Abstract
Although typically considered a neurotransmitter, there is substantial evidence that serotonin (5-HT) plays an important role in the pathogenesis of inflammatory disorders. Despite these findings, the precise role of 5-HT in modulating immune function, particularly T-cell function, remains elusive. We report that naive T cells predominantly express the type 7 5-HT receptor (5-HTR), and expression of this protein is substantially enhanced on T-cell activation. In addition, T-cell activation leads to expression of the 5-HT1B and 5-HT2A receptors. Significantly, exogenous 5-HT induces rapid phosphorylation of extracellular signal-regulated kinase-1 and -2 (ERK1/2) and IκBα in naive T cells. 5-HT–induced activation of ERK1/2 and NFκB is inhibited by preincubation with a specific 5-HT7 receptor antagonist. Thus, 5-HT signaling via the 5-HT7 receptor may contribute to early T-cell activation. In turn, 5-HT synthesized by T cells may act as an autocrine factor. Consistent with this hypothesis, we found that inhibition of 5-HT synthesis with parachlorophenylalanine (PCPA) impairs T-cell activation and proliferation. Combined, these data demonstrate a fundamental role for 5-HT as an intrinsic cofactor in T-cell activation and function and suggest an alternative mechanism through which immune function may be regulated by indoleamine 2,3-dioxygenase–mediated catabolism of tryptophan.
Introduction
The central and peripheral nervous systems can modulate immune function by releasing soluble factors such as hormones, neuropeptides, and neurotransmitters.1,2 Serotonin (5-HT) is a classical neurotransmitter and vasoactive amine best known for its role in the regulation of variety of physiologic states and behaviors, including pain, appetite, mood, and sleep.3 Despite these major roles for 5-HT in the central nervous system, the vast majority of 5-HT is produced in the periphery (> 90%), primarily by enterochromaffin cells in the gut.4 Consistent with its abundance in the periphery, 5-HT is recognized as an important inflammatory mediator with significant immune-modulatory effects.2,5 Mast cells and platelets both express the 5-HT specific transporter (SERT) which enables them to sequester 5-HT from the microenvironment. In turn, 5-HT is released in response to injury and/or inflammatory signals such as platelet activating factor, complement components (C3a and C5a), and IgE complexes.5,6 Released 5-HT has been shown to regulate platelet aggregation7 and to promote the accessory function of macrophages, and it is a potent eosinophil chemoattractant.8,9 Consistent with these effects, 5-HT is implicated in the pathogenesis of inflammatory disorders, including asthma, inflammatory bowel syndrome, allergic diarrhea, and chronic eczema.5,10-14
Several studies have demonstrated that T and B lymphocytes are functionally responsive to 5-HT, implicating a role for this monoamine in the generation of adaptive immune responses. Inhibition of endogenous 5-HT synthesis can impair rodent T-cell proliferation.2,15,16 Conversely, exogenous 5-HT is reported to suppress T-cell proliferation.3 5-HT triggers the increased release of preformed IL-16 from CD8+ T cells and can initiate delayed-type hypersensitivity reactions through the local recruitment and activation of CD4+ T cells.17-19 Thus, the precise role of 5-HT in modulating lymphocyte activation or function is currently ambiguous. Moreover, the identity of 5-HTRs expressed by T cells and the intracellular signaling pathways that transduce this signal remain unclear. 5-HTR signaling is complex with 7 recognized receptor subfamilies.2 With the exception of the 5-HT3 receptor, which is a ligand-gated ion channel, they belong to the family of 7 transmembrane G protein–coupled receptors. Both 5-HT1 and 5-HT2 receptor subfamilies have been implicated in signaling T cells.3,15-17,20,21 With rare exception, however,20 most studies have only demonstrated the expression of 5-HTR gene transcripts or pharmacologic sensitivity to 5-HTR agonists or antagonists.3
To elucidate the function of 5-HT signaling in T cells, we have made a comprehensive analysis of 5-HTR expression and signaling in primary mouse T cells. We show that naive T cells primarily express the 5-HT7 receptor. Exogenous 5-HT leads to rapid phosphorylation of extracellular signal related kinase-1 and -2 (ERK1/2) and IκBα in bulk naive T cells, early steps that are recognized to lead to T-cell activation. Activation of ERK1/2 and NFκB is inhibited by a specific 5-HT7 receptor antagonist. Consistent with these data, inhibition of 5-HT synthesis by T cells impairs their capacity for ex vivo proliferation and expression of CD25. In sum, these data strongly support the hypothesis that 5-HT is an endogenous accessory signal that promotes T-cell activation and proliferation. In addition, we provide the first direct evidence that the 5-HT7 receptor transduces the initial 5-HT signal in naive T cells. As T cells become activated, we hypothesize that 5-HT1B and/or 5-HT2A receptors may provide additional accessory signals that sustain T-cell proliferation and/or direct T-cell differentiation.
Materials and methods
Animals
Male C57BL/6 or BALB/c mice (7-12 weeks; Charles River Laboratory, Wilmington, MA) were used in accordance with protocols approved by the University of Western Ontario Animal Care and Use Subcommittee, and the Canadian Council on Animal Care. Endogenous 5-HT synthesis was inhibited by administration of para-chlorophenlyalanine (250 mg/kg, intraperitoneally; Sigma, St Louis, MO)22 in saline on days −5, −3, and −1, and spleens were removed the following day (day 0).
T-cell isolation and culture
Spleen T cells were purified (SpinSep T-cell enrichment kit; StemCell Technologies, Vancouver, BC) and cultured at 1 × 106 cells/mL in RPMI 1640 (Biosource, Rockville, MD) supplemented with 10% FCS (Hyclone, Logan, UT), nonessential amino acids, L-glutamine, sodium pyruvate, penicillin-streptomycin, and 2-ME (Invitrogen, Carlsbad, CA). In some experiments, FCS was pretreated with charcoal-coated dextran (0.25% wt/vol; 12 hours, 4°C; C6241; Sigma) to absorb exogenous 5-HT.23 Complete media supplemented with 10% FCS contains 299 ± 23 nM 5-HT, and this was reduced to 4.32 ± 2.6 nM following treatment with charcoal-coated dextran.
T cells were activated by culture for 48 hours with 5 μg/mL Concanavalin A (Con A; Sigma), a combination of PMA (10 nM) together with ionomycin (1 μM; Sigma), or by culture in the presence of plate-bound anti-CD3 mAb (5 μg/mL, 145-2C11; BD Biosciences, San Jose, CA). To assess T-cell activation and expansion, 1 × 105 T cells were placed in the wells of a 96-well, round-bottom plate and incubated in complete medium for 48 to 72 hours. In some experiments, cultures were supplemented with 5-HT (1.0 μM), or the selective 5-HT7 receptor agonist AS 19 (1.0 μM; Tocris Bioscience, Ellisville, MO). Proliferation was determined by the addition of 1 μCi (0.037 MBq) [3H] thymidine (TdR) to each well for the final 18 hours of culture. Cells were harvested using a multiple-well harvester, and [3H]TdR incorporation was determined in a liquid scintillation counter. T-cell activation was determined by labeling with rat anti–mouse CD4 FITC (RM4-5) and biotinylated rat anti–mouse CD25 (PC 61) followed by secondary labeling with SA-APC (all from BioLegend, San Diego, CA). Samples were analyzed using a FACSCalibur flow cytometer and Cell Quest software (BD Biosciences).
RT-PCR
RNA was extracted using TRIzol (Invitrogen), and first-strand cDNA synthesis was performed using Advantage RT-for-PCR (BD Biosciences). For conventional reverse transcription–polymerase chain reaction (RT-PCR) cDNA was amplified using Taq DNA polymerase (Eppendorf, Hamburg, Germany). Comparable quantities of cDNA were ensured by amplification of GAPDH (forward, 5′-GCC GCC TGG AGA AAC CTG CCA AGT-3′, and reverse, 5′-TAT TCA AGA GAG TAG GGA GGG CTC-3′). All other primers are as follows: 5-HT1A (forward, 5′-ACC CCA ACG AGT GCA CCA TCA G-3′, and reverse, 5′-GCA GGC GGG GAC ATA GGA G-3′), 5-HT1B (forward, 5′-CGA TGC GGT GGA GTA TTC TGC-3′, and reverse, 5′-TAG CGG CCA TGA GTT TCT TCT TTT-3′), 5-HT1D (forward, 5′-CTC TGA GAC CCG GGT TGA TTT G-3′, and reverse, 5′-GGG CCA CTG ATA CCA CTT TCC TTA-3′), 5-HT1F (forward, 5′-CGC AGC GAT CAC GAG GGA GTT-3′, and reverse, 5′-CGG CGG GCT AGG GAC CAG A-3′), 5-HT2A (forward, 5′-CAG CCC TCC CTC CTC GTT TTG-3′, and reverse, 5′-GCC GGA AGT TGT AGC AGA TGA AGT-3′), 5-HT2B (forward, 5′-AGG ATA ATT TGG CCC GAG TGC TG-3′, and reverse, 5-GGT TTC CTT GTC ATG CCC GTG T-3′) 5-HT2C (forward, 5′-TCG TGA TTA TAA TCA TGA CAA TAG GGG GC-3′, and reverse, 5′-TGA GCA CGC AGG TAG TAT TAT TCA CGA AC-3′), 5-HT3 (forward, 5′-CAA CGT GGA TGA GAA GAA CCA GG-3′, and reverse, 5′-AGC AAG AGG CTG ACT GCA TAG AAT AAA G-3′), 5-HT4 (forward, 5′-CTG GGC TTA TGG GGA GAT GTT CT-3′, and reverse, 5′-GCT GGG GCC TGC TTT CAG AG-3′), 5-HT5A (forward, 5′-TGC AGG CGA CCA GAC AAC AG-3′, and reverse, 5′-CCG ACT AGG GCG CAG GAC TT-3′), 5-HT5B (forward, 5′-TCG CCC TGG ATC GCT ACT GGA C-3′, and reverse, 5′-GCT CGG CGA CGG GCT GTG AAC-3′), 5-HT6 (forward, 5′-CTT TGG ACC GCC TTC GAC GTG ATG TGC T-3′, and reverse, 5′-GGC TGG CCT TCA AGG CCT TCC TGC TAT G-3′), 5-HT7 (forward, 5′-CCG TGA GGC AGA ATG GGA AAT GTA T-3′, and reverse, 5′-CAC TGC GGT GGA GTA GAT CGT GTA GC-3′). Primers and cycling conditions for SERT, tryptophan hydroxylase type 1 (TPH-1), and TPH-2 were reported previously.24 Transcripts were amplified by 32 cycles for 5-HT7 or 38 cycles for 5-HT1-6 of the following: cDNA denaturation (20 seconds at 95°C), followed by 30 seconds of primer annealing and 30 seconds extension at 72°C. Annealing temperatures were as follows: GAPDH (57°C), 5-HT1A (62°C), 5-HT1B (56°C), 5-HT1D (56°C), 5-HT1F (60°C), 5-HT2A-C (57°C), 5-HT3 (55°C), 5-HT4 (55°C), 5-HT5A (57°C), 5-HT5B (60°C), 5-HT6 (64°C), and 5-HT7 (57°C). PCR products were resolved as single bands by agarose gel electrophoresis and visualized with SYBR Safe (Invitrogen) fluorescence.
For quantitative PCR cDNA was amplified using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) in an MJ Research Chromo4 System (Bio-Rad Laboratories). All reactions were performed in triplicate, with cycling conditions as for conventional RT-PCR. Expression of TPH-1 was normalized to the housekeeping gene 18S using the comparative threshold cycle25 and is presented relative to naive T cells.
5-HT detection
T cells were activated with PMA plus ionomycin, cytocentrifuged onto glass slides, and fixed with 4% paraformaldehyde. 5-HT was labeled with 5-HT antisera (72 hours, room temperature; S-5545; Sigma) followed by biotinylated anti–rabbit Ig (24 hours, 4°C) and SA-Alexa Fluor 546 (1 hour, room temperature; Invitrogen).24 For use as a negative control, 5-HT antisera was preabsorbed with 5-HT (∼ 5 mM, 18 hours, 4°C). Slides were examined using a Zeiss LSM510 META microscope (Oberkochen, Germany) equipped with a Plan-Apochromat 63 × /1.4 numerical aperture oil immersion objective. Pinhole settings were 1 airy unit. Images were acquired with LSM510 software (Zeiss). Figures were compiled using Adobe Photoshop (Adobe Systems, San Jose, CA).
To quantify 5-HT, T cells (5 × 106 cells/mL) were incubated for 6 hours at 37°C in complete media supplemented with 10% charcoal/dextran-treated FBS, 100 μM L-ascorbic acid, 30 μM tranylcypromine (Sigma), and 10 IU/mL IL-2 (PeproTech, Rocky Hill, NJ). 5-HT released into the T-cell culture supernatants was quantified using the Ultra-Sensitive Serotonin enzyme immunoassay (EIA; Labor Diagnostika Nord, Nordhorn, Germany).
Western blot
T cells were lysed in ice-cold buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl pH 7.5, 5 mM EDTA, 1 mM sodium o-vanadate) containing protease inhibitors (Complete Mini; Roche Diagnostics, Mannheim Germany). Debris were sedimented by centrifugation (15 500g, 10 minutes, 4°C), supernatants were collected, and their protein content was determined (Bio-Rad Laboratories). Samples were diluted in 4 × sample buffer (8% SDS, 8% β-mercaptoethanol, 250 mM Tris pH 6.8, 40% glycerol, 2% Bromophenol blue), boiled for 10 minutes, and stored at −20°C until use. Proteins (20 μg/sample) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to supported-nitrocellulose membranes (0.45 μM; Bio Rad Laboratories), and blocked for 2 hours at room temperature with 4% BSA in TBS (50 mM Tris pH 7.6, 150 mM NaCl). Blots were immunolabeled with 1 μg/mL polyclonal rabbit anti-5HT7 or anti–5-HT1B receptor Abs (IM6-368 or IM6-366, respectively; Imgenex, San Diego, CA) overnight at 4°C in TBS containing 0.1% Tween-20 and 4% BSA (TBS-T). Membranes were rinsed 3 times in TBS-T and incubated with secondary Ab, horseradish peroxidase–conjugated goat anti–rabbit IgG (affinity purified H&L; Cell Signaling Technology, Beverly MA) diluted 1:20 000 in TBS-T. Membranes were rinsed 3 times with wash buffer and then incubated with SuperSignal (West Femto Maximum sensitivity substrate; Pierce, Rockford, IL). Membranes were stripped and reprobed with anti–β-actin mAb (1 μg/mL; Clone Ac-15; Sigma) for 3 hours at room temperature, followed by incubation with secondary Ab. Immunoblot signals were quantified using a Bio-Rad GS-700 Image densitometer and analyzed using Molecular Analyst (v2.1.2; Bio-Rad). Densitometry units for each band are expressed as OD adjusted by surface (OD × mm2).
ERK1/2 and IκBα phosphorylation
T cells (5 × 106 cells/200 μL) were stimulated with 5-HT (10 μM) or Con A (10 μg/mL) for 5, 15, or 30 minutes at 37°C. In some experiments 5-HT7 or 5-HT1B receptor signaling was blocked by preincubation with the specific antagonist, SB 269970 (Sigma; S7389; 10-100 nM) or SB 216641 (Sigma; S8942; 10-100 nM) for 60 minutes at 37°C. Cell lysates were prepared, and proteins were resolved by SDS-PAGE. Blots were immunolabeled overnight at 4°C using 1 μg/mL polyclonal rabbit anti–phospho-p44/p42 MAP kinase Ab (Thr 202/Tyr 204) or a polyclonal rabbit anti–phospho-IκBα (Ser 32) Ab (Cell Signaling Technology) diluted in TBS-T. Secondary labeling, detection, and analysis were performed as previously described. Membranes were stripped and reprobed with polyclonal rabbit anti–total ERK1/2 Ab (1 μg/mL; Stressgen Biotechnologies, Victoria, BC) or with mouse anti–β-actin mAb.
Statistical analysis
Errors are the SD of the mean unless otherwise indicated. Statistical comparisons are based on Student t test.
Results
Naive T cells express 5-HT7
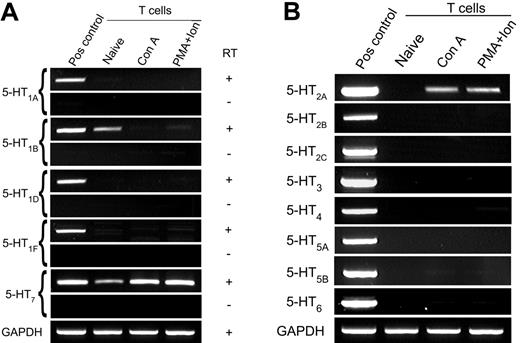
Although 5-HT is implicated in the regulation of T-cell function, the underlying signaling pathways of this monoamine remain unclear. In particular, there are conflicting reports of the identity of 5-HTRs expressed by T cells.3,15-17,20,21 To resolve this controversy, we performed a comprehensive analysis of 5-HTR gene expression in mouse splenic T cells immediately following isolation (naive T cells) and after stimulation with Con A or PMA plus ionomycin for 2 days (activated T cells). The 5-HTRs are grouped into 7 subfamilies.2 For 5-HTR subfamilies 3, 4, 6, and 7, we designed primer pairs that recognized all known variants within the subfamily. With the exception of subfamilies 1 and 7, primers spanned intron-exon boundaries and thus do not amplify genomic sequences. RT-negative samples were amplified in parallel with cDNA samples for analysis of 5-HT1 and 5-HT7 receptor expression.
Using 32 cycles of RT-PCR, we determined that naive T cells express the 5-HT7 receptor, and levels of this transcript increased following T-cell activation (Figure 1A). In contrast to previous reports, however,16,20,21 the 5-HT1A receptor was not detected using either our own primers or the primer sequences and amplification conditions as reported by Abdouh et al21 (data not shown). To confirm this result was not mouse strain specific, we analyzed T cells from both C57Bl/6 and BALB/c mice (data not shown). Small amounts of 5-HT1B receptor gene transcripts were observed in naive T cells after 38 cycles of amplification. For direct comparison with 5-HT7 receptors, detection of 5-HT1B receptor gene transcripts using 32 amplification cycles is shown as supplementary data (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, and consistent with reports that some mouse effector T cells display sensitivity to 5-HT2 receptor agonist/antagonists,3,17 we show that activated T cells express the 5-HT2A receptor (Figure 1B).
Expression of 5-HTR by naive and activated T cells. T cells were negatively purified and activated with Con A or PMA plus ionomycin, and gene expression for 5-HTR subtype was examined by RT-PCR. (A) Primers for the type 1 and type 7 5-HTR families amplify a region with 1 exon; thus, both cDNA and RT-negative (RNA) samples are shown. (B) Gene expression of the 5-HT2-6 receptor subfamilies. Comparable quantities of cDNA were ensured by amplification of GAPDH. Data are representative of 3 independent experiments.
Expression of 5-HTR by naive and activated T cells. T cells were negatively purified and activated with Con A or PMA plus ionomycin, and gene expression for 5-HTR subtype was examined by RT-PCR. (A) Primers for the type 1 and type 7 5-HTR families amplify a region with 1 exon; thus, both cDNA and RT-negative (RNA) samples are shown. (B) Gene expression of the 5-HT2-6 receptor subfamilies. Comparable quantities of cDNA were ensured by amplification of GAPDH. Data are representative of 3 independent experiments.
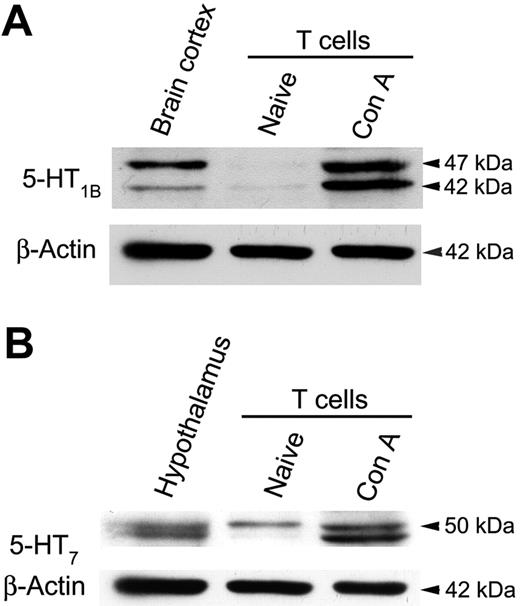
To confirm 5-HTR protein expression we used Western blot and immunolabeling to examine protein lysates from naive and activated T cells. Although 5-HT1B receptor gene transcripts were only detected in naive T cells, immunolabeling revealed that protein was largely expressed by activated T cells (Figure 2A). These data imply that 5-HT1B receptor expression is subject to post-transcriptional regulation. As expected, 5-HT7 receptor protein was expressed by naive T cells, and its expression was increased following T-cell activation (Figure 2B). Two protein products were observed for both 5-HT1B and 5-HT7 receptors, between 41 and 50 kDa, and are consistent with reports of splice variants and post-translational modifications.26-29 This conclusion is supported by observations of similar doublets in the positive control samples (Figure 2A-B).
Naive T cells primarily express 5-HT7 receptors. Representative immunoblots showing relative expression of (A) 5-HT1B and (B) 5-HT7 receptors by T cells. Blots were stripped and reprobed for β-actin to ensure comparable quantities of protein were analyzed (lower blots). Data are 1 of 2 similar experiments.
Naive T cells primarily express 5-HT7 receptors. Representative immunoblots showing relative expression of (A) 5-HT1B and (B) 5-HT7 receptors by T cells. Blots were stripped and reprobed for β-actin to ensure comparable quantities of protein were analyzed (lower blots). Data are 1 of 2 similar experiments.
T cells synthesize 5-HT in an activation-dependent manner
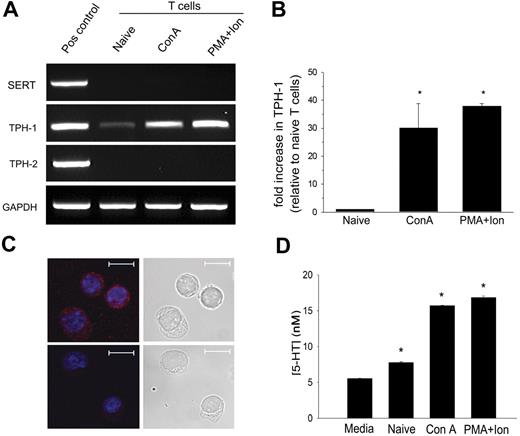
We considered the possibility that some reported inconsistencies of 5-HT signaling in T cells might result from a 5-HT source endogenous to T cells. First, we determined whether T cells can selectively take-up 5-HT. Using RT-PCR, we determined that neither naive nor activated T cells express the high-affinity serotonin transporter, SERT, and therefore presumably do not selectively sequester 5-HT (Figure 3A). The norepinephrine transporter was not detected. Small amounts of the dopamine transporter (DAT) were identified in naive and Con A–activated T cells but only after sequence-specific reverse transcription (Figure S2). Given the rarity of DAT gene transcripts, coupled with the low efficacy of 5-HT transport by DAT (5-HT uptake by SERT KO neuronal cultures is approximately 10% of wild-type),30 it appears unlikely that T cells efficiently sequester 5-HT.
Activated T cells are capable of 5-HT synthesis. (A) Expression of gene transcripts for SERT, TPH-1, and TPH-2 was determined by RT-PCR from naive T cells and from T cells activated with Con A or PMA plus ionomycin. (B) The relative expression of TPH-1 was quantified by q-PCR. Data are means ± 1 SD (n = 2, P < .001). (C) 5-HT was visualized by confocal microscopy in T cells activated with PMA plus ionomycin by labeling with 5-HT antisera (Alexa Fluor 546; red) (upper left). 5-HT antisera was preabsorbed with 5-HT as the negative control (lower left). Nuclei were counterstained with TO-PRO-3 (blue). Scale bar represents 10 μm. Data are representative of 3 independent experiments. (D) 5-HT concentrations in T-cell culture supernatants were determined by EIA. Data are mean from triplicate assays ± 1 SD (n = 2, *P < .001).
Activated T cells are capable of 5-HT synthesis. (A) Expression of gene transcripts for SERT, TPH-1, and TPH-2 was determined by RT-PCR from naive T cells and from T cells activated with Con A or PMA plus ionomycin. (B) The relative expression of TPH-1 was quantified by q-PCR. Data are means ± 1 SD (n = 2, P < .001). (C) 5-HT was visualized by confocal microscopy in T cells activated with PMA plus ionomycin by labeling with 5-HT antisera (Alexa Fluor 546; red) (upper left). 5-HT antisera was preabsorbed with 5-HT as the negative control (lower left). Nuclei were counterstained with TO-PRO-3 (blue). Scale bar represents 10 μm. Data are representative of 3 independent experiments. (D) 5-HT concentrations in T-cell culture supernatants were determined by EIA. Data are mean from triplicate assays ± 1 SD (n = 2, *P < .001).
Previously we have shown that T cells express mRNA for TPH-1,24,30 the rate-limiting enzyme that catalyzes hydroxylation of tryptophan to 5-hydroxy-tryptophan, the immediate precursor of 5-HT. Synthesis of peripheral and central nervous system 5-HT is regulated separately by 2 isoenzymes, TPH-1 and TPH-2, respectively.31 To further characterize TPH-1 expression we performed quantitative RT-PCR. Figure 3A shows that T cells produce mRNA for TPH-1 but not TPH-2. TPH-1 is barely detectable in naive T cells, but it is up-regulated by approximately 30-fold following T-cell activation (Figure 3B).
To confirm 5-HT synthesis, we used immunolabeling and fluorescence microscopy to directly visualize 5-HT within T cells activated with PMA and ionomycin. As shown in Figure 3C, granules of 5-HT labeled with 5-HT antisera are readily visible in the cytosol. To quantify the amount of 5-HT produced we performed EIA of T-cell culture supernatants (Figure 3D). Activated T cells produced 5-HT in amounts approximately 3-fold greater than the baseline signal from complete media alone. Thus, unlike dendritic cells (DCs),24 T cells are able to synthesize 5-HT, and this activity is enhanced following their activation. This finding supports the hypothesis that 5-HT may play an important role in T-cell activation.
Exogenous 5-HT leads to rapid phosphorylation of ERK1/2 and IκBα
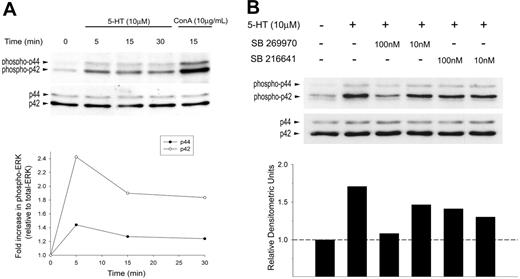
Next, we investigated a functional signal transduction pathway for 5-HT7 receptors in naive T cells. Activation of ERK1/2 is a key step in the signaling cascade that leads to the activation and translocation of transcription factors required for T-cell activation, IL-2 synthesis, and proliferation. As shown in Figure 4A, transient exposure to 5-HT (10 μM) led to the rapid (≤ 5 minutes) phosphorylation of ERK1/2. To identify the 5-HTR responsible for ERK1/2 activation we pretreated T cells with the specific 5-HT7 receptor antagonist, SB 269970 (10-100 nM) or the 5-HT1B receptor antagonist, SB 216641 (10-100 nM). Figure 4B shows that SB 269970 fully inhibited 5-HT–induced ERK1/2 phosphorylation. In contrast, SB 216641 induced only a partial inhibition of ERK1/2 activation that may represent the known cross-reactivity of 5-HT1B receptor ligands with the 5-HT7 receptor. Alternatively, this may reflect concomitant signaling via small amounts of 5-HT1B receptors.
5-HT induces rapid phosphorylation of ERK1/2 that is inhibited by a 5-HT7 receptor–selective antagonist. Freshly isolated naive T cells were incubated with exogenous 5-HT (10 μM) at 37°C. Samples were lysed, immunoblotted, and probed for (A) phospho-ERK1/2 (top). Blots were stripped and reprobed for total ERK1/2 to confirm equal loading in each lane (bottom). Densitometric analysis was performed showing a maximal increase in phospho-ERK1/2 at 5 minutes following stimulation with exogenous 5-HT (relative to total ERK). (B) Freshly isolated naive T cells were incubated with SB 269970 (5-HT7 receptor antagonist) or SB 216641 (5-HT1B receptor antagonist) for 1 hour, 37°C. Samples were then pulsed with 5-HT (10 μM) for 5 minutes at 37°C) and analyzed for phospho-ERK1/2 (top) and total ERK1/2 (bottom). Densitometric analysis was performed, showing phospho-ERK p42 (relative to total ERK).
5-HT induces rapid phosphorylation of ERK1/2 that is inhibited by a 5-HT7 receptor–selective antagonist. Freshly isolated naive T cells were incubated with exogenous 5-HT (10 μM) at 37°C. Samples were lysed, immunoblotted, and probed for (A) phospho-ERK1/2 (top). Blots were stripped and reprobed for total ERK1/2 to confirm equal loading in each lane (bottom). Densitometric analysis was performed showing a maximal increase in phospho-ERK1/2 at 5 minutes following stimulation with exogenous 5-HT (relative to total ERK). (B) Freshly isolated naive T cells were incubated with SB 269970 (5-HT7 receptor antagonist) or SB 216641 (5-HT1B receptor antagonist) for 1 hour, 37°C. Samples were then pulsed with 5-HT (10 μM) for 5 minutes at 37°C) and analyzed for phospho-ERK1/2 (top) and total ERK1/2 (bottom). Densitometric analysis was performed, showing phospho-ERK p42 (relative to total ERK).
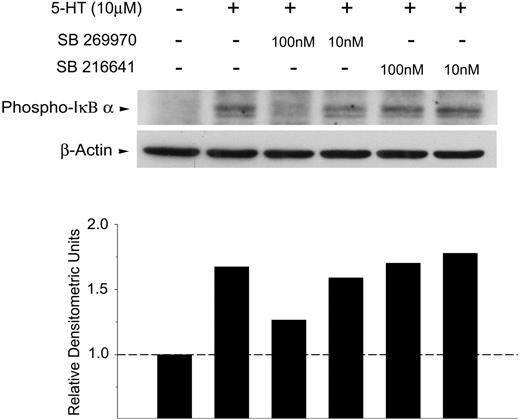
To further dissect the components of 5-HT signaling in T cells, we examined the activation of NFκB, a transcription factor involved in the up-regulation of IL-2 gene transcription. Signals that activate NFκB lead to the phosphorylation and dissociation of the negative regulatory subunit IκBα and thereby allow NFκB to translocate into the nucleus. As shown in Figure 5, exogenous 5-HT induces the rapid phosphorylation of IκBα, which is substantially inhibited by the selective 5-HT7 receptor antagonist, SB 269970 (100 nM). In contrast, no inhibition of phospho-IκBα was observed in response to the 5-HT1B receptor antagonist, SB 216641. Combined, these data indicate that naive T cells express functional 5-HT7 receptors that can trigger early T-cell activation events.
Exogenous serotonin induces rapid phosphorylation of IκBα that is inhibited by a 5-HT7 receptor–selective antagonist. Freshly isolated naive T cells were incubated with SB 269970 (5-HT7 receptor antagonist) or SB 216641 (5-HT1B receptor antagonist) for 1 hour, 37°C. Samples were then pulsed with exogenous 5-HT (10μM) for 5 minutes at 37°C and analyzed for phospho-IκBα (top). Blots were stripped and reprobed for β-actin to confirm equal loading in each lane (bottom). Densitometric analysis was performed showing phospho-IκBα (relative to β-actin).
Exogenous serotonin induces rapid phosphorylation of IκBα that is inhibited by a 5-HT7 receptor–selective antagonist. Freshly isolated naive T cells were incubated with SB 269970 (5-HT7 receptor antagonist) or SB 216641 (5-HT1B receptor antagonist) for 1 hour, 37°C. Samples were then pulsed with exogenous 5-HT (10μM) for 5 minutes at 37°C and analyzed for phospho-IκBα (top). Blots were stripped and reprobed for β-actin to confirm equal loading in each lane (bottom). Densitometric analysis was performed showing phospho-IκBα (relative to β-actin).
Inhibition of 5-HT signaling impairs T-cell activation and proliferation
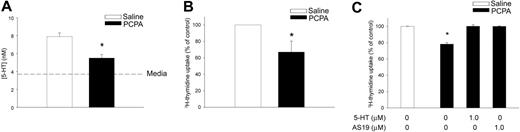
To examine the physiologic relevance of 5-HT signaling, we examined T-cell activation and proliferation. In preliminary studies we identified a subtle enhancement of T-cell proliferation in vitro in response to exogenous 5-HT; however, these data were not reliably significant from vehicle controls (data not shown). It is conceivable that autocrine synthesis of 5-HT by T cells may have confounded these experiments and many earlier studies exploring the role of 5-HT in T-cell–mediated immune responses, reviewed by Meredith et al.2 To inhibit endogenous 5-HT production we treated C57Bl/6 mice with PCPA, a well-established specific inhibitor of TPH activity in vivo.22 Naive splenic T cells were isolated and cultured for 18 hours in the presence of IL-2 (10 IU/mL) to support their viability. As shown in Figure 6A, the basal synthesis of 5-HT was significantly reduced in naive T cells from PCPA-treated mice, compared with saline-treated controls.
Inhibition of endogenous 5-HT synthesis impairs T-cell proliferation ex vivo. Splenic CD3+ T cells were negatively enriched from C57BL/6 mice pretreated with PCPA or saline. (A) Basal 5-HT released from naive T cells isolated from saline- (□) or PCPA-treated mice (▪) after overnight culture with IL-2 (10 IU/mL). Dashed line indicates background 5-HT resulting from complete media alone. Data are mean ± 1 SD from triplicate results (n = 2, *P < .01). (B) T cells from saline- or PCPA-treated mice were incubated for 48 hours with plate-bound anti-CD3 mAb (5 μg/mL). Data are the mean proliferation ± 1 SE from 6 independent assays, performed in triplicate (*P < .05). (C) T-cell proliferation in the presence of exogenous 5-HT (1 μM) or the 5-HT7 receptor agonist, AS19 (1 μM). Data are mean ± 1 SD from 1 representative experiment (n = 3) performed in triplicate (*P < .05).
Inhibition of endogenous 5-HT synthesis impairs T-cell proliferation ex vivo. Splenic CD3+ T cells were negatively enriched from C57BL/6 mice pretreated with PCPA or saline. (A) Basal 5-HT released from naive T cells isolated from saline- (□) or PCPA-treated mice (▪) after overnight culture with IL-2 (10 IU/mL). Dashed line indicates background 5-HT resulting from complete media alone. Data are mean ± 1 SD from triplicate results (n = 2, *P < .01). (B) T cells from saline- or PCPA-treated mice were incubated for 48 hours with plate-bound anti-CD3 mAb (5 μg/mL). Data are the mean proliferation ± 1 SE from 6 independent assays, performed in triplicate (*P < .05). (C) T-cell proliferation in the presence of exogenous 5-HT (1 μM) or the 5-HT7 receptor agonist, AS19 (1 μM). Data are mean ± 1 SD from 1 representative experiment (n = 3) performed in triplicate (*P < .05).
Next, we determined whether inhibition of 5-HT synthesis impairs T-cell activation and initial proliferation. Bulk, naive T cells were negatively enriched from PCPA- or saline-treated mice and cultured for 48 hours with plate-bound anti-CD3 mAb. As shown in Figure 6B, T-cell proliferation from PCPA-treated mice was significantly reduced compared with saline-treated controls. The efficacy of PCPA inhibition ranged from approximately 18% to 49% (data not shown). Addition of exogenous 5-HT (1 μM) fully restored T-cell proliferation (Figure 6B). Because 5-HT is nonselective, we tested whether T-cell proliferation from PCPA-treated mice could be restored using the 5-HT7 receptor agonist, AS19. Similar to exogenous 5-HT, addition of AS19 (1 μM) completely restored T-cell proliferation after 48 hours (Figure 6C).
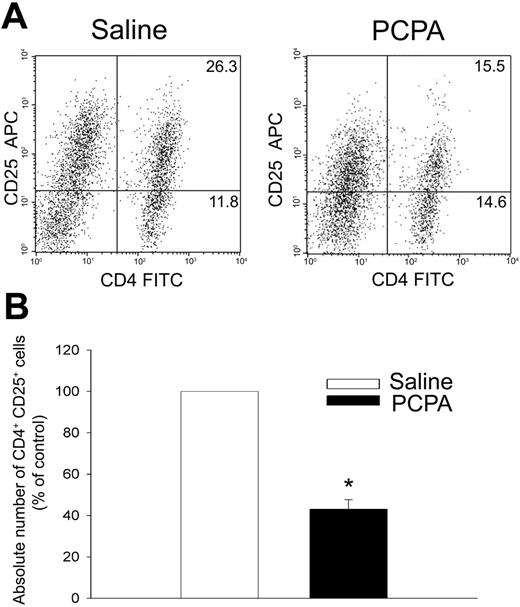
Next, we used immunolabeling and flow cytometry to examine expression of the IL-2Rα chain (CD25) as an indicator of T-cell activation. As shown in Figure 7, PCPA treatment significantly reduced expression of CD25 by CD4+ T cells. The percentage and the absolute number of CD4+ CD25+ T cells were both significantly reduced (Figure 7A-B). In addition, the mean fluorescence of labeled CD25 by CD4+ T cells was also reduced, indicating that fewer molecules of CD25 were expressed per cell (Figure 7A). Together, these data strongly support the hypothesis that 5-HT is an important autocrine stimulus that promotes T-cell activation and proliferation.
Inhibition of endogenous 5-HT synthesis impairs T-cell expression of CD25. T cells from saline- or PCPA-treated mice were incubated for 48 hours with plate-bound anti-CD3 mAb (5 μg/mL). (A) Expression of CD25 and CD4 by T cells analyzed by flow cytometry. (B) Absolute number of CD25+ CD4+ T cells expressed as mean (percentage of control) ± 1 SE from triplicate assays (n = 4, *P < .001).
Inhibition of endogenous 5-HT synthesis impairs T-cell expression of CD25. T cells from saline- or PCPA-treated mice were incubated for 48 hours with plate-bound anti-CD3 mAb (5 μg/mL). (A) Expression of CD25 and CD4 by T cells analyzed by flow cytometry. (B) Absolute number of CD25+ CD4+ T cells expressed as mean (percentage of control) ± 1 SE from triplicate assays (n = 4, *P < .001).
Discussion
Self-inflammatory molecules produced in response to microbial insult or cellular stress, and those released by dead and dying cells, produce profound effects on both the innate and adaptive immune systems.32-35 Similarly, neuropeptides and neurotransmitters that have key functions in the central and peripheral nervous systems can exert immune modulatory effects.2,5,36 Indeed, emerging data demonstrate that these molecules can be transported or expressed by hematopoietic cells.24,37,38 In our previous study, we demonstrated that DCs take up and sequester 5-HT in lysosomal vesicles for subsequent release via Ca2+-triggered exocytosis.24 Because DCs are specialized to stimulate naive T cells and initiate primary adaptive immune responses,39 we postulated that 5-HT released from DCs may modulate T-cell function.24
We have now extended this initial study to examine 5-HT signaling in T cells and reveal several novel findings. First, we show that the 5-HT7 receptor is the predominant 5-HTR expressed by naive mouse T cells and is dramatically up-regulated following T-cell activation. The 5-HT7 receptor is the most recently identified member of the G protein–coupled 5-HTR family.40 Although the 5-HT7 receptor is identified in gross lymphoid tissues and bulk hematopoietic cells, its expression by purified lymphocytes has not been reported.40,41 We find that T-cell–expressed 5-HT7 receptors are functional. Signaling through the 5-HT7 receptor induces the second messenger phospho-ERK1/2 and activates the transcription factor NFκB, known early events that converge in de novo IL-2 synthesis and T-cell proliferation. Second, we demonstrate that T cells synthesize 5-HT, following up our earlier identification of TPH-1 in these cells.24 Importantly, endogenous production of 5-HT appears to contribute to T-cell activation; inhibition of TPH-1 by PCPA leads to reduced proliferation, lower levels of CD25 expression, and fewer CD4+ CD25+ T cells. Because 5-HT alone had no apparent effect on T-cell proliferation (M.L.-P. and P.J.O., unpublished observations, January 2005), we conclude that 5-HT acts as a cofactor that interacts synergistically with T-cell receptor (TCR) signaling to promote T-cell activation and proliferation.
Our finding that naive T cells primarily express 5-HT7 receptors is inconsistent with several studies that demonstrate pharmacologic sensitivity to type 1 and type 2 5-HTR ligands.16,17,20,24 5-HT7 receptors share sensitivity to a number of important and widely used 5-HT1 and 5-HT2 receptor ligands.40,42,43 Thus, some 5-HT effects attributed to 5-HT1 or 5-HT2 receptors in T cells may actually be signaled by the 5-HT7 receptor. At this time we cannot exclude 5-HT1B expression and signaling by naive T cells. Faint signals characteristic of this 5-HTR were detected by Western blot, and a selective 5-HT1B receptor antagonist partially inhibits 5-HT–mediated phosphorylation of ERK1/2. It is conceivable, however, that this signal is contributed by small numbers of resting, memory T cells (CD62L negative) present in the spleen of our naive, specific pathogen-free mice. These are key questions that will be addressed in ongoing studies that examine 5-HTR expression by purified T-cell subsets.
As T cells become activated, the interpretation of 5-HT signal transduction becomes complex. First, on T-cell activation it is primarily expression of the lower molecular weight 5-HT7 receptor species that is increased. This may represent differential regulation of 5-HT7 receptor splicing (3 splice variants are recognized in mice), and/or post-translational modification.26 Furthermore, although expression of 5-HT1B and 5-HT2A is low or undetectable in naive T cells, respectively, we find that protein and/or mRNA for these receptors is substantially increased following T-cell activation. Unlike 5-HT7 receptors, 5-HT1B and 5-HT2A receptors are coupled to Gi and Gq proteins and thus likely trigger distinct signaling events in activated T cells, which potentially contribute to sustained proliferation and/or differentiation. Although this study focuses on 5-HT signaling in naive T cells, it will be important in the future to investigate the relative expression and functional significance of 5-HT1B, 5-HT2A, and 5-HT7 receptors in activated T cells. Ideally this would be performed using mice selectively deficient in these receptors.
Although the cross-selectivity of 5-HTR ligands explains many prior reports regarding naive or resting T cells, T cells have been shown to express 5-HT1A gene transcripts and protein.16,21 Despite an exhaustive analysis we are unable to confirm expression of the 5-HT1A receptor by either naive or activated T cells. This finding is consistent with Stefulj et al,41 who also reported that 5-HT1A receptors could not be detected in bulk rodent hematopoietic cells. Although the precise reason for this discrepancy is currently unclear, there are a number of likely contributory factors. In our contemporaneous analysis of all known 5-HTR families we used highly purified, negatively enriched samples of both naive and activated T cells, from both C57Bl/6 and BALB/c mice. We have been extremely cautious in our use of positive and negative RT-PCR controls and high stringency cycling conditions to avoid amplification of spurious PCR products. All PCR products were identified as single bands of correct molecular size. For analysis of the 5-HT1A receptor we used multiple primer pairs of our own design and previously published sequences.21 In support of our PCR findings, functional analysis of cAMP signaling in naive T cells also argues against the expression of 5-HT1A receptors. Signaling through these receptors is well known to reduce cAMP levels via a Gi protein–coupled pathway. However, we have found that neither 5-HT nor a 5-HT1A receptor agonist (S14506), could inhibit elevations in cAMP induced by forskolin or ATP. In contrast, 5-HT but not S14506 inhibits approximately 50% of the cAMP rise produced by rolipram (a type-4 phosphodiesterase inhibitor). This latter effect was not sensitive to pertussis toxin and is thus unlikely mediated by a Gi protein pathway (G.P.A., unpublished observations, May 2006). In neurons, 5-HT1A receptors can signal independently of cAMP to activate inwardly rectifying K+ channels; however, a similar signaling mechanism has not been demonstrated in hematopoietic cells.44 On the basis of the preponderance of evidence, therefore, we conclude that the 5-HT1A receptor is not expressed by mouse T cells.
Although there is evidence that T cells are functionally responsive to 5-HT, until recently the source of this 5-HT was unclear. A recent study in rhesus macaques revealed that CD3+ cells (T cells) localize in close proximity to enterochromaffin cells that secrete 5-HT.45 Previously, we hypothesized that DCs shuttle 5-HT from sites of inflammation to naive T cells.24 Consistent with this, B cells, which also serve as antigen-presenting cells, express functional SERT and sequester 5-HT.38 In this study, we show that T cells can directly synthesize 5-HT which they release into their microenvironment. Importantly, synthesis of 5-HT is increased in response to T-cell activation, a finding that suggests this monoamine may normally play an important role in T-cell function. In future studies we will dissect the physiologic triggers that induce and enhance 5-HT synthesis by T cells. One previous report has demonstrated that IFNγ, a type-1 T-cell–produced cytokine, induces the release of 5-HT from bulk peripheral blood lymphocytes.46 Although this report is of considerable interest, whether this represents de novo synthesis or the release of 5-HT sequestered by B cells is currently unknown. Furthermore, in considering the physiologic relevance of 5-HT synthesis by T cells, it is important to note that 5-HT is a tryptophan metabolite. Catabolism of tryptophan by indoleamine 2,3-dioxygenase has been identified as a regulatory mechanism that controls T-cell activation, proliferation, and functional responses.47 Our findings reveal that the immune regulatory role attributed to indoleamine 2,3-dioxygenase may reflect a more complex mechanism. Rather than leading to a simple deficit of tryptophan, indoleamine 2,3-dioxygenase may impair the synthesis of 5-HT by T cells, thereby limiting the potential for autocrine 5-HT signaling.
In summary, as novel data we show that T cells can synthesize the monoamine 5-HT. In turn, 5-HT signals via the 5-HT7 receptor in naive T cells to augment early T-cell activation events. We propose that an autocrine 5-HT signaling pathway may play an important role in the regulation of T-cell function and activation. In addition, these findings may lead to an improved understanding of the role of 5-HT in the pathogenesis of some inflammatory disorders5,10-14 and tumor cell growth.48
Authorship
Contribution: M.L.-P. designed and performed the research and analyzed the data; and G.P.A. and P.J.O. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peta J. O'Connell, Robarts Research Institute, PO Box 5015, 100 Perth Dr, London, ON, N6A 5K8, Canada; e-mail: peta@robarts.ca.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Drs Joaquin Madrenas and Michael Poulter for advice and discussion, Eilis Hamilton for guidance with Western blotting, and Meaghan Stolk for the design of some primers and quantitative PCR for TPH-1. We thank Mia Merrill and Xiangbin Wang for their technical support.
This work was supported by the National Institutes of Health (grant NIAID AI054450) (G.P.A. and P.J.O.) and by the Premiers' Research Excellence Award (PREA) (M.L.-P. and P.J.O.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal