Abstract
We identified a family with gray platelet syndrome (GPS) segregating as a sex-linked trait. Affected males had a mild bleeding disorder, thrombocytopenia, and large agranular platelets characteristic of GPS, while obligate carrier females were asymptomatic but had dimorphic platelets on peripheral smear. Associated findings included mild erythrocyte abnormalities in affected males. Linkage analysis revealed a 63 cM region on the X chromosome between markers G10578 and DXS6797, which segregated with the platelet phenotype and included the GATA1 gene. Sequencing of GATA1 revealed a G-to-A mutation at position 759 corresponding to amino acid change Arg216Gln. This mutation was previously described as a cause of X-linked thrombocytopenia with thalassemia (XLTT) but not of gray platelet syndrome. Our findings suggest that XLTT is within a spectrum of disorders constituting the gray platelet syndrome, and we propose that GATA1 is an upstream regulator of the genes required for platelet α-granule biogenesis.
Introduction
Gray platelet syndrome (GPS) is a rare, mild bleeding diathesis characterized by large dysfunctional, agranular platelets that appear gray on Wright-stained peripheral blood smears. Patients exhibit bruising and mucocutaneous bleeding out of proportion to their mild to moderate thrombocytopenia. Since the first description in 1971,1 many sporadic cases as well as a few autosomal dominant2 and recessive3 pedigrees, totaling approximately 60 cases, have been reported. While GPS has been described biochemically and pathologically, no mutation has been associated with this disorder. This report describes a new GPS pedigree wherein the disorder segregates with a missense mutation in GATA1.
Patients, materials, and methods
A healthy 1-year-old female presented for evaluation of a family history of thrombocytopenia. Her father and paternal uncle, both with lifelong histories of bruising and bleeding, had been diagnosed with GPS previously; in fact, her uncle has been described previously (Figure 1A).4 The family history was notable for multiple male relatives in the paternal lineage with bleeding symptoms (Figure 1A). The proband had no bleeding history and had a normal physical examination and complete blood count (CBC), including a platelet count of 382 × 109/L (382 000/μL). Review of her peripheral blood smear revealed 2 distinct populations of platelets: one normal appearing; the other large, agranular, and pale (Figure 1B).
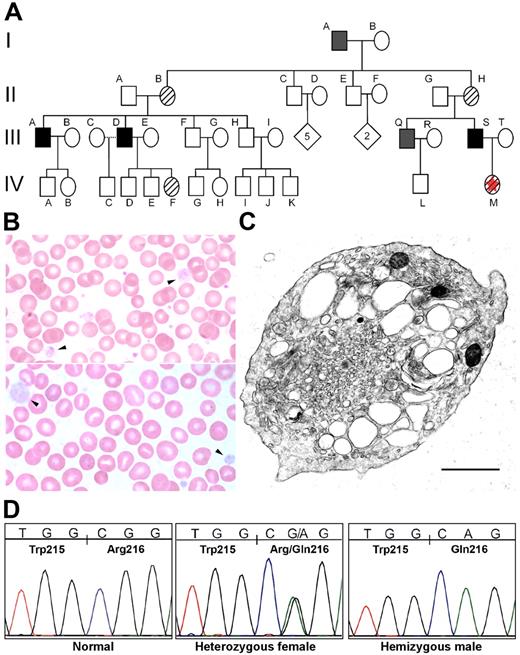
Familial GPS. (A) This pedigree reflects the X-linked pattern of inheritance that was suspected clinically and later confirmed by DNA analysis. Subject I-A was diagnosed with idiopathic thrombocytopenic purpura (ITP) in childhood and died of complications of chronic myelogenous leukemia. His medical record contains frequent references to his abnormally large platelets. Subject III-A has been extensively evaluated for his history of chronically low platelets without a definitive diagnosis. Subject III-D had a splenectomy to manage persistent thrombocytopenia. ▪, affected; ▨, carrier; ▪, inferred from clinical history; ⋄, not tested; □, not affected; ☆, proband. (B) The proband's blood smear (IV-M) contains normal-appearing platelets and a population of abnormally large, pale, agranular platelets (arrowheads). A blood smear from the proband's father (III-S) contains decreased platelets, and those present have similarly abnormal morphology. (C) Electron micrograph (EM) of a peripheral blood platelet from subject III-S shows a large platelet with enlarged elements of the open canalicular system and the virtual absence of α-granules. The α-granules that are present lack the characteristic electron density, appearing “empty.” Scale bar, 1 μm. Original magnification, 35 100×. A range of platelet EM morphologies was seen in this individual and his brother (subject III-Q), including some platelets with more abundant, better-formed α-granules (Figure S2). (D) DNA sequencing of GATA1 exon 4 demonstrated a G-to-A transition segregating with the phenotype and predicted to result in an arginine-to-glutamine change at amino acid 216 (R216Q).
Familial GPS. (A) This pedigree reflects the X-linked pattern of inheritance that was suspected clinically and later confirmed by DNA analysis. Subject I-A was diagnosed with idiopathic thrombocytopenic purpura (ITP) in childhood and died of complications of chronic myelogenous leukemia. His medical record contains frequent references to his abnormally large platelets. Subject III-A has been extensively evaluated for his history of chronically low platelets without a definitive diagnosis. Subject III-D had a splenectomy to manage persistent thrombocytopenia. ▪, affected; ▨, carrier; ▪, inferred from clinical history; ⋄, not tested; □, not affected; ☆, proband. (B) The proband's blood smear (IV-M) contains normal-appearing platelets and a population of abnormally large, pale, agranular platelets (arrowheads). A blood smear from the proband's father (III-S) contains decreased platelets, and those present have similarly abnormal morphology. (C) Electron micrograph (EM) of a peripheral blood platelet from subject III-S shows a large platelet with enlarged elements of the open canalicular system and the virtual absence of α-granules. The α-granules that are present lack the characteristic electron density, appearing “empty.” Scale bar, 1 μm. Original magnification, 35 100×. A range of platelet EM morphologies was seen in this individual and his brother (subject III-Q), including some platelets with more abundant, better-formed α-granules (Figure S2). (D) DNA sequencing of GATA1 exon 4 demonstrated a G-to-A transition segregating with the phenotype and predicted to result in an arginine-to-glutamine change at amino acid 216 (R216Q).
Subjects provided written, informed consent in accordance with Children's Hospital Committee of Clinical Investigation and the Declaration of Helsinki. May-Grünwald-Giemsa (Sigma, St. Louis, MO)–stained peripheral blood smears were photographed using a Nikon Eclipse E600 microscope equipped with a 100×/0.30 numerical aperture oil immersion lens and an RT Slider SPOT 2.3.1 camera (Diagnostic Instruments, Sterling Heights, MI) using SPOT Advanced software (version 3.5.9). DNA was extracted from peripheral blood leukocytes using the Puregene DNA Purification Kit (Gentra, Minneapolis, MN). For linkage analysis, X chromosome markers DXS6807, DXS9902, DXS9896, DXS6810, G10578, DXS7132, DXS6800, DXS6789, DXS6799, DXS6797, DXS6804, and G10699 were amplified by polymerase chain reaction (PCR). Forward primers for each marker were labeled with 32P and amplified in 10.2 μL PCR reactions each containing 20 ng genomic DNA. PCR products were separated on a 6% acrylamide gel.
The 5 coding exons and the 3′ region of the promoter of GATA1 were sequenced from genomic PCR products. Primer sequences and PCR conditions are listed in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Products were purified by microcentrifuge column filtration (Qiagen, Valencia, CA) and agarose gel electrophoresis. Sequencing was performed on the Applied Biosystems 3730 DNA Analyzer (Applied Biosystems, Foster City, CA).
Electron microscopy studies of platelets were performed on a Philips 400 electron microscope (Philips, Eindhoven, The Netherlands).
Results and discussion
Eleven members of the extended kindred participated in the study. In clinically affected males, CBC revealed thrombocytopenia and increased mean platelet volume (MPV) (Table 1; subjects III-A, III-D, III-S). Mild microcytosis and elevated red cell distribution width (RDW) were also apparent in those subjects. These data are also represented in ADVIA graph format (Bayer, Tarrytown, NY) in Figure S1. Hemoglobin electrophoresis in subject III-S was consistent with a mild β-thalassemia–like phenotype due to a hemoglobin A2 (Hb A2) of 4.5% (Table 1). No female subjects had automated CBC values outside the normal range. Review of peripheral blood smears revealed large, abnormal, pale platelets in clinically affected males and 2 populations of platelets in female subjects II-B, II-H, IV-F, and IV-M (Figure 1B). This suggested a pattern of sex-linked inheritance, because all affected subjects were male, females had dimorphic pathology, and no male-to-male transmission was apparent. Electron microscopy of peripheral blood platelets in 2 affected males (Figure 1A; subjects III-Q and III-S) showed large agranular or paucigranular platelets (Figures 1D and S3) characteristic of gray platelets.
CBC results
| Patient . | Platelet count,× 109/L . | MPV, fL . | Hematocrit, proportion of 1 . | Red blood cell count, × 1012/L . | Mean corpuscular volume, fL . | RDW, % . |
|---|---|---|---|---|---|---|
| Affected males | ||||||
| III-A | 76 | 9.5 | .402 | 5.1 | 78.9 | 18.4 |
| III-D | 177 | 10.8 | .451 | 5.7 | 79.6 | 19.3 |
| III-S | 58 | 9.8 | .424 | 5.4 | 78.6 | 17.1 |
| Mean | 67 | 10.0 | .426 | 5.4 | 79.1 | 18.3 |
| Obligate carrier females | ||||||
| II-B | 236 | 8.1 | .456 | 5.1 | 90.0 | 13.4 |
| II-H | 178 | 7.6 | .384 | 4.7 | 81.3 | 13.7 |
| IV-F | 312 | 8.0 | .373 | 4.8 | 77.1 | 13.9 |
| IV-M | 354 | 7.7 | .335 | 4.2 | 78.9 | 14.0 |
| Mean | 227 | 7.9 | .390 | 4.7 | 82.1 | 13.7 |
| Unaffected | ||||||
| II-C | 316 | 8.9 | .355 | 4.4 | 81.4 | 18.2 |
| III-T | 273 | 8.6 | .354 | 4.4 | 80.4 | 13.7 |
| IV-D | 266 | 7.5 | .466 | 5.7 | 82.3 | 12.9 |
| IV-E | 347 | 8.1 | .458 | 5.5 | 83.9 | 12.7 |
| Patient . | Platelet count,× 109/L . | MPV, fL . | Hematocrit, proportion of 1 . | Red blood cell count, × 1012/L . | Mean corpuscular volume, fL . | RDW, % . |
|---|---|---|---|---|---|---|
| Affected males | ||||||
| III-A | 76 | 9.5 | .402 | 5.1 | 78.9 | 18.4 |
| III-D | 177 | 10.8 | .451 | 5.7 | 79.6 | 19.3 |
| III-S | 58 | 9.8 | .424 | 5.4 | 78.6 | 17.1 |
| Mean | 67 | 10.0 | .426 | 5.4 | 79.1 | 18.3 |
| Obligate carrier females | ||||||
| II-B | 236 | 8.1 | .456 | 5.1 | 90.0 | 13.4 |
| II-H | 178 | 7.6 | .384 | 4.7 | 81.3 | 13.7 |
| IV-F | 312 | 8.0 | .373 | 4.8 | 77.1 | 13.9 |
| IV-M | 354 | 7.7 | .335 | 4.2 | 78.9 | 14.0 |
| Mean | 227 | 7.9 | .390 | 4.7 | 82.1 | 13.7 |
| Unaffected | ||||||
| II-C | 316 | 8.9 | .355 | 4.4 | 81.4 | 18.2 |
| III-T | 273 | 8.6 | .354 | 4.4 | 80.4 | 13.7 |
| IV-D | 266 | 7.5 | .466 | 5.7 | 82.3 | 12.9 |
| IV-E | 347 | 8.1 | .458 | 5.5 | 83.9 | 12.7 |
For patient III-S, Hb A2 was 4.5% and reticulocytes were 3.5%; for patient II-H, Hb A2 was 2.6% and reticulocytes were 2.5%.
Using linkage analysis with 15 markers on the X chromosome, we identified a common haplotype in the 63 cM region between markers G10578 and DXS6797, including GATA1 in all affected and carrier subjects. Sequencing GATA1 revealed a G-to-A transition at position 759 in the cDNA located within exon 4. This is predicted to result in an arginine to glutamine change at codon 216 of the protein within the amino-terminal zinc finger of GATA1.
The diagnosis of GPS in this family was determined by the characteristic appearance of platelets on peripheral blood smear, abnormal platelet function studies, and the absence or paucity of α-granules with retained dense granules on electron microscopy.4 In addition to this platelet phenotype, some patients have exhibited nonprogressive myelofibrosis and/or splenomegaly, neither of which was assessed in this family. The variability of the phenotype suggests GPS is a genetically heterogeneous disorder. While Arg216Gln is the first mutation associated with GPS, it is highly likely that mutations in a diverse set of genes could result in a similar phenotype. We propose that GATA1 regulates transcription of the genes necessary for normal platelet granule morphogenesis or retention. Therefore, the set of GATA1 cofactors as well as transcriptional targets are likely candidate genes for other forms of GPS.
Arg216Gln was previously reported5-7 in association with the syndrome of X-linked thrombocytopenia with thalassemia (XLTT).8 XLTT is characterized by thrombocytopenia, splenomegaly, and an increased α/non-α globin chain ratio resulting in a mild β-thalassemia–like pathology. Platelets and megakaryocytes from patients with XLTT are large and deficient in α-granules, identical to the cardinal phenotype of GPS.7 Furthermore, and similar to GPS, in XLTT α-granular proteins are found in reduced concentrations diffusely throughout platelets.7,9 Given the phenotypic similarities and now the genetic correlation provided by the family reported here, it is likely that all patients with the Arg216Gln mutation, and therefore XLTT, have GPS.
GATA1 mutations may not be uncommon in GPS, because the family we report here represents the fourth incidence of this mutation described in the literature. The frequency of the G759A transition may be accounted for by the fact that it occurs in a CpG dinucleotide and therefore is prone to mutation.10,11
The R216Q mutation is known to affect DNA binding, diminishing the ability of the transcription factor to bind GATA binding sites.5 A second mutation at the same residue, Arg216Trp, results in a dramatic erythropoietic porphyria phenotype with morphologically normal platelets.12 Other amino-terminal zinc finger mutations affect cofactor binding and cause thrombocytopenia and dyserythropoiesis.13-16 While we do not know that any of these cofactor binding face mutations result in a definitive gray platelet phenotype, these mutations do result in macrothrombocytopenia and/or α-granule deficiency. Therefore, we suggest that GATA1 mutations should be considered in seemingly sporadic, male cases of GPS. Continuing investigations of these mutations may elucidate the difference between DNA binding and cofactor binding in the function of GATA1.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark D. Fleming, Enders 1116.1, Children's Hospital Boston, 320 Longwood Ave, Boston, MA 02115; e-mail: mark.fleming@childrens.harvard.edu.
Presented in abstract form at the 47th annual meeting of the American Society of Hematology, Atlanta, GA, December 11, 2005.17
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr Alan Cantor for critical comments regarding this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal