and present them to nonmalignant T cells. In our CTCL model, we show that malignant cells do not respond directly to bacterial superantigens but do, indeed, function as potent stimulators of superantigen-reactive nonmalignant T cells. In turn, superantigen stimulation of nonmalignant T cells triggered back-stimulation of the malignant cells, indicating that superantigens trigger a bidirectional cross talk between malignant and nonmalignant T cells that promotes growth of malignant cells. Therefore, our data suggest a novel mechanism by which infections with superantigen-producing microbes might contribute to the pathogenesis of CTCL.
Materials and methods
Antibodies and reagents
Monoclonal antibodies (mAbs) against IL-2, IL-4, IL-7, IL-15, CD1, CD2, CD3, CD5, CD8, CD29, HLA-DP (B7/21), HLA-DQ (1a3), secondary antibodies, and nonbinding isotype controls were obtained from Leinco Technologies (St Louis, MO). Antibodies from Leinco Technologies were directly conjugated with FITC or phycoerythrin (for flow cytometry) or unconjugated, and were reconstituted in azide- and endotoxin-free PBS (for cell culture experiments). Anti–LFA-3, anti-CD4 (G17-2), and anti–CTLA-4-Ig mAbs were generous gifts from Jeffrey A. Ledbetter (CDR Therapeutics, Seattle, WA). Anti–HLA-DR (L243) and anti–HLA class I (W6/32) antibodies were generous gifts from Soren Buus (IMMI, Panum Institute, University of Copenhagen). Staphylococcal enterotoxins were from Toxin Technologies (Sarasota, FL), and staphylococcal enterotoxin A (SEA) wt and diverse mutants27 were generous gifts from Mikael Dohlsten (Active Biotech, Lund, Sweden).
Cells
Malignant and nonmalignant T-cell lines isolated from skin and blood from patients with mycosis fungoides and Sezary syndrome have been described elsewhere.28-32 The origin and features of the cell lines are shown in Table 1. The malignant T-cell line MyLa 2039 and the MyLa 2059 subline were established from a plaque biopsy specimen of a patient with MF. A combinatorial approach that included phenotyping, genotyping, HLA typing, and karyotyping verified the tumor origin of the cell lines.29-32 The MyLa 2059 cell line has been kept in long-term culture and shows some karyotypic changes. The malignant T-cell line MyLa 1929 was established from an independent biopsy (3 month later) and resembles closely (karyotypically and otherwise) the MyLa 2039 cell line and the original tumor. All cell lines were seronegative for human T-cell leukemia virus type 1 (HTLV-1) and were HTLV-1 negative as determined by reverse-transcriptase–polymerase chain reaction (PCR). The SeAx cell line is derived from peripheral blood of a patient with SS.28-32 The molecular and functional features of the malignant T-cell lines include an aberrant activity of Janus kinase 3 (Jak-3) and signal transducers and activators of transcription 3 (Stat3), a constitutive production of cytokines (IL-5, IL-6, IL-13, and VEGF), and resistance to apoptosis and growth inhibition by interferons.33-38 Primary tumor cells (CD3+, CD4+, CD7−, CD26− malignant T cells) were obtained from peripheral blood from a 75-year-old female diagnosed with SS and with a CD3 count of 13 300, a CD4/CD8 ratio of 99:1, and a percentage of CD4+, CD7− and CD4+, CD26− cells of 76% and 96%, respectively.
Characteristics and phenotypes of cell lines
| Cell line . | Type of cell line . | Location . | CD2 . | CD3 . | CD4 . | CD8 . | HLA class I . | HLA class II . |
|---|---|---|---|---|---|---|---|---|
| SeAx | CTCL tumor cell line | Peripheral blood | +++ | + | + | − | ++ | +++ |
| MyLa 1929 | CTCL tumor cell line | Skin | ++ | + | − | + | ++ | +++ |
| MyLa 2039 | CTCL tumor cell line | Skin | ++ | − | − | − | ++ | +++ |
| MyLa 2059 | CTCL tumor cell line | Skin | ++ | − | − | − | ++ | +++ |
| MyLa 1928 | Nonmalignant T-cell line | Skin | +++ | ++ | +++ | − | ++ | +++ |
| MyLa 1850 | Nonmalignant T-cell line from CTCL patient | Skin | +++ | +++ | +++ | − | +++ | ++ |
| MySi | Nonmalignant T-cell line from CTCL patient | Skin | +++ | ++ | − | +++ | +++ | +++ |
| MyLa 2355 | Nonmalignant T-cell line from CTCL patient | Peripheral blood | +++ | +++ | +++ | − | +++ | ++ |
| MyLa 1885 | Nonmalignant T-cell line | Peripheral blood | +++ | ++ | +++ | − | +++ | ++ |
| MyLa 3241 | Nonmalignant T-cell line | Peripheral blood | +++ | ++ | ++ | − | +++ | ++ |
| PSOR2 | Psoriasis T-cell line | Skin | +++ | +++ | − | ++ | ++ | ++ |
| PSOR3069 | Psoriasis T-cell line | Skin | +++ | +++ | − | +++ | +++ | ++ |
| AH1 | Atopic dermatitis T-cell line | Skin | +++ | +++ | +++ | − | +++ | ++ |
| P1119 | Healthy donor T-cell line | Peripheral blood | +++ | +++ | +++ | − | +++ | ++ |
| P1183 | Healthy donor T-cell line | Peripheral blood | +++ | +++ | +++ | − | +++ | ++ |
| P1675 | Healthy donor T-cell line | Peripheral blood | +++ | +++ | +++ | +++ | ++ |
| Cell line . | Type of cell line . | Location . | CD2 . | CD3 . | CD4 . | CD8 . | HLA class I . | HLA class II . |
|---|---|---|---|---|---|---|---|---|
| SeAx | CTCL tumor cell line | Peripheral blood | +++ | + | + | − | ++ | +++ |
| MyLa 1929 | CTCL tumor cell line | Skin | ++ | + | − | + | ++ | +++ |
| MyLa 2039 | CTCL tumor cell line | Skin | ++ | − | − | − | ++ | +++ |
| MyLa 2059 | CTCL tumor cell line | Skin | ++ | − | − | − | ++ | +++ |
| MyLa 1928 | Nonmalignant T-cell line | Skin | +++ | ++ | +++ | − | ++ | +++ |
| MyLa 1850 | Nonmalignant T-cell line from CTCL patient | Skin | +++ | +++ | +++ | − | +++ | ++ |
| MySi | Nonmalignant T-cell line from CTCL patient | Skin | +++ | ++ | − | +++ | +++ | +++ |
| MyLa 2355 | Nonmalignant T-cell line from CTCL patient | Peripheral blood | +++ | +++ | +++ | − | +++ | ++ |
| MyLa 1885 | Nonmalignant T-cell line | Peripheral blood | +++ | ++ | +++ | − | +++ | ++ |
| MyLa 3241 | Nonmalignant T-cell line | Peripheral blood | +++ | ++ | ++ | − | +++ | ++ |
| PSOR2 | Psoriasis T-cell line | Skin | +++ | +++ | − | ++ | ++ | ++ |
| PSOR3069 | Psoriasis T-cell line | Skin | +++ | +++ | − | +++ | +++ | ++ |
| AH1 | Atopic dermatitis T-cell line | Skin | +++ | +++ | +++ | − | +++ | ++ |
| P1119 | Healthy donor T-cell line | Peripheral blood | +++ | +++ | +++ | − | +++ | ++ |
| P1183 | Healthy donor T-cell line | Peripheral blood | +++ | +++ | +++ | − | +++ | ++ |
| P1675 | Healthy donor T-cell line | Peripheral blood | +++ | +++ | +++ | +++ | ++ |
Malignant and nonmalignant T-cell lines from skin and blood from CTCL patients, patients with inflammatory skin diseases (psoriasis and atopic dermatitis), and healthy donors were subjected to flow cytometric analysis and scored according to the relative median fluorescence intensity (MFI) of positive cells compared with nonbinding, isotype-matched control antibodies as described in “Materials and methods.” Positive scores were arbitrarily defined as high (+++ or ++++), medium (++), or low (+). Negative (−) scores were defined as less than 5% positive cells.
Nonmalignant T-cell lines from skin and blood were derived from 2 patients with mycosis fungoides and from patients with inflammatory disorders (psoriasis and atopic dermatitis).31,32,39 Skin biopsies were washed twice in sterile phosphate-buffered saline and once in RPMI-1640 and placed in culture flasks containing 90% RPMI-1640, 10% human AB serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 500 ng/mL ciprofloxacin (culture medium = CM) supplemented with 2000 U/mL recombinant human IL-2 and 500 U/mL recombinant human IL-4. The T cells were initially expanded in 5 mL CM with cytokines and when cell density reached 1.5 × 106/mL, the culture was split at a 1:2 ratio. Neither antigen nor feeder cells were initially added to the culture. However, when proliferation of the nonmalignant T-cell lines from MF patients ceased, growth could be restored by further addition of gamma-irradiated autologous lymphoma (malignant) cell lines.30 Nonmalignant skin T cells from MF patients are likely to represent tumor-infiltrating lymphocytes (TILs) as these T cells were isolated from plaques/tumors and expanded in tissue culture with cytokines but without TCR stimulation by mitogens or antigens in vitro.31,32,40,41 Likewise, skin T cells and nonmalignant T cells from blood are also likely to represent in vivo–activated lymphocytes as these cells also responded directly to cytokines without prior TCR stimulation in vitro.31,32,40,41 The molecular and functional features of nonmalignant T cells are distinctly different from malignant T cells. Thus, the nonmalignant T-cell lines do not have constitutive Jak3/Stat3 activity or spontaneous production of cytokines, and they stop to proliferate and undergo apoptosis following cytokine starvation33-38 (and data not shown). The alloantigen-specific, CD4+ human T-cell lines derived from healthy donors have been described and characterized previously.42 All nonmalignant cell lines were depleted of plastic adherent cells, frozen in liquid nitrogen until use, and washed extensively prior to testing in proliferation assays (see “Proliferation assay”), and none proliferated significantly in response to stimulation with PHA, anti-CD3 mAb in soluble form, or staphylococcal enterotoxins A and B42,43 (N.Ø., unpublished observations, January 2006).
Approval was obtained from the University of Copenhagen for these studies. Informed consent was provided according to the Declaration of Helsinki. The project part concerning establishment and study of CTCL cell lines by Dr Keld Kaltoft has been approved by “Den videnskabsetiske Kommite i Århus Amt” (the science ethics committee in Århus County).
Proliferation assay
Assays were performed in culture medium (RPMI 1640; Life Technologies, Roskilde, Denmark) supplemented with 10% pooled human serum, 2 mM l-glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin (NOVO Nordic, Copenhagen, Denmark) in 96-well round-bottom tissue culture plates (Nunc, Roskilde, Denmark) with a final volume of 200 μL.42 Malignant and nonmalignant T cells were cultured at 2.5 or 5.0 × 104 cells/well for 24, 48, or 72 hours with or without cytokines, bacterial superantigens, mAbs, culture supernatants, and/or reagents as indicated. In parallel, cocultures of malignant or nonmalignant T cells and irradiated (22 Gy) malignant or nonmalignant T cells or Epstein-Barr virus (EBV)–transformed lymphoblastoid cell lines (EBV-LCLs) were performed for 24, 48, or 72 hours with or without cytokines, bacterial superantigens, mAbs, culture supernatants, and/or reagents as indicated.
In some experiments, 50 or 100 μL culture supernatants were harvested at different time points and added to fresh cultures of malignant T cells with or without SEA and/or irradiated nonmalignant T cells. In other experiments, malignant and nonmalignant T cells were separated by cell-impermeable membranes (Trans-well) allowing free diffusion of macromolecules but not cells.
Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicated cultures and the percentage inhibition by mAbs calculated as described elsewhere.43
Flow cytometry
For surface staining, cells were stained with the appropriate antibodies for 30 minutes at 4°C, washed, and occasionally stained with secondary polyclonal rabbit anti–mouse immunoglobulin/FITC or polyclonal rabbit anti–human IgG/FITC (DakoCytomation, Glostrup, Denmark) for 30 minutes at 4°C. Flow cytometric measurement of cell proliferation was performed in quadruplicates in 96-well flat-bottomed plates. Cells were stimulated at 106 cell/mL in a final volume of 200 μL. Carboxy fluorescein succinimidyl ester (CFSE) labeling was performed as described priously.38 In brief, malignant T cells were stained with 1 μM carboxy fluorescein diacetate–succinimidyl ester (CFDA-SE, which is transformed to CFSE within living cells) for 10 minutes at 25°C and washed extensively prior to culture for up to 96 hours with or without SEA (100 ng/mL) and with or without nonmalignant T cells at a 1:1 ratio in tissue culture plates with CM without exogenous cytokines. Some cultures were double-stained with CD4 and CD7 antibodies to identify the CD4+, CD7− population of SS cells. Data acquisition and flow cytometric analysis were done on a BD FACSCalibur using CellQuest software (both from Becton Dickinson, Franklin Lakes, NJ).43,44
Results
Malignant cells have a deficient TCR/CD3 function
In the early stages of CTCL, malignant and nonmalignant T cells express functional antigen receptors, whereas a state of anergy may develop as the disease progresses.9 As shown in Figure 1A (a representative result) and summarized in Table 1, CD3 expression is significantly lower in malignant T cells than in nonmalignant T cells isolated from affected skin or blood from patients with tumor-stage MF and SS. Furthermore, ligation of the TCR/CD3 complex by the plate-bound CD3 antibody induced a vigorous proliferation in nonmalignant T cells but had little effect on the spontaneous proliferation of malignant T cells (Figure 1B and data not shown). In contrast, malignant T cells responded to growth factors such as IL-2, IL-7, and IL-15 (Figure 1B and data not shown).
Malignant T cells have deficient expression and function of the TCR/CD3 complex. (A) Nonmalignant (MyLa 1885) and malignant (MyLa 2039) T-cell lines from skin from a patient with tumor-stage MF and malignant T cells from blood of a patient with Sezary syndrome (SeAx) were stained with anti-CD3 mAb, anti–MHC class I (HLA-A, HLA-B, and HLA-C) mAb, or isotype IgG control. Flow cytometry results are shown for viable cells gated from Fsc/Ssc-scatter plots. The data are representative of 3 independent experiments. (B) Nonmalignant (P1183) and malignant (MyLa 2039) T cells were grown in microtiter plates coated with or without anti-CD3 mAb or isotype control mAb and with or without cyclosporine A (10 nM final concentration) or with rIL-2 (100 U/mL) for 48 hours at 37°C in a humidified 5% CO2 atmosphere. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute (+ SD) of triplicate cultures. The data are representative of 5 independent experiments with 3 malignant and 3 nonmalignant T-cell lines.
Malignant T cells have deficient expression and function of the TCR/CD3 complex. (A) Nonmalignant (MyLa 1885) and malignant (MyLa 2039) T-cell lines from skin from a patient with tumor-stage MF and malignant T cells from blood of a patient with Sezary syndrome (SeAx) were stained with anti-CD3 mAb, anti–MHC class I (HLA-A, HLA-B, and HLA-C) mAb, or isotype IgG control. Flow cytometry results are shown for viable cells gated from Fsc/Ssc-scatter plots. The data are representative of 3 independent experiments. (B) Nonmalignant (P1183) and malignant (MyLa 2039) T cells were grown in microtiter plates coated with or without anti-CD3 mAb or isotype control mAb and with or without cyclosporine A (10 nM final concentration) or with rIL-2 (100 U/mL) for 48 hours at 37°C in a humidified 5% CO2 atmosphere. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute (+ SD) of triplicate cultures. The data are representative of 5 independent experiments with 3 malignant and 3 nonmalignant T-cell lines.
Malignant cells express MHC class II and present SE and alloantigens to nonmalignant T cells
Malignant T cells express MHC class II molecules (Table 1), which are high-affinity receptors for bacterial superantigens such as SEs.22,25,26 As shown in Figure 2A, malignant T cells were able to present SEs to nonmalignant T cells. Indeed, SEA-treated malignant T cells were as potent as EBV-LCLs to induce proliferation in SEA-responsive CD4+ T-cell lines. Moreover, the ability to present SEA was strictly dependent on MHC class II binding as indicated by the inability to present SEA mutants with altered MHC class II–binding sites.27 The superantigen presentation was not confined to SEA because malignant T cells were able to present a range of SEs (SEB, SEC, SEE) to the SE-responsive CD4+ T cells (data not shown). Moreover, malignant T cells were able to present alloantigens to alloantigen-specific CD4+ T cells. Thus, malignant T cells from an HLA-DPB*0401–positive CTCL patient induced vigorous proliferation in an HLA-DPB0401–specific CD4+ T-cell line but not in HLA-DPB*0501– and HLA-DPB*0601–specific T cells (Figure 2B). As expected, alloantigen presentation to HLA-DPB*0401 was almost completely blocked by HLA-DP framework antibody but not HLA-DR and HLA-DQ antibodies (Figure 2C). Furthermore, alloantigen presentation was strongly inhibited by an anti–LFA-3 (CD58) antibody, indicating that cell-cell interaction involving the CD58-CD2 complex may be involved in the response (Figure 2D). A chimeric CTLA-4 Ig fusion protein had no effect, suggesting that CD80/86-CD28 axis is not involved and that alloantigen presentation by the malignant T cells differs from the conventional antigen presentation.
Malignant T cells function as superantigen- and alloantigen-presenting cells. (A) SE presentation by malignant to nonmalignant T cells is MHC class II dependent. Nonmalignant T cells (P1119) from healthy donors were grown in microtiter plates with or without malignant T cells (MyLa 2059) or EBV-LCLs and with or without SEA (wt) or SEA (F47A, D227A) with mutations in the HLA class II–binding sites. Malignant T cells and EBV-LCLs were irradiated (22 Gy, as indicated by *) to avoid proliferation. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. The data are representative of 3 independent experiments. (B) Malignant T cells present HLA-DP allospecific T cells. HLA-DPB*0301–, HLA-DPB*0401–, HLA-DPB*0501–, and HLA-DPB*0601–specific CD4+ T-cell lines from healthy donors were grown in microtiter plates with or without HLA-DPB0401–positive irradiated (to avoid proliferation) malignant T cells (MyLa 2039). 3H-thymidine uptake was performed and measured. The data are representative of 3 independent experiments. (C) HLA-DP presentation is blocked by HLA-DP mAb. HLA-DPB*0401–positive malignant T cells (MyLa 2039) were irradiated and incubated with or without HLA-DP, HLA-DQ, or HLA-DR framework mAbs (10 μg/mL) prior to coculture for 48 hours with HLA-DPB*0401–specific nonmalignant T cells in microtiter plates. The data are representative of 3 independent experiments. (D) Alloantigen stimulation by malignant T cells is inhibited by LFA-3 mAb. HLA-DPB*04.01–positive malignant T cells (MyLa 2039) were irradiated and incubated with or without CD5, CD8, LFA-3 mAb (10 μg/mL), or CTLA4-Ig (1 μg/mL) prior to coculture for 48 hours with HLA-DPB*0401–specific nonmalignant T cells in microtiter plates. The data are representative of 3 independent experiments. Error bars represent SD.
Malignant T cells function as superantigen- and alloantigen-presenting cells. (A) SE presentation by malignant to nonmalignant T cells is MHC class II dependent. Nonmalignant T cells (P1119) from healthy donors were grown in microtiter plates with or without malignant T cells (MyLa 2059) or EBV-LCLs and with or without SEA (wt) or SEA (F47A, D227A) with mutations in the HLA class II–binding sites. Malignant T cells and EBV-LCLs were irradiated (22 Gy, as indicated by *) to avoid proliferation. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. The data are representative of 3 independent experiments. (B) Malignant T cells present HLA-DP allospecific T cells. HLA-DPB*0301–, HLA-DPB*0401–, HLA-DPB*0501–, and HLA-DPB*0601–specific CD4+ T-cell lines from healthy donors were grown in microtiter plates with or without HLA-DPB0401–positive irradiated (to avoid proliferation) malignant T cells (MyLa 2039). 3H-thymidine uptake was performed and measured. The data are representative of 3 independent experiments. (C) HLA-DP presentation is blocked by HLA-DP mAb. HLA-DPB*0401–positive malignant T cells (MyLa 2039) were irradiated and incubated with or without HLA-DP, HLA-DQ, or HLA-DR framework mAbs (10 μg/mL) prior to coculture for 48 hours with HLA-DPB*0401–specific nonmalignant T cells in microtiter plates. The data are representative of 3 independent experiments. (D) Alloantigen stimulation by malignant T cells is inhibited by LFA-3 mAb. HLA-DPB*04.01–positive malignant T cells (MyLa 2039) were irradiated and incubated with or without CD5, CD8, LFA-3 mAb (10 μg/mL), or CTLA4-Ig (1 μg/mL) prior to coculture for 48 hours with HLA-DPB*0401–specific nonmalignant T cells in microtiter plates. The data are representative of 3 independent experiments. Error bars represent SD.
Nonmalignant T cells enhance growth of SE- and alloantigen-presenting malignant cells
The findings presented in Figure 2 indicate that malignant T cells can present superantigens and alloantigens to nonmalignant T cells. Because malignant T cells respond to T-cell growth factors such as IL-2, IL-7, and IL-15 (Figure 1B and data not shown), we hypothesized that SE and alloantigen presentation by malignant cells triggers nonmalignant T cells to produce growth factors that could be used by the malignant T cells. Therefore, we cocultured malignant T cells from an SS patient (SeAx) with or without SEA and with or without irradiated (to be devoid of the proliferative capacity) nonmalignant T cells (Figure 3A). Coculture of malignant and nonmalignant T-cell lines in the presence of SEA induced a significant increase in proliferation of the malignant T cells when compared with cultures without SEA, indicating that SEA triggered a growth-stimulating cross talk between malignant and nonmalignant T cells. In contrast, SEA had no effect on proliferation of the malignant T cells in the absence of nonmalignant T cells, indicating that SEA did not directly stimulate proliferation of the malignant T cells (Figure 3A). Similar results were obtained in the cocultures of skin-derived malignant T cells and irradiated nonmalignant T cells (TILs). Thus, SEA induced an enhanced proliferation of malignant T cells (MyLa 2039 and MyLa 2059) in cocultures with autologous, nonmalignant skin-derived T cells (MyLa 1850) or blood-derived T cells (MyLa 2355), whereas SEA had no direct effect on the proliferation of malignant and nonmalignant T cells (Figure 3B and data not shown). Likewise, SEA induced enhanced proliferation of malignant T cells derived from an independent biopsy (MyLa 1929) in cocultures with autologous, nonmalignant skin-derived T cells (MyLa 1850) (Figure 3C) or TILs from another MF patient (MySi cell line) (data not shown). Essentially similar results were obtained in 8 experiments with different combinations of malignant and autologous, nonmalignant T cells with or without SEA and SEE (Figure 4A and data not shown).
Nonmalignant T cells stimulate growth of malignant T cells in the presence of SEA. (A) Malignant T cells (SeAx) were grown with or without irradiated (to be devoid of the proliferative capacity) nonmalignant T cells (P1675) and/or SEA (100 ng/mL) in microtiter plates. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. The data are representative of 3 independent experiments. (B) Malignant T cells (MyLa 2039) and nonmalignant T cells were grown with or without irradiated nonmalignant T cells (MyLa 2355) and/or SEA (100 ng/mL) in microtiter plates. The data are representative of 4 independent experiments. (C) Malignant T cells (MyLa 1929) were grown with or without irradiated nonmalignant T cells (MyLa 1850) and/or SEA (100 ng/mL) in microtiter plates. Error bars represent SD.
Nonmalignant T cells stimulate growth of malignant T cells in the presence of SEA. (A) Malignant T cells (SeAx) were grown with or without irradiated (to be devoid of the proliferative capacity) nonmalignant T cells (P1675) and/or SEA (100 ng/mL) in microtiter plates. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. The data are representative of 3 independent experiments. (B) Malignant T cells (MyLa 2039) and nonmalignant T cells were grown with or without irradiated nonmalignant T cells (MyLa 2355) and/or SEA (100 ng/mL) in microtiter plates. The data are representative of 4 independent experiments. (C) Malignant T cells (MyLa 1929) were grown with or without irradiated nonmalignant T cells (MyLa 1850) and/or SEA (100 ng/mL) in microtiter plates. Error bars represent SD.
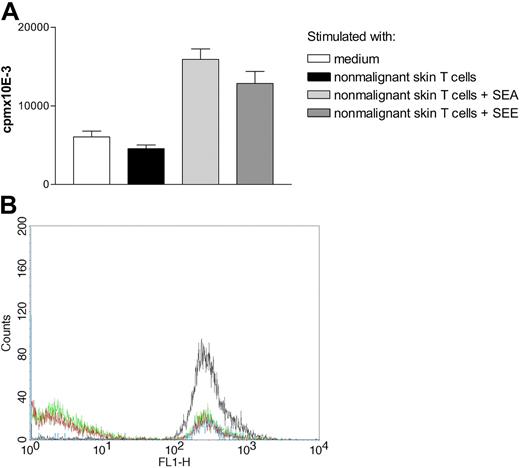
Nonmalignant T cells stimulate growth of malignant T cells in the presence of SEE. (A) Malignant T cells (MyLa 2059) were grown with or without irradiated nonmalignant T cells (MyLa 3241) with or without SEA (100 ng/mL) or SEE (100 ng/mL) for 48 hours in microtiter plates. The data are representative of 3 independent experiments. Error bars represent SD. (B) Nonmalignant T cells stimulate growth of primary SS T cells in the presence of SEA. Flow cytometric analyses of CFSE expression in malignant cells grown for 3 days in medium (blue line), or with SEA (100 ng/mL) (black line), nonmalignant, blood-derived T cells (MyLa 1885) (green line), or SEA and nonmalignant T cells (red line). The following MFI values were obtained: MFI = 264 (blue line); MFI = 339 (black line); MFI = 90 (green line); and MFI = 70 (red line). The data are representative of 2 independent experiments.
Nonmalignant T cells stimulate growth of malignant T cells in the presence of SEE. (A) Malignant T cells (MyLa 2059) were grown with or without irradiated nonmalignant T cells (MyLa 3241) with or without SEA (100 ng/mL) or SEE (100 ng/mL) for 48 hours in microtiter plates. The data are representative of 3 independent experiments. Error bars represent SD. (B) Nonmalignant T cells stimulate growth of primary SS T cells in the presence of SEA. Flow cytometric analyses of CFSE expression in malignant cells grown for 3 days in medium (blue line), or with SEA (100 ng/mL) (black line), nonmalignant, blood-derived T cells (MyLa 1885) (green line), or SEA and nonmalignant T cells (red line). The following MFI values were obtained: MFI = 264 (blue line); MFI = 339 (black line); MFI = 90 (green line); and MFI = 70 (red line). The data are representative of 2 independent experiments.
To study primary tumor cells, we cocultured CFSE-labeled CD4+, CD7−, CD26− malignant T cells from peripheral blood from an SS patient with or without SEA and nonmalignant, blood-derived T cells (MyLa 1885). More than 95% of the SS tumor cells died following labeling/culture, and no spontaneous proliferation was measured (data not shown). Yet, coculture of primary SS cells with nonmalignant T cells induced a proliferative response as indicated by a shift in fluorescence intensity in CFSE-labeled cells following 3 days of culture (Figure 4B, blue line [MFI = 264] versus green line [MFI = 90]). Coculture of primary SS cells and nonmalignant T cells in the presence of SEA induced a further increase in proliferation of SS cells when compared with cultures without SEA (Figure 4B, red line [MFI = 70] versus green line [MFI = 90]). In contrast, SEA did not enhance proliferation of SS cells in the absence of nonmalignant T cells, indicating that SEA did not directly stimulate proliferation of malignant T cells (Figure 4B, black versus blue).
Coculture of HLA-DPB*0401–positive malignant T cells with allospecific CD4+ T cells from healthy donors showed that HLA-DP presentation by malignant T cells to irradiated HLA-DPB*0401–specific T cells induced a significantly greater proliferation of malignant T cells when compared with (1) cultures without nonmalignant T cells, (2) cocultures with HLA-DPB*0501–specific T cells, and (3) cocultures with HLA-DPB*0601–specific T cells (Figure 5A left). Thus, nonmalignant T cells provide growth signals for malignant cells only when recognizing relevant MHC class II molecules on the malignant cells. Indeed, an antibody against HLA-DP framework strongly inhibited the enhanced growth of malignant cells, whereas antibodies against HLA-DR and HLA-DQ framework did not (Figure 5B). Because the HLA-DP–specific T-cell lines were all responsive to SEA, SEA was added to cocultures of malignant T cells and irradiated allospecific T cells (Figure 5A right). HLA-DPB*0501 and HLA-DPB*0601 T cells induced a significantly increased proliferation of malignant T cells, in the presence, but not in the absence, of SEA. In contrast, the enhanced growth of malignant T cells induced by HLA-DPB*0401–specific T cells was not further increased by the presence of SEA (Figure 5A right).
Alloantigen presentation by malignant T cells to nonmalignant T cells stimulates growth of malignant T cells. (A) HLA-DPB*0401–positive malignant T cells (MyLa 2039) were grown with and without SEA (100 ng/mL) and/or HLA-DP*0401–, HLA-DP*0501–, and HLA-DP*0601–specific nonmalignant T cells (irradiated to avoid proliferation) for 48 hours in microtiter plates. The data are representative of 3 independent experiments. (B) HLA-DPB*0401–positive malignant T cells (MyLa 2039) were grown with or without HLA-DPB*0401–specific T cells (irradiated) with or without HLA-DP, HLA-DQ, HLA-DR, CD8, LFA-3, or CD29 mAb (10 μg/mL) for 48 hours in microtiter plates. The percentage inhibition of growth stimulation by coculture was calculated as described in “Materials and methods.” The data are representative of 3 independent experiments. Error bars indicate SD.
Alloantigen presentation by malignant T cells to nonmalignant T cells stimulates growth of malignant T cells. (A) HLA-DPB*0401–positive malignant T cells (MyLa 2039) were grown with and without SEA (100 ng/mL) and/or HLA-DP*0401–, HLA-DP*0501–, and HLA-DP*0601–specific nonmalignant T cells (irradiated to avoid proliferation) for 48 hours in microtiter plates. The data are representative of 3 independent experiments. (B) HLA-DPB*0401–positive malignant T cells (MyLa 2039) were grown with or without HLA-DPB*0401–specific T cells (irradiated) with or without HLA-DP, HLA-DQ, HLA-DR, CD8, LFA-3, or CD29 mAb (10 μg/mL) for 48 hours in microtiter plates. The percentage inhibition of growth stimulation by coculture was calculated as described in “Materials and methods.” The data are representative of 3 independent experiments. Error bars indicate SD.
Mechanisms involved in cross talk between malignant and nonmalignant T cells
To elucidate the mechanisms involved in the growth stimulation of malignant CTCL cells, cocultures were performed with or without antibodies against accessory molecules and growth factors. As shown in Figure 5B, anti–LFA-3 antibodies strongly inhibited (> 60%) the growth-promoting effect. A β1 integrin (CD29) antibody also had some inhibitory effect, whereas CD5 and CD8 antibodies had no inhibitory effect on growth stimulation of malignant T cells (Figure 5B and data not shown). SEA had no effect on the growth of malignant T cells (MyLa cell lines) when separated from nonmalignant T cells by cell-impermeable filters in Trans-well tissue culture plates (data not shown), indicating that SEA-mediated costimulation was critically dependent on direct cell-cell contact. Although neutralizing antibodies against cytokines such as IL-2, IL-7, and IL-15 had no effect on the spontaneous growth of all malignant T cells tested (Figure 6 and data not shown), an IL-2 antibody inhibited the SEA-induced proliferation of SeAx cells in cocultures (Figure 6). In contrast, this antibody had no effect on the SEA-induced proliferation of MyLa cells, indicating that growth factors other than IL-2 might also be involved (data not shown).
Costimulation of SeAx cells by nonmalignant cells and SEA is blocked by an anti–IL-2 mAb. Malignant T cells (SeAx) were grown with or without irradiated (to avoid proliferation) nonmalignant CD4+ T cells (P1675), and/or SEA (100 ng/mL) with or without control mAb or an IL-2–blocking (25 μg/mL) mAb in microtiter plates. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. Essentially identical results were obtained in 2 independent experiments. Error bars represent SD.
Costimulation of SeAx cells by nonmalignant cells and SEA is blocked by an anti–IL-2 mAb. Malignant T cells (SeAx) were grown with or without irradiated (to avoid proliferation) nonmalignant CD4+ T cells (P1675), and/or SEA (100 ng/mL) with or without control mAb or an IL-2–blocking (25 μg/mL) mAb in microtiter plates. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. Essentially identical results were obtained in 2 independent experiments. Error bars represent SD.
Discussion
In the present study, we provide the first evidence that SE stimulates proliferation of malignant T cells through a novel mechanism involving collaboration between malignant and nonmalignant T cells. Thus, SE had no direct effect on the proliferation of malignant T cells—but enhanced their growth in cocultures with nonmalignant T cells. Because the malignant cells used in this study have a deficient expression of the TCR-CD3 complex, it is likely that SE-mediated growth stimulation is not mediated through the antigen receptors. This conclusion is supported by our finding that malignant T cells (as opposed to nonmalignant T cells) do not respond to the immobilized CD3 antibody. Malignant cells display, however, constitutive expression of high-affinity receptors for SE. Accordingly, malignant CTCL cells express a high concentration of MHC class II molecules. Of importance, mutations in the MHC class II–binding domains of SEA27 almost completely blocked presentation of SEA by malignant T cells to nonmalignant T cells. In our allogeneic model, allospecific (nonmalignant) CD4 T cells also stimulated growth of malignant cells but only if the malignant cells expressed the relevant MHC class II molecule, indicating that growth stimulation involves MHC class II on malignant cells and antigen receptors on nonmalignant T cells. This conclusion was supported by our observation that pretreatment of malignant cells with the relevant MHC class II antibody inhibited cross talk between malignant and nonmalignant T cells.
In an attempt to elucidate how cross talk between malignant and nonmalignant T cells takes place, we used several different blocking antibodies as well as the coculture chambers with cell-impermeable membranes. The enhanced proliferation of malignant T cells was critically dependent on direct cell-cell contact between malignant and nonmalignant T cells. Indeed, inclusion of an LFA-3 antibody or separation via cell-impermeable filters inhibited or abolished the effect of nonmalignant T cells on the growth of malignant cells, suggesting that hitherto unidentified cell surface molecules are involved in the growth stimulation. Because nonmalignant T cells express CD2, a natural ligand for LFA-3, it is likely that LFA-3–CD2 interactions are involved in the cross talk between malignant and nonmalignant T cells. In case of SeAx cells, the growth promotion was almost completely blocked by an IL-2 antibody, indicating that SEA presentation by malignant T cells triggered nonmalignant T cells to produce IL-2, which, in turn, stimulated growth of malignant T cells. In contrast, anti–IL-2, anti–IL-7, and anti–IL-15 antibodies had no effect on growth stimulation of MyLa cells. Thus, the malignant T cells seem to differ in terms of growth factor requirements, with SeAx cells being highly responsive and MyLa cells less responsive to IL-2. It is therefore likely that other, as-yet-unidentified, growth factors are also involved in the cross talk between malignant and nonmalignant T cells.
Although SEA had no direct effect on the proliferation of malignant T cells, we cannot exclude the possibility that SEA binding to MHC class II triggers signals involved in the cross talk between malignant and nonmalignant T cells. It is well known that MHC class II ligation in human T cells (nonmalignant) activates a signal transduction cascade involving protein tyrosine (src) kinases, phospholipase C, and elevations in intracellular free calcium,43,45 and studies are in progress to elucidate which genes are activated by SEs in malignant T cells.
Clinical experience indicates that bacterial infection is a complicating factor in CTCL, and systemic antibiotic therapy has been associated with a decrease in tumor size and tumor-associated symptoms such as erythroderma.18,46 For example, Jackow et al19 showed that more than 75% of CTCL patients had a positive staphylococcal culture from skin and blood. Of interest, one half of the patients with positive cultures grew SE-producing Staphylococcus aureus, and most of these patients had a severe disease such as Sezary syndrome and rapidly enlarging MF plaques or tumors. As the malignant T-cell lines used in this study have a deficient surface expression of CD3/TCR, we cannot exclude the possibility that SE may provide growth signals via the CD3-TCR complex in vivo. However, our preliminary findings in primary tumor cells from SS patients suggest that this may not be the case. Instead, our findings indicate that superantigens stimulate cancer growth via an indirect pathway involving cross talk between malignant and nonmalignant T cells.
In conclusion, we provide evidence that SE triggers growth of malignant T cells via a novel mechanism involving cross talk between malignant and nonmalignant T cells. Our findings point toward the use of aggressive antibiotic therapy in patients with SE-producing infections. Moreover, they suggest blockage of bacterial superantigens and nonmalignant “bystander” T cells (eg, by antibodies) as a new therapeutic strategy in CTCL.
Authorship
Contribution: A.W. designed and performed research and wrote the paper; P.L., K.W.E., T.K., T.L., Q.Z., and A.-M.M. performed research; C.G. and A.S. contributed vital new reagents; M.A.W. analyzed data; N.O. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niels Ødum, IMMI, Panum 22.5.34, University of Copenhagen, Blegdamsvej 3c, DK2200 Copenhagen N, Denmark; e-mail: n.odum@immi.ku.dk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research grants from the University of Copenhagen, The Danish Research Councils, The Novo Nordic Foundation, Fabrikant Vilhelm Pedersen og Hustrus Mindelegat, The Danish Cancer Research Foundation (Dansk Kræftsforsknings Fond), The Danish Cancer Society (Kræftens Bekæmpelse), Direktør Jacob Madsen & Hustru Olga Madsens Fond, Købmand i Odense Johann og Hanne Weimann f. Seedroff′s Legat (T.L.), and the National Cancer Institute-CA89194 (M.A.W.).
We wish to thank Keld Kaltoft (Århus Universitet and CellCure Århus, Denmark) for the generous gift of MF, MySi, and SeAx cell lines and Mikael Dohlsten (Active Biotech, Lund Sweden) for the generous gift of SEA wt and SEA mutants.

![Figure 1. Malignant T cells have deficient expression and function of the TCR/CD3 complex. (A) Nonmalignant (MyLa 1885) and malignant (MyLa 2039) T-cell lines from skin from a patient with tumor-stage MF and malignant T cells from blood of a patient with Sezary syndrome (SeAx) were stained with anti-CD3 mAb, anti–MHC class I (HLA-A, HLA-B, and HLA-C) mAb, or isotype IgG control. Flow cytometry results are shown for viable cells gated from Fsc/Ssc-scatter plots. The data are representative of 3 independent experiments. (B) Nonmalignant (P1183) and malignant (MyLa 2039) T cells were grown in microtiter plates coated with or without anti-CD3 mAb or isotype control mAb and with or without cyclosporine A (10 nM final concentration) or with rIL-2 (100 U/mL) for 48 hours at 37°C in a humidified 5% CO2 atmosphere. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute (+ SD) of triplicate cultures. The data are representative of 5 independent experiments with 3 malignant and 3 nonmalignant T-cell lines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-04-017863/4/m_zh80080711170001.jpeg?Expires=1769751878&Signature=OMOBLQyWETmNykWsFZkLy4IJ6thHM2PAS69ughEovOEZ-5FPkjj7W1kdDQ0mA4v0o~EI3kLJQZFvSkfQ6g6G45XR-lnaX8lU02ONRQLP9jsTE3CT9tWe4rgVOLOGeViX321ViJsn7nPmEYBvc5M-Z67aeS3YUqnRYMy6tjoiSPobcH~bVu8u2974-9F1ug1mEOeq7uQNQm2JbpkF0EJ8Hw0l5TnV~8cIACtPr5wqh4HTv8cgT5zUHSRmFB9tLZUf0DyHvH4X2dd~FXZQn-19HL8uERbkHIk-sizUslH3aqQWREUjJMXUZF2tYygmVuwDALhuQZOZnaIZMVzwABzfKg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Malignant T cells function as superantigen- and alloantigen-presenting cells. (A) SE presentation by malignant to nonmalignant T cells is MHC class II dependent. Nonmalignant T cells (P1119) from healthy donors were grown in microtiter plates with or without malignant T cells (MyLa 2059) or EBV-LCLs and with or without SEA (wt) or SEA (F47A, D227A) with mutations in the HLA class II–binding sites. Malignant T cells and EBV-LCLs were irradiated (22 Gy, as indicated by *) to avoid proliferation. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. The data are representative of 3 independent experiments. (B) Malignant T cells present HLA-DP allospecific T cells. HLA-DPB*0301–, HLA-DPB*0401–, HLA-DPB*0501–, and HLA-DPB*0601–specific CD4+ T-cell lines from healthy donors were grown in microtiter plates with or without HLA-DPB0401–positive irradiated (to avoid proliferation) malignant T cells (MyLa 2039). 3H-thymidine uptake was performed and measured. The data are representative of 3 independent experiments. (C) HLA-DP presentation is blocked by HLA-DP mAb. HLA-DPB*0401–positive malignant T cells (MyLa 2039) were irradiated and incubated with or without HLA-DP, HLA-DQ, or HLA-DR framework mAbs (10 μg/mL) prior to coculture for 48 hours with HLA-DPB*0401–specific nonmalignant T cells in microtiter plates. The data are representative of 3 independent experiments. (D) Alloantigen stimulation by malignant T cells is inhibited by LFA-3 mAb. HLA-DPB*04.01–positive malignant T cells (MyLa 2039) were irradiated and incubated with or without CD5, CD8, LFA-3 mAb (10 μg/mL), or CTLA4-Ig (1 μg/mL) prior to coculture for 48 hours with HLA-DPB*0401–specific nonmalignant T cells in microtiter plates. The data are representative of 3 independent experiments. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-04-017863/4/m_zh80080711170002.jpeg?Expires=1769751878&Signature=1yqj3yxZ0em5sky5DcExHqOdOg2P7L2YHOZAGCRVX3L1JbeTXEDuLQx1lB3Y-rDotQk4aWM5w7Fw7G4rH9--5-e-jqBb8I7ycGropWa5djtE3kGOYIe5TTbkuXzm3VHwjmSMl2OVby3kvvXBcae5b6zV6Mm2~3ifoQEKEyjugqb3K87p7WZFcuBP8tuW2gXOeCJC-WwFb1Zj~qGWEEqDF7yZXHchiyBoIgxVcltpghmKh4ACydyh0XwgM49H-Fc~134jSMdueSC69aWL3tSlmERNgTMFYQVyfhyKyDrs5uXe0QP4CSVYTdUxuNXpiuCKVnazVYx3lEpfI9Q6NDLPgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Nonmalignant T cells stimulate growth of malignant T cells in the presence of SEA. (A) Malignant T cells (SeAx) were grown with or without irradiated (to be devoid of the proliferative capacity) nonmalignant T cells (P1675) and/or SEA (100 ng/mL) in microtiter plates. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. The data are representative of 3 independent experiments. (B) Malignant T cells (MyLa 2039) and nonmalignant T cells were grown with or without irradiated nonmalignant T cells (MyLa 2355) and/or SEA (100 ng/mL) in microtiter plates. The data are representative of 4 independent experiments. (C) Malignant T cells (MyLa 1929) were grown with or without irradiated nonmalignant T cells (MyLa 1850) and/or SEA (100 ng/mL) in microtiter plates. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-04-017863/4/m_zh80080711170003.jpeg?Expires=1769751878&Signature=JRReQ4-orBr9XxCGjzXO8LFbyJ4cWPJ3xD65SW7TL917FKOfqAFzCeBgd5LDbvw1XEWvCJQUO1UCoqggfQlcve4Lj8Cchwv7i777kmK0RcpBF61aTPsylByQL6wpvCXqZ~YiWm7MWt38h28lWAn~GkOId8Q9d25TdkFKDwmAKoxVFAy8BxOuHJ68CT7mS3atLLAUArIW2fgTHBTzS~DXzCeXL1hiC7g~pUP6RwFg9FrNbrSOsumubwECJaqPOaMnLrbi~eB03LbE-z3VpMKl6CNMoXYwl8n7G98IRhsW0ab1EvLH0-neqPZXQRPqbuAHcKsW4yw2VtQmhJ-zh6-H3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Costimulation of SeAx cells by nonmalignant cells and SEA is blocked by an anti–IL-2 mAb. Malignant T cells (SeAx) were grown with or without irradiated (to avoid proliferation) nonmalignant CD4+ T cells (P1675), and/or SEA (100 ng/mL) with or without control mAb or an IL-2–blocking (25 μg/mL) mAb in microtiter plates. Twelve hours prior to harvest, 3H-thymidine (1 μCi [0.037 MBq]/well) was added, the cells were harvested onto glass fiber filters, and 3H-thymidine incorporation was measured. The proliferation was expressed as mean counts per minute of triplicate cultures. Essentially identical results were obtained in 2 independent experiments. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/8/10.1182_blood-2006-04-017863/4/m_zh80080711170006.jpeg?Expires=1769751878&Signature=ms~QhvJbgvD8z2tjr2KmB85ZaODmYjLuessGX5zY8gDceU9MxWvcTNLw5Nl88Ms27Z0IZHY27GqSDPPdXFeCE2fB-yUu3AySA1CVETDI0Reb1wUmn8AcTNjLOLN2k3L6aA1Nbt~SANxFxJIqKt-5tzFu0ZvA0d~nBrL9EVWBexJZpQmSkZ3qYxXcXj4JI43gkFLYe-YzTaDwCBx6XyURvJN5DVr6AdDEezcpVnHnoztlTSzwpNdBoKAS1prmihfZdfcNgTY~arqRUQ5Znx7~zi4zY0e481cT-6uy261HdVNOmqSnX1a-DDPpKxvmJ2f73iXlMXtbOBBmknvM1Uc8yQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal