As the butterfly flaps its wings, so can a small latent change within hematopoietic cells have major effects on a pathological outcome such as leukemia.
The paper by Zhao and colleagues in this issue of Blood indicts a leukemia-related chimeric protein for selectively killing one cell type while sparing another to cause a cell type–specific disease.1 The runt-related transcription factor 1 (RUNX1/AML1) and its cofactor core-binding factor subunit β (CBFβ) are essential regulators of hematopoiesis. Genetic defects including chromosomal translocations that affect RUNX1 (t(8;21)) or CBFB (Inv16) genes have each been causally linked to the etiology of acute myelogenous leukemia. Both proteins are expressed in the myeloid and lymphoid lineages, yet pathological defects in RUNX1 or CBFβ function are evident only in the myeloid lineage. Why are the tumorigenic effects of RUNX1 and CBFβ lineage restricted? The spatiotemporal expression of RUNX1 and the extent of redundancy with other RUNX regulatory factors (eg, RUNX2 and RUNX3) could account in part for preferential effects in myeloid cells. However, CBFβ is ubiquitously expressed, and genetic aberrations that produce dominant-negative forms of CBFβ may affect normal myeloid growth and differentiation at any stage. The paper by Zhao and colleagues provides a cogent model to explain how a dominant-negative CBFβ protein causes tumors in the myeloid but not lymphoid lineage.
The CBFβ-SMMHC fusion protein is a prototypical dominant-negative factor, which is generated from the chimeric CBFB-MYH11 gene as a result of the chromosome 16 inversion that is characteristic of human acute myeloid leukemia subtype M4Eo. The CBFβ-SMMHC related fusion protein inactivates RUNX1 and acts as an oncogene that promotes proliferation of immature myeloid progenitors. Mouse models based on CBFB-MYH11 have been effectively used to examine the roles of RUNX1 and CBFβ in hematopoiesis and progression of leukemia. Heterozygous expression of Cbfb-MYH11 in a knock-in mouse model results in a phenotype similar to that of mice with complete loss of Runx1 or Cbfb.2 Moreover, Lucio Castilla's group (Kuo et al3 ) showed that early expression of Cbfb-MYH11 causes abnormalities in myeloid progenitors, thereby predisposing cells to leukemia. Recent studies by the research groups led by Nancy Speck (Zhao et al1 ) and Paul Liu (Talebian et al4 ) have revealed that, in addition to its role in myeloid differentiation, CBFβ is required for the T-lymphoid lineage.
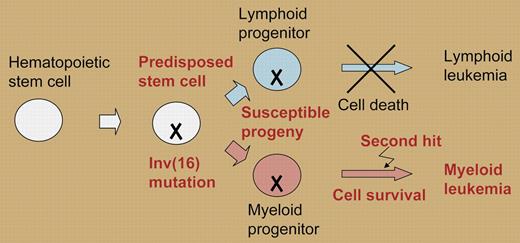
Of importance, the chromosome 16 inversion occurs in hematopoietic stem cells, and thus the CBFB-MYH11 fusion gene should affect both the lymphoid and myeloid lineages. In the current paper from Zhao and colleagues, T-cell development was examined in mice that express a conditional Cbfb-MYH11 fusion gene, and the resulting phenotypes were compared with mice with severe Cbfb deficiency due to mutations generating null or hypomorphic alleles.1 Cbfb-MYH11 suppresses Cbfβ function at several stages of T-cell development. Hypomorphic mice with Cbfβ deficiency have a 20-fold reduction in thymocytes due to a differentiation block at the DN1 and DN2 stages. In contrast, Cbfb-MYH11 knock-in chimeras are blocked at the DN1 stage of thymocyte development. Cre activation of the conditional Cbfb-MYH11 knock-in reduces the number of adult thymocytes by an order of magnitude due to increased apoptosis of CD4+/CD8+ thymocytes. Consistent with these findings, the authors propose that the mechanistic basis for the association of CBFB-MYH11 with myeloid leukemias rather than lymphoid leukemias is due to a molecular butterfly effect (see figure): cellular context determines that the fusion protein increases cell death in lymphoid cells, but does not decrease survival of myeloid cells that hence remain susceptible to secondary hits that cause leukemia.
The CBFB-MYH11 butterfly effect. The Inv(16) mutation causes a latent lesion that decreases cell survival in lymphoid cells (blue) but not in myeloid cells (red).
The CBFB-MYH11 butterfly effect. The Inv(16) mutation causes a latent lesion that decreases cell survival in lymphoid cells (blue) but not in myeloid cells (red).
The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal