Mouse T lymphocytes unexpectedly produce the classic neurotransmitter serotonin, which—upon binding the constitutively expressed 5-HT7 Gs-coupled receptor-subtype—signals ERK1/2 phosphorylation and NFκB activation to boost their stimulation.
Serotonin/5-hydroxytryptamine (5-HT), often perceived as the brain's “happy chemical,” is—increasingly—assuming a prominent role in immune regulation. In fact, this “neurotransmitter” is primarily a product of the periphery, with gut enterochromaffin cells the principal factories. Intestinal lymphocytes could certainly be exposed directly to their output, though platelets are typically proffered as the source of the monoamine within the immune system: these 5-HT–loaded reservoirs delivering their potent cargo at sights of inflammation and immunologic reactivity.1,2
In this issue of Blood, León-Ponte and colleagues not only consolidate serotonin's importance to T-cell activation but also tender a paradigm whereby the monoamine is provided by the immune cell itself. Critical to any outcome from 5-HT exposure—whether the source be autocrine or paracrine—is a capacity for target cells to sense the monoamine. This could be via the serotonin transporter (SERT) or one or more of 14 receptor subtypes (13 in rodents).1,2 Using reverse transcription–polymerase chain reaction (RT-PCR), the authors ruled out SERT and all but 3 receptor subtypes: only 5-HT1B, 5-HT2A, and 5-HT7 receptors remaining to deliver the 5-HT hit. While not excluding contributions from 5-HT1B and 5-HT2A receptors, the authors homed in on 5-HT7 as the major player: this 7-transmembrane domain G protein–coupled receptor (GPCR) assisted T-cell stimulation by promoting ERK1/2 phosphorylation and canonical NFκB activation. Not only were the authors able to attenuate 5-HT–dependent change with a selective 5-HT7 receptor antagonist but, by using a 5-HT7 receptor agonist, were able to reverse otherwise defective T-cell stimulation arising as a consequence of having blocked tryptophan hydroxylase (TPH), the rate-limiting enzyme in the conversion of tryptophan to 5-HT. Tryptophan is also the catabolic substrate for indolamine-2,3-dioxygenase (IDO), and the relative abundance/activity of TPH, IDO, and, additionally, metabolizing monoamine oxidases (MAOs) could determine 5-HT output from immune cells.
While the present study marks a significant advance, the authors themselves address seeming inconsistencies between this and isolated reports on the relative importance of individual 5-HT receptor subtypes to T-cell function, and rightly posit additional questions. Issues include strain and species differences, tissue and subset heterogeneity, and methodological concerns.
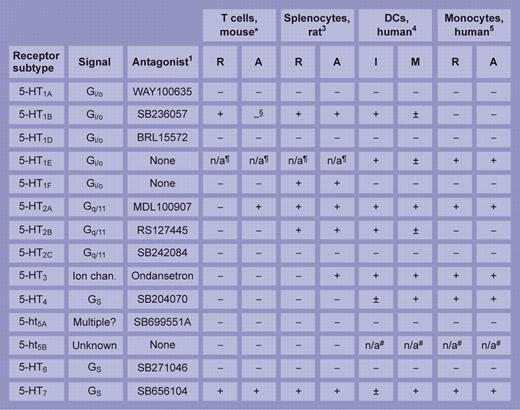
Few studies have attempted such an extensive survey of an immune cell's 5-HT receptor repertoire as the one here. The table summarizes and compares the study's salient features with 3 others of merit: a pioneering study on rat splenocytes3 and, from Idzko and colleagues, separate analyses of receptor subtype distribution on human dendritic cells (Idzko et al4 ) and monocytes (Durk et al5 ). Of the discordant and common themes, the near-universal expression of 5-HT7 receptors—irrespective of cell type or animal species—is striking. A built-in knowledge of the pharmacology and signaling properties of 5-HT receptors (see table), driven by and gained largely through their contribution to CNS pathways, holds promise for the ready translation of 5-HT receptor therapeutics to immunology and hematology clinics once a more robust integration of their place in immune physiology—and pathology—has been secured.
Reported expression of 5-HT receptor transcripts in immune cells. Synthesis of findings from studies by *León-Ponte et al in this issue of Blood and from Stefulj et al,3 Idzko et al,4 and Durk et al5 highlighting the repertoire of 5-HT receptor subtype of mRNA expressed in immune cells. R indicates resting; A, activated; I, immature; M, mature; Ion chan., ligand-gated ion channel; −, negative; +, strong signal; and ±, weak signal. §5-HT1B protein increased on activation. ¶Absent from mouse and rat. #Truncated transcript in human. Illustration by Frank Forney.
The authors are directors and cofounders of Celentyx Ltd, a spinout company whose activities include the profiling of serotonergic compounds against immune cells. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal